Introduction
Glaucoma is a member of a group of eye diseases
characterized by an increased intraocular pressure (IOP),
degeneration of the optic nerve and irreversible retinal ganglion
cell (RGC) death (1). Elevated
pressure is a significant risk factor (2,3).
Although the role of glial cells in glaucomatous retinae remains
under debate, certain research has found that the expression of
various genes by Müller cells can be altered in glaucomatous
models, including the glial fibrillary acidic protein (GFAP),
glutamine synthetase (GS) and nestin (1,4–6).
However, the situation in inwardly rectifying potassium (Kir)
channels remains unclear.
Müller cells, the major glial cells of the retina,
provide functional and structural support to the retinal neurons
and constitute a functional link between neurons and vessels. One
of the key roles of these cells is the spatial buffering of
extracellular K+ ions. Müller cells transport
K+ through their cell bodies away from excited neurons,
from extracellular regions of ‘high K+’ to those of ‘low
K+’ in the retina. In Müller cells, the most significant
mediators of K+ buffering are the Kir channels,
particularly the Kir 2.1 and Kir 4.1 channels (7–10).
Kir 2.1 channels are strongly rectifying Kir channels, which allow
inward K+ currents even at high extracellular
K+ concentrations. However, Kir 4.1 channels are weakly
rectifying Kir channels at negative potentials, allowing either
‘inward’ or ‘outward’ K+ currents, depending on the
concentration of extracellular K+. The present study
suggests that the acceleration of K+ clearance through
the Kir 2.1 and Kir 4.1 channels in Müller cells can prevent the
effects of neuronal information processing by depolarization caused
by glia-derived K+ (8,11,12).
Adenosine is a natural chemical messenger, which
binds to four subtypes (A1, A2A,
A2B and A3) of adenosine receptors (ARs), and
regulates the physiological functions of cells. In the retina,
adenosine is capable of dilating vessels and serves an
autoregulatory role in mediating the compensatory dilation in
response to hypoxia, ischemia, hypoglycemia and high hydrostatic
pressure (13–15).
The aim of this study was to investigate the
expression of Kir 2.1 and Kir 4.1 channels at an elevated
hydrostatic pressure in vitro, and determine whether
adenosine can modulate the expression of Kir 2.1 and Kir 4.1
channels in retinal Müller cells at an elevated hydrostatic
pressure in vitro.
Materials and methods
Reagents
Adenosine was purchased from Sangon Biotech Co. Ltd.
(Shanghai, China). A total of 1 μM of adenosine was dissolved in
serum-free medium prior to usage (16,17).
Pressure system
T75 culture flasks, equipped with manometers, were
placed in incubators and maintained at 37°C, as the pressure
mechanism, as described in detail in our previous study (3). A mixture of 95% air and 5%
CO2 was pumped into the flasks to obtain the pressure
(2). To determine the short-term
effects of elevated pressure on the Müller cells, we exposed our
cultures to a wide range of pressures (20, 40, 60 and 80 mmHg). In
our previous study, we evaluated the effects of glutamine
synthetase (GS) at each pressure for 24 or 48 h and determined that
a hydrostatic pressure of 40 mmHg/24 h produced the most reliable
and measurable effects on the Müller cells. Thus, we used 40
mmHg/24 h as the experimental condition.
Cell separation and culture
Eyeballs from post-natal 0–3 day Sprague-Dawley rats
(Slaccas Laboratory Animal Co. Ltd, Shanghai, China) were
enucleated, and the retina of each was freely dissected and stored
on ice in D-Hank’s solution (Anresco). Tissue was dissociated by
centrifugation and incubated for 15 min at 37°C in
phosphate-buffered saline (PBS) containing 0.125% trypsin
(Anresco). Finally, the cell suspension was cultured in T75 culture
flasks at 37°C in humidified air containing 5% CO2.
Following primary initial outgrowth, the cell culture medium was
replaced every 48 h, and maintained in DMEM/F12 medium (Invitrogen,
Carlsbad, CA, USA) supplemented with 100 U/ml penicillin, 100 μg/ml
streptomycin and 10% fetal bovine serum (FBS) (Sijiqing).
Following 5–8 days, all of the flasks were agitated
at 37°C, 100 rpm for 1 h, and the cell culture medium was
refreshed. Through agitation the other cell types (microglial cells
and RGCs), which were initially adhered to the surface of the
Müller cells, were rinsed off and a purified flat cell population
was obtained. For passage, cell cultures were incubated at 37°C
with PBS containing 0.125% trypsin.
Using immunocytochemisty, early passage Müller cells
and the Müller cell marker, glutamine synthetase (GS), were
characterized (3,18). Contamination from other cell types
was also tested and reported, and revealed <10% of cells
expressing specific makers for other cell types, including
astrocytes and microglial cells.
Experiments were performed following the second
passage when cell confluence was 80–90%. Cells were cultured in
serum-free medium for 16 h. Then, the cells were cultured in 0 mmHg
(the control) or 40 mmHg in the presence or absence of 1 μM
adenosine for 24 h.
Immunofluorescence
The cultured cells which had grown to 80% confluence
on the coverslips were fixed in sodium phosphate buffer (100 mM, pH
7.4) containing 4% paraformaldehyde for 10 min. The cells were
washed in PBS, then incubated with various primary GS antibodies
(Abcam, 1:5000, polyclonal rabbit anti-GS antibody) overnight at
4°C. Subsequently, the cells were washed three times (5 min each)
in PBS, and immunolabeled with fluorescein isothiocyanate Cy3
(BioLegend,1:200) linked with anti-mouse or anti-rabbit IgG. The
labeled cells were visualized and processed using an Axio
microscope (Zeiss).
RNA extraction
Total RNA from cultures was isolated using TRIzol
reagent (Gibco) according to the manufacturer’s instructions. RNA
was treated with RNase-free DNase (Sangon Biotech) to remove any
genomic DNA contamination. The isolated RNA had an optical density
(OD) 260/280 ratio of ≥2.0.
Real-time PCR
To synthesize a cDNA template for PCR, we
reverse-transcribed 2 μg total RNA to a cDNA probe. The primer
sequences were as follows: Kir 2.1 channels: sense,
5′-gcctcctggttgctgttc-3′ and antisense, 5′-tggtggtctgcgtctcaat-3′;
Kir 4.1 channels: sense, 5′-agttcgcacttcctatctaccg-3′ and
antisense, 5′-gggacgccactttcacaa-3′; β-actin: sense, 5′-cccatctat
gagggttacgc-3′ and antisense, 5′-tttaatgtcacgcacgatttc-3′.
Real-time PCR was performed using a LightCycler instrument
(Rotor-Gene), with a SYBR-Green PCR Master mix (Shuiyuan Biotech),
according to the manufacturer’s instructions. The PCR conditions
were as follows: initial denaturation at 94°C for 5 min and 40
cycles performed at 94°C for 30 sec, 55°C for 30 sec and 72°C for
30 sec.
Statistical analysis
Data were reported as the means ± standard error of
the mean (each group, n=3–4). All analyses were performed with the
SPSS statistical package. Data were analyzed using one-way analysis
with a P<0.05 used to indicate a statistically significant
difference.
Results
Identification of cultured retinal Müller
cells
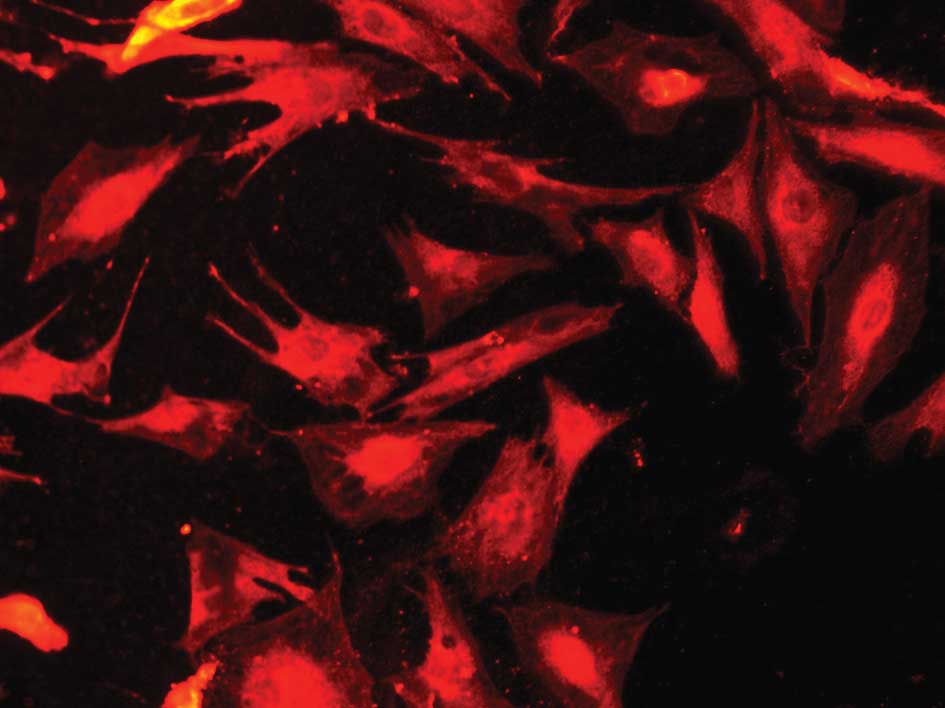
We used immunofluorescence to identify the cultured
Müller cells. The cultured cells demonstrated positive labeling for
GS, the molecular markers for Müller cells in the retina. From the
immunocytochemical labeling, the cultured cells were considered to
be Müller cells (Figs. 1 and
2).
Effect of adenosine on the expression of
Kir 2.1 channels in the cultured retinal Müller cells
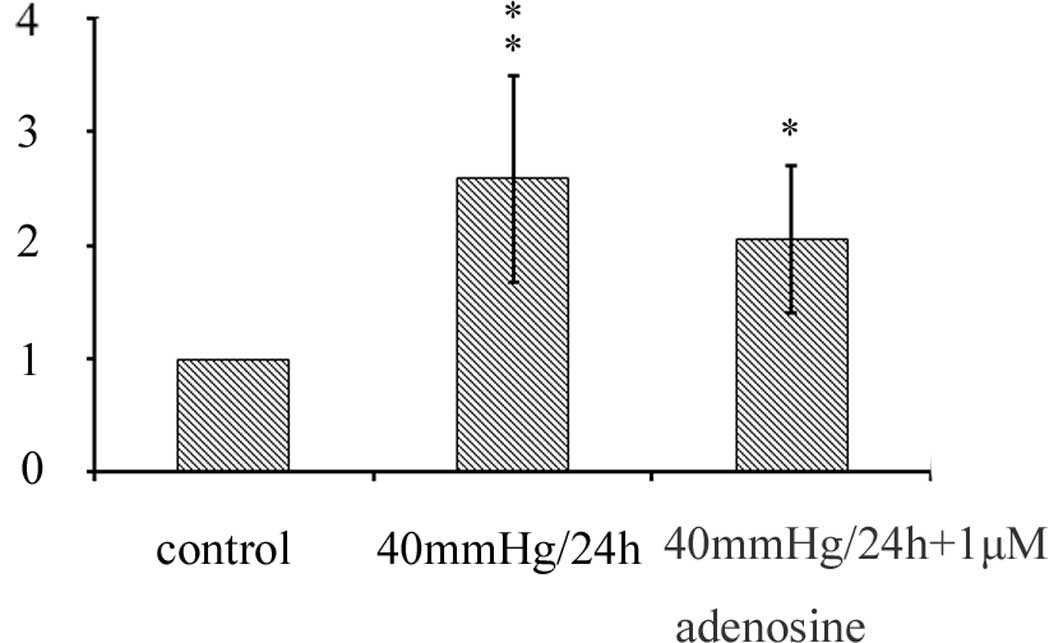
In our study, the real-time PCR data revealed that
the mRNA expression of Kir 2.1 channels was significantly increased
in the Müller cells cultured in the presence or absence of 1 μM
adenosine at 40 mmHg for 24 h, compared with the normoxia control.
However, there were no significant changes between the groups in
which Müller cells were cultured in the presence or absence of 1 μM
adenosine at 40 mmHg pressure for 24 h; there was even a decline
(Fig. 3).
Effect of adenosine on the expression of
Kir 4.1 channels in the cultured retinal Müller cells
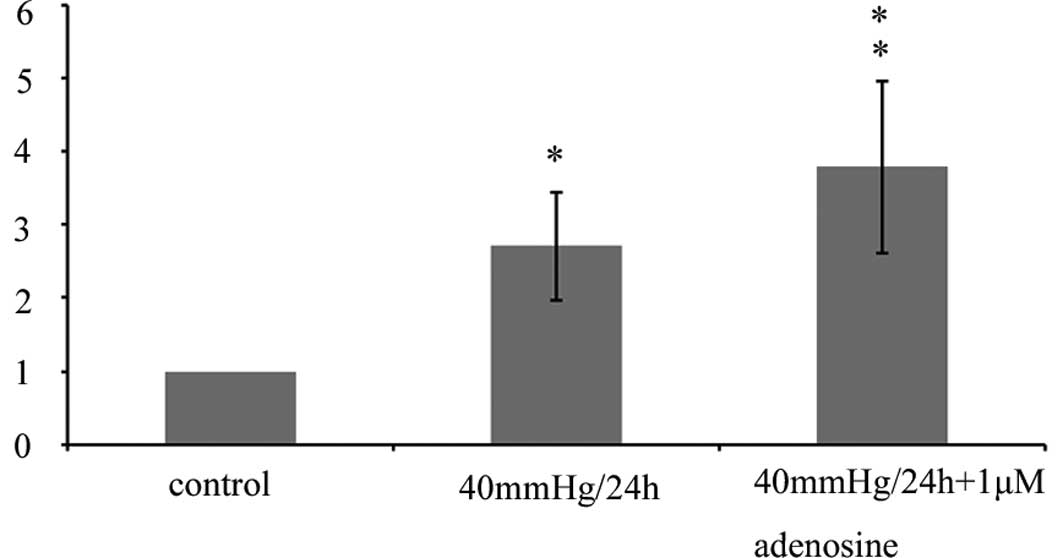
The real-time PCR data revealed that the mRNA
expression of Kir 4.1 channels was significantly increased in the
Müller cells cultured with 1 μM adenosine at 40 mmHg pressure for
24 h, compared with the normoxia control or at 40 mmHg pressure, in
the absence of adenosine (Fig.
4).
Discussion
The results of this study demonstrated that in
Müller cells, the mRNA expression of Kir 2.1 and Kir 4.1 channels
significantly increased at 40 mmHg in vitro. Continually,
when cells were further treated with 1 μM adenosine at 40 mmHg
pressure, the mRNA expression of Kir 2.1 channels decreased;
however, the mRNA expression of Kir 4.1 channels continued to
increase, compared to when treated without adenosine at 40 mmHg
pressure (Figs. 3 and 4).
GS is predominantly expressed in the retina and has
been used as a specific marker for Müller cells. In our study, more
than 90% of cells in this culture system demonstrated positive
markers for GS, therefore these cells were identified to be Müller
cells.
The degeneration of RGCs in glaucoma is accompanied
by morphological and functional changes in Müller cells, the main
type of glial cell in the retina (18). Müller cells are responsible for the
maintenance of homeostasis in the extracellular medium of the
retina and protection of the neurons through the release of
neurotrophins (19–22). Moreover, Müller cells can also have
altered expression and functioning potassium channels, with
consequential alteration in ion homeostasis and development of
edema in the retina. Activated neurons release potassium ions. To
avoid potassium-induced depolarization of neurons, Müller cells
take up excess potassium from the extracellular space, particularly
in the plexiform layers of the retina, and release an appropriate
amount of potassium into the spaces outside the retina. Kir
channels localized in Müller cell membranes are used to mediate
extracellular potassium. Müller cells express various types of
potassium channels. Kir 2.1 channels are expressed in
neuron-abutting membranes, through which Müller cells take up
excess potassium. Kir 4.1 channels are expressed in membranes which
are in close contact with spaces outside the neural retina. On the
basis of the present results, we presumed that when the pressure
elevated rapidly, Müller cells would remain capable of taking up
K+, and mediate the potassium concentration of the
retina.
Kir 2.1 channels are strongly rectifying Kir
channels, which mediate only inward potassium currents into Müller
cells, while Kir 4.1 channels are weakly rectifying Kir channels,
which mediate bidirectional currents between the extraretinal
tissues and the interior of the Müller cells (23,24).
Based on the present results it has been suggested that adenosine
can upregulate the expression of Kir 4.1 channels, but weakly
affect the expression of Kir 2.1 channels. The upregulation of Kir
4.1 channels should accelerate retinal K+ clearance to
prevent neuronal hyperexcitation and excessive release of
glutamate.
There are still certain problems which require
resolution, including whether adenosine is capable of affecting the
expression of Kir 2.1 channels, and what would happen if the
concentration of adenosine was changed. Moreover, the effect of
adenosine on the protein expression of Kir 2.1 and Kir 4.1 channels
require further study.
Acknowledgements
This study was supported by the
National Natural Science Foundation of China (81170860), the
Shanghai Municipal Education Committee Projects (10YZ38), the
Shanghai Natural Science Foundation (11ZR1422000) and the Shanghai
‘Science and Technology Innovation Action Plan’ Basic Research Key
Project (Nos. 11JC1407700 and 11JC1407701).
References
|
1.
|
Bolz S, Schuteeauf F, Fires J, et al:
K+ currents fail to change in reactive retinal glial
cells in a mouse model of glaucoma. Graefes Arch Clin Exp
Ophthalmol. 246:1249–1254. 2008.
|
|
2.
|
Sapptington R, Chan M and Calikns D:
Interleukin-6 protects retinal ganglion cells from pressure-induced
death. Invest Ophthalmol Vis Sci. 47:2932–2942. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Yu J, Zhong Y, Cheng Y, et al: The effect
of high hydrostatic pressure on the expression of glutamine
synthetase in rat retinal Müller cells cultured in vitro.
Exp Ther Medicine. 2:513–516. 2011.PubMed/NCBI
|
|
4.
|
Wang X, Tay S and Ng Y: An electron
microscopic study of neuronal degeneration and glial cell reaction
in the retina of glaucomatous rats. Histol Histopathol.
17:1043–1052. 2002.PubMed/NCBI
|
|
5.
|
Kanamori A, Nakamura M, Nakanishi Y, et
al: Long-term glial reactivity in rat retinas ipsilateral and
contralateral to experimental glaucoma. Exp Eye Res. 81:48–56.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Ishii M, Fujita A, Iwai K, et al:
Differential expression and distribution of Kir 5.1 and Kir 4.1
inwardly rectifying K+ channels in retina. Am J Physiol
Cell Physiol. 285:260–267. 2003. View Article : Google Scholar
|
|
7.
|
Kofuji P, Biedermann B, Siddharthan V, et
al: Kir potassium channel subunit expression in retinal glial
cells: implications for spatial potassium buffering. Glia.
39:292–303. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Bringmann A, Francke M, Pannicke T, et al:
Role of glial K+ channels in ontogeny and gliosis: a
hypothesis based upon studies on Müller cells. Glia. 29:35–44.
2000.
|
|
9.
|
Chen K and Nicholson C: Spatial buffering
of potassium ions in brain extracellular space. Biophys J.
78:2776–2797. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Fakler B, Bond C, Adelman J, et al:
Heterooligomeric assembly of inward-rectifier K+
channels from subunits of different subfamilies: Kir2.1 (IRK1) and
Kir4.1 (BIR10). Pflügers Arch. 433:77–83. 1996.PubMed/NCBI
|
|
11.
|
Yang D, Sun F, Thomas L, et al: Molecular
cloning and expression of an inwardly rectifying K+
channel from bovine corneal endothelial cells. Invest Ophthalmol
Vis Sci. 41:2936–2944. 2000.PubMed/NCBI
|
|
12.
|
Shuck M, Piser T, Bock J, et al: Cloning
and characterization of two K+ inward rectifier (Kir)
1.1 potassium channel homologs from human kidney (Kir1.2 and
Kir1.3). J Biol Chem. 272:586–593. 1997.PubMed/NCBI
|
|
13.
|
Hou X, Pal S, Choi W, et al: Design and
synthesis of truncated 4′-thioadenosine derivatives as potent and
selective A3 adenosine receptor antagonists. Nucleic Acids Symp
Ser. 52:641–642. 2008.
|
|
14.
|
Daines B, Kent A, McAleer M, et al:
Intraocular adenosine levels in normal and ocular-hypertensive
patients. J Ocul Pharmacol Th. 19:113–119. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Wang Z, Che P, Du J, et al: Static
magnetic field exposure reproduces cellular effects of the
Parkinson’s disease drug candidate ZM241385. PLoS One.
5:138832010.PubMed/NCBI
|
|
16.
|
Shearer T and Crosson C: Adenosine A1
receptor modulation of MMP-2 secretion by trabecular meshwork
cells. Invest Ophthalmol Vis Sci. 43:3016–3020. 2002.PubMed/NCBI
|
|
17.
|
Crosson C, Sloan C and Yates P: Modulation
of conventional outflow facility by the adenosine A1 agonist
N6-cyclohexyladenosine. Invest Ophthalmol Vis Sci. 46:3795–3799.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Tezel G and Wax MB: Hypoxia-inducible
factor 1α in the glaucomatous retina and optic nerve head. Arch
Ophthalmol. 122:1348–1356. 2004.
|
|
19.
|
Fletcher E, Downie L, Ly A, et al: A
review of the role of glial cells in understanding retinal disease.
Clin Exp Optom. 91:67–77. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Kawasaki A, Otori Y and Barnstable C:
Müller cell protection of rat retinal ganglion cells from glutamate
and nitric oxide neurotoxicity. Invest Ophthalmol Vis Sci.
41:3444–3450. 2000.
|
|
21.
|
Newman E and Reinchenbach A: The Müller
cell: a functional element of the retina. Trends Neurosci.
19:307–312. 1996.
|
|
22.
|
Vidal L, Díaz F, Villena A, et al:
Reaction of Müller cells in an experimental rat model of increased
intraocular pressure following timolol, latanoprost and
brimonidine. Brain Res Bull. 82:18–24. 2010.
|
|
23.
|
Reichenbach A, Wurm A, Pannicke T, et al:
Müller cells as players in retinal degeneration and edema. Graefes
Arch Clin Exp Ophthalmol. 245:627–636. 2007.
|
|
24.
|
Nagelhus E, Horio Y, Inanobe A, et al:
Immunogold evidence suggests that coupling of K+
siphoning and water transport in rat retinal Müller cells is
mediated by a coenrichment of Kir4.1 and AQP4 in specific membrane
domains. Glia. 26:47–54. 1999.
|