Introduction
Methicillin-resistant Staphylococcus aureus
(MRSA) is a serious and urgent clinical problem worldwide. Few new
drugs are available against MRSA, since it has the ability to
acquire resistance to most antibiotics. An effective method for
treating MRSA may be to develop antibiotics from natural products
which are likely to have lower toxicity and fewer side effects.
Rheum palmatum, popularly known as Dahuang, has
traditionally been used as an oriental folk medicine. Rhein
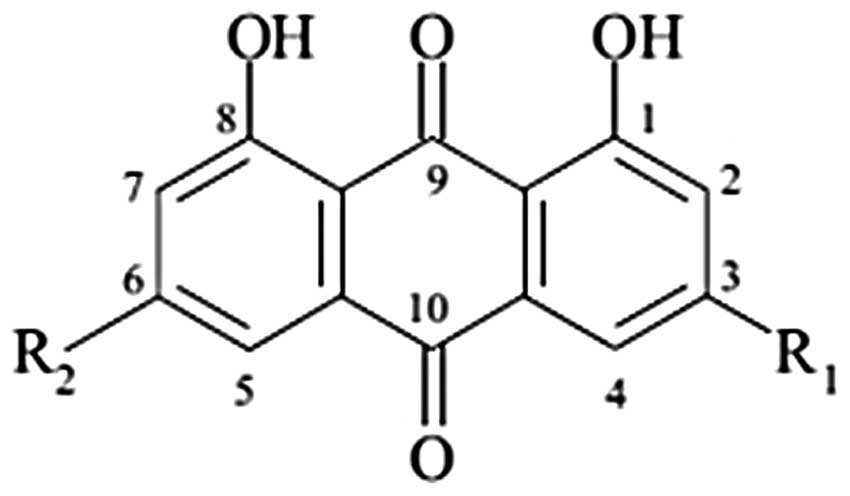
naturally occurs in anthraquinone (1,3,8-trihydroxy-6-methyl
anthraquinone) (Fig. 1), which is
found in Rheum palmatum and related plants such as rhubarb.
Anthraquinone components include aloe-emodin, rhein, emodin,
chrysophanol and physcion (1).
Rhein is used for treating numerous complaints, including
constipation, jaundice, gastrointestinal hemorrhage and ulcers
(2). It has also been shown to
have good antitumor (3–6) and anti-inflammatory properties
(7,8), anticancer (9), purgative (10), nephric protection (11,12),
liver protection (13),
antimicrobial and hemostatic properties (14,15).
As such, it has been widely used for the treatment of
gastrointestinal diseases, hepatitis and chronic renal failure.
However, little is known about its antimicrobial effects on MRSA.
Thus, we present the current study demonstrating the antimicrobial
activity of rhein against MRSA and methicillin-sensitive S.
aureus (MSSA) strains, as well as its synergistic effect.
Materials and methods
Test microorganisms
Among the 16 S. aureus strains used in this
study, 14 clinical isolates of MRSA were obtained from 14 different
patients at Wonkwang University Hospital (Iksan, Republic of
Korea). The other two strains used were S. aureus ATCC 33591
(methicillin-resistant strain) and S. aureus ATCC 25923
(methicillin-susceptible strain). ATCC 25923 (American Type Culture
Collection, Manassas, VA, USA) and ATCC 33591 were commercially
purchased. Before use, all bacteria were stored in 30% glycerol and
frozen at −70°C. The bacteria were cultured in Mueller-Hinton broth
(MHB) and Mueller-Hinton agar (MHA) (Difco Laboratories, Baltimore,
MD, USA). Bacteria were suspended in MHB and then incubated at 37°C
for 24 h.
Disc diffusion method
The disc diffusion method was performed as described
by the Clinical and Laboratory Standards Institute (CLSI) and by
using a modified agar well diffusion method (CLSI, 2001) (16). Bacterial strains grown on MHA at
37°C for 18 h were suspended in MHB and adjusted to a turbidity of
0.5 on the McFarland standard scale (approximately
1.5×108 CFU/ml). The MHA was poured into Petri dishes
and inoculated with 100 μl of the suspension. Sterile paper discs
(diameter, 6 mm; Tokyo Roshi Kaihsa, Japan) were punched into the
agar and each well was filled with 500 and 250 μg of each drug. The
dissolution of the ampicillin (AM), oxacillin (OX) and rhein was
facilitated by the addition of 50% (v/v) DMSO (50% DMSO was not
active against all strains) (DMSO; Sigma, St. Louis, MO, USA). AM
and OX were used as the positive controls, and the discs treated
with DMSO were used as the negative control. The plates were placed
in an incubator at 37°C for 18 h in the dark. The inhibition zone
diameter around each of the discs was measured and recorded at the
end of the incubation period.
Minimum inhibitory concentration
The minimum inhibitory concentration (MIC) was
determined using the broth micro-dilution method according to the
CLSI guidelines, 2000 (17).
Briefly, the inoculation of the microorganisms was carried out on
24 h broth cultures, and the suspensions were adjusted to a 0.5
McFarland standard turbidity (approximately 1.5×108
CFU/ml). Final inoculum size was adjusted to 1.5×106
CFU/ml. These serially diluted cultures were then incubated at 37°C
for 18 h. MIC was defined at the lowest concentration of AM, OX and
rhein. At the end of the incubation period, the well plates were
visually examined for turbidity. Cloudiness indicated that
bacterial growth had not been inhibited by the concentration of the
antimicrobial agent contained in the medium.
Checkerboard dilution test
The synergistic combinations were investigated in
the preliminary checkerboard method performed using the MRSA and
MSSA strains, and one clinical isolate strain via MIC
determination, according to the CLSI guidelines (18). The MIC was defined as the lowest
concentration of drug alone or in combination that inhibited the
visible growth. The in vitro interaction was quantified by
determining the fractional inhibitory concentration (FIC). The FIC
index was calculated as follows: FIC = (MIC of drug A in
combination/MIC of drug A alone) + (MIC of drug B in
combination/MIC of drug B alone). FIC indices (FICIs) were
interpreted as follows: <0.5, synergy; 0.5–0.75, partial
synergy; 0.76–1.0, additive effect; >1.0–4.0, indifference; and
>4.0, antagonism. Finally, the varying rates of synergy between
the two agents were determined. All experiments were independently
repeated three times.
Colorimetric assay using MTT test
A colorimetric assay based on MTT for the rapid
detection of the presence of bacteria was performed as previously
described (19–21). Briefly, a stock solution of 5 mg/ml
MTT (Sigma) was prepared in phosphate-buffered saline and kept at
−70°C. A final concentration of 1 mg/ml of MTT was used in the
assay. Following 24 h of incubation a 37°C, 20 μl of the yellow MTT
was added to the 96-well microtiter plate (0.3 ml volume) and
incubated for an additional 20 min. The presence of a blue color
indicated the presence of bacteria.
Results
Table I shows the
S. aureus strains used in the experiments. Table II shows the means of inhibition
zones produced against the tested bacteria ranged between 20 and 29
mm. The growth of all the tested strains of MRSA and MSSA was
inhibited at 500 and 250 μg/disc. As shown in Table III, rhein demonstrated
antimicrobial activity against all the tested strains of MRSA as
well as the MSSA strain. The MICs of rhein against S. aureus
ranged from 7.8–31.25 μg/ml. As shown in Tables IV and V, tests were performed to determine the
action of rhein alone as well as its synergistic action with AM or
OX against the MRSA clinical isolate, the standard MRSA strain or
the standard MSSA strain. Rhein lowered the MICs of AM and OX
against the MRSA strains. For the standard MSSA strain, rhein
lowered the MICs of both AM and OX. The combined activity of rhein
and the two antimicrobial agents (AM and OX) against all strains
resulted in a FICI ranging from 0.28–1 and from 0.18–1,
respectively. In conclusion, the combination effect of rhein with
AM and OX was found to be synergistic or partially synergistic.
 | Table I.The S. aureus strains used in
the experiments. |
Table I.
The S. aureus strains used in
the experiments.
| S. aureus
strain | Class | mecA gene | Antibiotic resistance
pattern |
|---|
| ATCC 25923 | MSSA | - | - |
| ATCC 33591 | MRSA | + | AM, OX |
| DPS-1a | MRSA | + | AM, OX |
| DPS-2 | MRSA | + | AM, OX |
| DPS-3 | MRSA | + | AM, OX |
| DPS-4 | MRSA | + | AM, OX |
| DPS-5 | MRSA | + | AM, OX |
| DPS-6 | MRSA | + | AM, OX |
| DPS-7 | MRSA | + | AM, OX |
| DPS-8 | MRSA | + | AM, OX |
| DPS-9 | MRSA | + | AM, OX |
| DPS-10 | MRSA | + | AM, OX |
| DPS-11 | MRSA | + | AM, OX |
| DPS-12 | MRSA | + | AM, OX |
| DPS-13 | MRSA | + | AM, OX |
| DPS-14 | MRSA | + | AM, OX |
 | Table II.The antimicrobial activity (as
inhibitory zone diameters) of rhein, AM and OX against the S.
aureus strain. |
Table II.
The antimicrobial activity (as
inhibitory zone diameters) of rhein, AM and OX against the S.
aureus strain.
| Zone of inhibition
(mm)
|
|---|
Rhein (μg/ml)
| Ampicillin (μg/ml)
| Oxacillin (μg/ml)
|
|---|
| S. aureus
strain | 500 | 250 | 500 | 250 | 500 | 250 |
|---|
| ATCC 25923 | 25 | 22 | 43 | 41 | 37 | 35 |
| ATCC 33591 | 29 | 23 | 16 | 15 | 18 | 15 |
| DPS-1a | 19 | 14 | 18 | 14 | ND | ND |
| DPS-2 | 25 | 20 | 18 | 17 | 17 | 15 |
| DPS-3 | 21 | 17 | 21 | 16 | ND | ND |
| DPS-4 | 21 | 18 | 20 | 15 | ND | ND |
| DPS-5 | 22 | 18 | 17 | 14 | ND | ND |
| DPS-6 | 20 | 16 | 17 | 14 | ND | ND |
| DPS-7 | 21 | 18 | 11 | 10 | ND | ND |
| DPS-8 | 21 | 17 | 12 | 11 | ND | ND |
| DPS-9 | 23 | 19 | 13 | 12 | ND | ND |
| DPS-10 | 25 | 20 | 12 | 11 | ND | ND |
| DPS-11 | 25 | 21 | 11 | 9 | ND | ND |
| DPS-12 | 24 | 20 | 12 | 10 | ND | ND |
| DPS-13 | 24 | 19 | 18 | 14 | ND | ND |
| DPS-14 | 20 | 16 | 15 | 11 | ND | ND |
 | Table III.The MICs of rhein, AM and OX against
the S. aureus strain. |
Table III.
The MICs of rhein, AM and OX against
the S. aureus strain.
| MICs (μg/ml)
|
|---|
| S. aureus
strain | Rhein | Ampicillin | Oxacillin |
|---|
| ATCC25923 | 15.62 | 7.8 | 7.8 |
| ATCC33591 | 15.62 | 1,000 | 250 |
| DPS-1a | 15.62 | 31.25 | 500 |
| DPS-2 | 15.62 | 1,000 | 500 |
| DPS-3 | 15.62 | 31.25 | 500 |
| DPS-4 | 31.25 | 31.25 | 500 |
| DPS-5 | 7.8 | 31.25 | 500 |
| DPS-6 | 7.8 | 31.25 | 250 |
| DPS-7 | 31.25 | 250 | 500 |
| DPS-8 | 31.25 | 250 | 500 |
| DPS-9 | 31.25 | 125 | 500 |
| DPS-10 | 31.25 | 250 | 500 |
| DPS-11 | 31.25 | 250 | 500 |
| DPS-12 | 31.25 | 250 | 500 |
| DPS-13 | 7.8 | 31.25 | 1,000 |
| DPS-14 | 7.8 | 250 | 500 |
 | Table IV.Result of the combined effect of
rhein and AM against S. aureus. |
Table IV.
Result of the combined effect of
rhein and AM against S. aureus.
| MICs (μg/ml)
|
|---|
| S. aureus
strain | Rhein alone | With AM | AM alone | With rhein | FICI |
|---|
| ATCC 25923 | 15.62 | 3.9 | 7.8 | 1.95 | 0.5 |
| ATCC 33591 | 15.62 | 0.97 | 1,000 | 250 | 0.31 |
| DPS-1a | 15.62 | 3.5 | 31.25 | 7.8 | 0.5 |
| DPS-2 | 15.62 | 3.9 | 1,000 | 250 | 0.5 |
| DPS-3 | 15.62 | 7.8 | 31.25 | 0.97 | 0.53 |
| DPS-4 | 31.25 | 15.62 | 31.25 | 0.97 | 0.53 |
| DPS-5 | 7.8 | 3.9 | 31.25 | 15.62 | 1 |
| DPS-6 | 7.8 | 3.9 | 31.25 | 7.9 | 0.75 |
| DPS-7 | 31.25 | 7.8 | 250 | 62.5 | 0.5 |
| DPS-8 | 31.25 | 7.8 | 250 | 7.8 | 0.28 |
| DPS-9 | 31.25 | 7.8 | 125 | 3.9 | 0.28 |
| DPS-10 | 31.25 | 7.8 | 250 | 7.8 | 0.28 |
| DPS-11 | 31.25 | 7.8 | 250 | 15.62 | 0.31 |
| DPS-12 | 31.25 | 7.8 | 250 | 62.5 | 0.5 |
| DPS-13 | 7.8 | 3.9 | 31.25 | 15.62 | 1 |
| DPS-14 | 7.8 | 0.48 | 250 | 125 | 0.56 |
 | Table V.Result of the combined effect of
rhein and OX against S. aureus. |
Table V.
Result of the combined effect of
rhein and OX against S. aureus.
| MICs (μg/ml)
|
|---|
| S. aureus
strain | Rhein alone | With OX | OX alone | With rhein | FICI |
|---|
| ATCC 25923 | 15.62 | 1.95 | 7.8 | 1.95 | 0.37 |
| ATCC 33591 | 15.62 | 1.95 | 250 | 15.62 | 0.18 |
| DPS-1a | 15.62 | 7.8 | 500 | 31.25 | 0.53 |
| DPS-2 | 15.62 | 3.9 | 500 | 125 | 0.5 |
| DPS-3 | 15.62 | 7.8 | 500 | 15.62 | 0.53 |
| DPS-4 | 31.25 | 7.8 | 500 | 125 | 0.5 |
| DPS-5 | 7.8 | 1.95 | 500 | 125 | 0.5 |
| DPS-6 | 7.8 | 1.95 | 250 | 7.8 | 0.53 |
| DPS-7 | 31.25 | 7.8 | 500 | 125 | 0.5 |
| DPS-8 | 31.25 | 7.8 | 500 | 62.5 | 0.37 |
| DPS-9 | 31.25 | 7.8 | 500 | 125 | 0.5 |
| DPS-10 | 31.25 | 7.8 | 500 | 62.5 | 0.37 |
| DPS-11 | 31.25 | 7.8 | 500 | 125 | 0.5 |
| DPS-12 | 31.25 | 7.8 | 500 | 250 | 0.75 |
| DPS-13 | 7.9 | 0.48 | 1,000 | 500 | 0.56 |
| DPS-14 | 7.9 | 3.9 | 500 | 250 | 1 |
Discussion
In the present study, we investigated the
antimicrobial activity of rhein against clinical isolates of MRSA
and a standard MSSA strain. We report that the rhein agent also
shows synergistic activity with AM and OX against MRSA. When
combined, these antibiotic effects were dramatically increased.
While the results obtained in the present study cannot currently be
applied in clinical practice, we consider that the combination
treatment of rhein isolated with AM or OX will prove to be helpful
in treating MRSA and MSSA. Further medicinal, clinical and
mechanism studies are required to verify the mechanisms by which
rhein enhances the antibacterial activity. At present, the
continued emergence of multi-drug resistant bacteria and the
infectious diseases caused by them are serious global problems. It
is hoped that it will be possible to reduce the use of existing
antibacterial drugs and increase the use of natural product drugs,
such as rhein. At this point in time, the product is still under
investigation. In our study, rhein markedly lowered the MICs of AM
and OX against the two MRSA strains and one MSSA strain. While the
product is still under investigation, the present results are
promising and may help to promote the use of natural products
rather than antibiotics.
Acknowledgements
This study was supported by the
Sunchon National University Research Fund (2011).
References
|
1.
|
Yan D, Ma Y, Shi R, Xu D and Zhang N:
Pharmacokinetics of anthraquinones in Xiexin decoction and in
different combinations of its constituent herbs. Phytother Res.
23:317–323. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Chinese Pharmacopoeia Commission:
Pharmacopoeia of the People's Republic of China. Chemical Industry
Press; Beijing: pp. 172005
|
|
3.
|
Dorsey JF and Kao GD: Aloe(-emodin) for
cancer? More than just a comforting salve. Cancer Biol Ther.
6:89–90. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Huang Q, Lu G, Shen HM, Chung MC and Ong
CN: Anti-cancer properties of anthraquinones from rhubarb. Med Res
Rev. 27:609–630. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Lee HZ, Hsu SL, Liu MC and Wu CH: Effects
and mechanisms of aloe-emodin on cell death in human lung squamous
cell carcinoma. Eur J Pharmacol. 431:287–295. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Shi YQ, Fukai T, Sakagami H, Kuroda J,
Miyaoka R, Tamura M, Yoshida N and Nomura T: Cytotoxic and DNA
damage-inducing activities of low molecular weight phenols from
rhubarb. Anticancer Res. 21:2847–2853. 2001.PubMed/NCBI
|
|
7.
|
Cuellar MJ, Giner RM, Recio MC, Manez S
and Rios JL: Topical anti-inflammatory activity of some Asian
medicinal plants used in dermatological disorders. Fitoterapia.
72:221–229. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Ding Y, Zhao L, Mei H, Zhang SL, Huang ZH,
Duan YY and Ye P: Exploration of emodin to treat
alpha-naphthylisothiocyanate-induced cholestatic hepatitis via
anti-inflammatory pathway. Eur J Pharmacol. 590:377–386. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Cai J, Razzak A, Hering J, Saed A, Babcock
TA, Helton S and Espat NJ: Feasibility evaluation of emodin
(rhubarb extract) as an inhibitor of pancreatic cancer cell
proliferation in vitro. JPEN J Parenter Enteral Nutr. 32:190–196.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Huang KC: The Pharmacology of Chinese
Herbs. CRC Press; Boca Raton, FL: pp. 233–234. 1993
|
|
11.
|
Yokozawa T, Suzuki N, Okuda I, Oura H and
Nishioka I: Changes in the urinary constituents in rats with
chronic renal failure during oral administration of rhubarb
extract. Chem Pharm Bull (Tokyo). 33:4508–4514. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Wang J, Zhao Y, Xiao X, Li H, Zhao H,
Zhang P and Jin C: Assessment of the renal protection and
hepatotoxicity of rhubarb extract in rats. J Ethnopharmacol.
124:18–25. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Zhao YL, Wang JB, Zhou GD, Shan LM and
Xiao XH: Investigations of free anthraquinones from rhubarb against
α-naphthylisothiocyanate-induced cholestatic liver injury in rats.
Basic Clin Pharmacol Toxicol. 104:463–469. 2009.PubMed/NCBI
|
|
14.
|
Dictionary of Traditional Chinese
Medicine: New Medical College of Jiangsu. 1st edition. People's
Publishing Co. of Shanghai; pp. 1021997
|
|
15.
|
WHO: Monographs on Selected Medicinal
Plants. World Health Organization; Geneva: pp. 231–240. 1999
|
|
16.
|
Clinical and Laboratory Standards
Institute: Performance standards for antimicrobial disk
susceptibility tests. Approved standards. CLSI document M2 A7.
Wayne, PA: 2001
|
|
17.
|
Clinical and Laboratory Standards
Institute: Methods for dilution antimicrobial susceptibility tests
for bacteria that grow aerobically. Approved standards. CLSI
document M7-A5.Wayne, PA: 2000
|
|
18.
|
Mazumdor K, Dutta NK, Kumar KA and
Dastidar SG: In vitro and in vivo synergism between tetracycline
and the cardiovascular agent oxyfedrine HCI against common
bacterial strains. Biol Pharm Bull. 28:713–717. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Abate G, Mshana RN and Miorner H:
Evaluation of a colorimetric assay based on
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT)
for rapid detection of rifampicin resistance in Mycobacterium
tuberculosis. Int J Tuberc Lung Dis. 2:1011–1016. 1988.
|
|
20.
|
Scheuber PH, Scheuber PH, Mossmann H, Beck
G and Hammer DK: Direct skin test in highly sensitized guinea pigs
for rapid and sensitive determination of staphylococcal enterotoxin
B. Appl Environ Microbiol. 46:1351–1356. 1983.PubMed/NCBI
|
|
21.
|
Shi YJ, Chen J and Xu M: A new method for
antimicrobial susceptibility testing of in vitro-cultured bacteria
by means of resonance light scattering technique. J Microbiol
Biotechnol. 18:118–123. 2008.
|