Introduction
Organophosphate compounds are the most extensively
used insecticides. Millions of cases of organophosphate poisoning
are reported annually worldwide, with the majority due to
insecticide exposure. The widespread use and easy accessibility of
these compounds result in a huge number of poisoning cases
(1). Methyl parathion
(O-O-dimethyl-O-p-nitrophenyl
phosphorothioate), an organophosphorus compound that may only be
lawfully used as an insecticide for agricultural crops, has
recently received attention as a consequence of its illegal use
(2). Tissue damage as a
consequence of organophosphate poisoning is frequently reported;
however, prevention of this potentially severe complication has not
been the subject of considerable research. Particularly, hepatic
dysfunction secondary to organophosphate exposure was reported in
animals a few years ago (3).
Mechanisms other than AChE inhibition may be
involved in the progression of acute organophosphate poisoning,
including oxidative stress, mitochondrial energy metabolism
impairment, microcirculation disturbance (4) and acute inflammation (5). Aesculus hippocastanum
(Hippocastanaceae) is a plant that is distributed worldwide due to
its excellent resistance to environmental conditions (6). Aescin, the major active agent from
Aesculus hippocastanum, has recently been used in clinical
therapy due to its anti-inflammatory and antioxidative effects.
However, few studies have examined the effect of aescin on liver
injury induced by phosphate pesticides. This study evaluates the
effect of sodium aescinate on liver injury induced by methyl
parathion poisoning.
Materials and methods
Chemicals
Methyl parathion (80%, w/w) was obtained from
Shandong Dacheng Co., Ltd (Zibo, China). Sodium aescinate (SA) was
supplied by Shandong Luye Pharmaceutical Co., Ltd. (Yantai, China).
Acetylcholinesterase (AChE), aspartate aminotransferase (AST),
alanine aminotransferase (ALT), nitric oxide (NO), superoxide
dismutase (SOD), glutathione peroxidase (GSH-Px), glutathione
(GSH), malondialdehyde (MDA) and protein level test kits were
purchased from the Institute of Jiancheng Bioengineering (Nanjing,
China). All other chemicals and reagents used in this study were of
analytical grade.
Animals and treatments
A total of 40 male Sprague-Dawley rats weighing
220±20 g were provided by the Experimental Animal Center of
Shandong Engineering Research Center for Natural Drugs (Yantai,
China), and the certificate number was 20030020. All experimental
procedures conducted in this study were performed in accordance
with the Guidelines for the Care and Use of Laboratory Animals of
Yantai University. The rats were provided with free access to food
and water on a 12-h light/dark cycle. They were housed in plastic
cages and randomly divided into 5 groups of 8 animals: the control
group; the methyl parathion (15 mg/kg) poisoning (MP) group; and
the MP plus SA at doses of 0.45, 0.9 and 1.8 mg/kg groups.
Rats received methyl parathion intragastrically to
establish the acute methyl parathion poisoning model. The animals
in the MP and SA groups were treated with SA via the tail vein at
2.5 h following methyl parathion poisoning, while the animals in
the other groups were treated with normal saline in equivalent
volumes. All animals were anesthetized with chloral hydrate (300
mg/kg, i.p.) 24 h following methyl parathion poisoning. A total of
5 ml heparinized blood (1% heparin, 100 μl) was collected
from the abdominal aorta, and then the animals were sacrificed
under anesthesia as a result of blood loss. The livers were excised
and immediately cut into two, and subsequently washed with chilled
normal saline. One section was fixed in paraformaldehyde (4%,
diluted in 0.1 mol/l phosphate buffer solution, pH 7.4). The other
section was weighed and homogenated.
Histopathological investigation
Paraformaldehyde-fixed, paraffin-embedded liver
samples were cut into 4-μm sections, deparaffinized in
xylene, and rehydrated through a series of descending
concentrations of ethanol. Sections were stained with hematoxylin
and eosin. Pathological observation of the tissues was performed
under light microscopy.
Biochemical analysis
Blood samples were drawn into heparinized tubes for
biochemical analysis. Following immediate centrifugation (2500 × g
for 10 min at 4°C), the plasma was stored at −80°C until
biochemical analysis. The livers were weighed and homogenized in
ice-cold normal saline (1/9, w/v) at a speed of 5000 rpm (15 sec ×
5). The suspension was centrifuged at a speed of 2500 × g for 10
min at 4°C, and the supernatant was stored at −80°C. The activities
of AChE, ALT, AST, SOD and GSH-Px, and the levels of NO, GSH and
MDA were determined, respectively, according to the manufacturer’s
instructions.
Statistical analysis
The one-way ANOVA test was used to analyze the
significant differences between the different groups. Comparisons
between two groups were determined using the Student’s unpaired
t-test, using SPSS 11.5 statistical software. P<0.05 was
considered to indicate a statistically significant difference. All
data in the study are expressed as the means ± SD.
Results
Effect of SA on the pathological changes
in rat livers
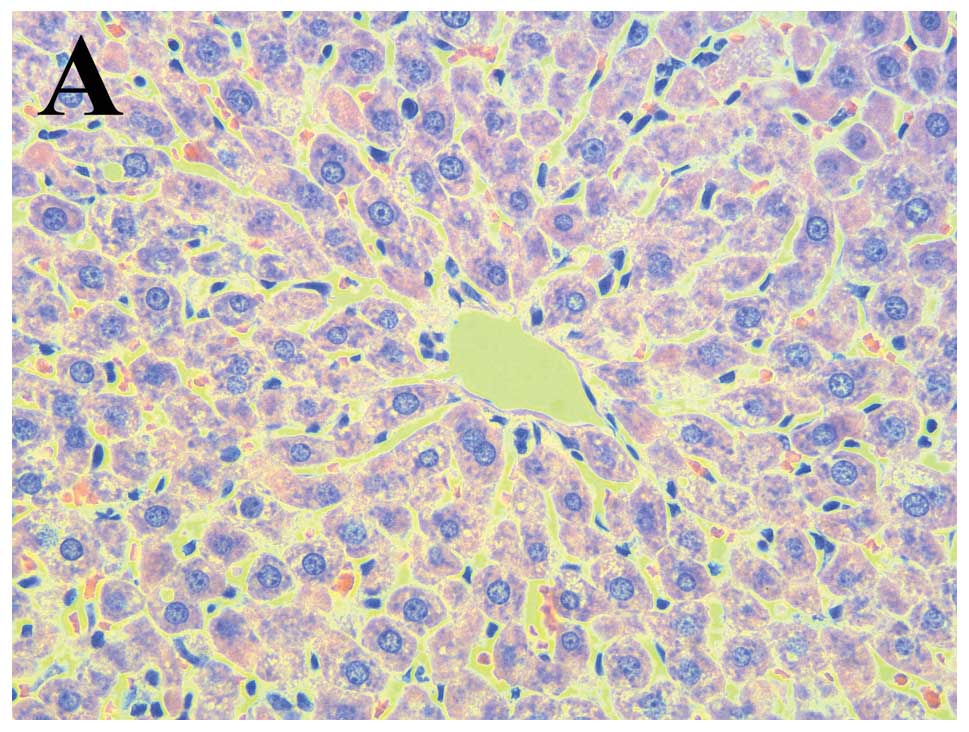
Normal architecture of the liver was observed in the
control group (Fig. 1A).
Inflammatory cell clusters, severe congestion of the hepatic
sinusoids, hepatocyte necrosis and steatosis were observed in the
MP group (Fig. 1B). SA
significantly ameliorated the pathological changes induced by
methyl parathion poisoning (Fig.
1C–E).
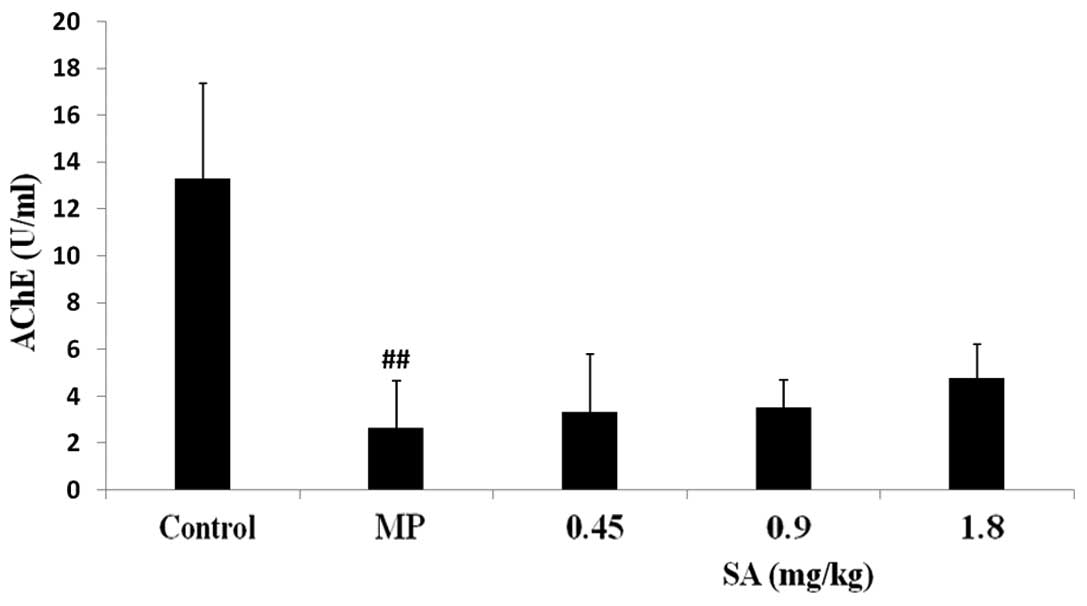
Effect of SA on AChE activity in the
plasma following methyl parathion poisoning
The AChE activity was significantly inhibited
following MP administration. SA had no effect on the reduction of
AChE activity (Fig. 2).
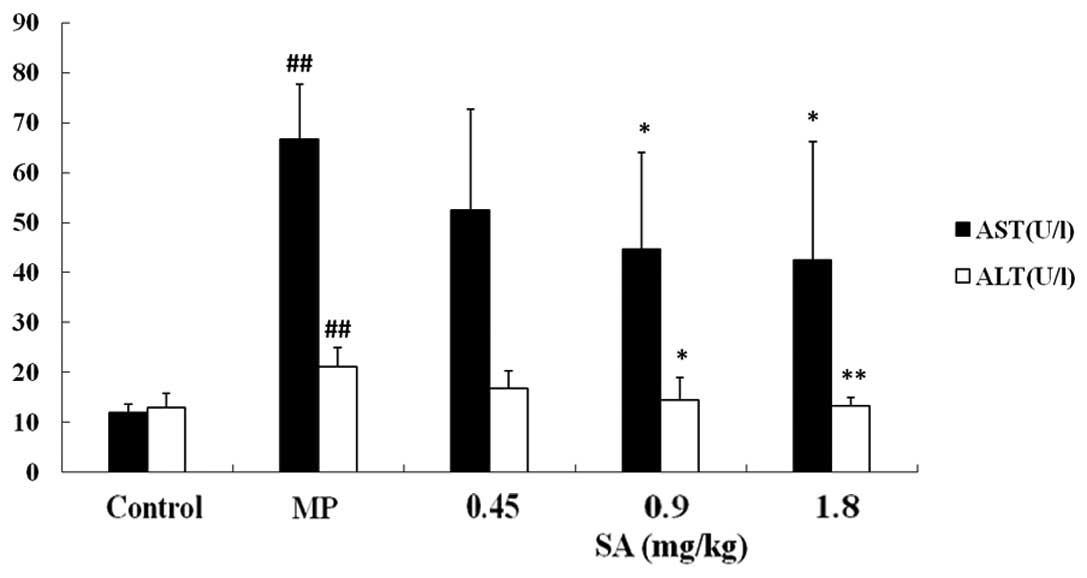
Effect of SA on ALT and AST activities in
the plasma following methyl parathion poisoning
The activities of ALT and AST increased markedly
following MP poisoning. SA (0.9 and 1.8 mg/kg) treatment decreased
ALT and AST activities (Fig.
3).
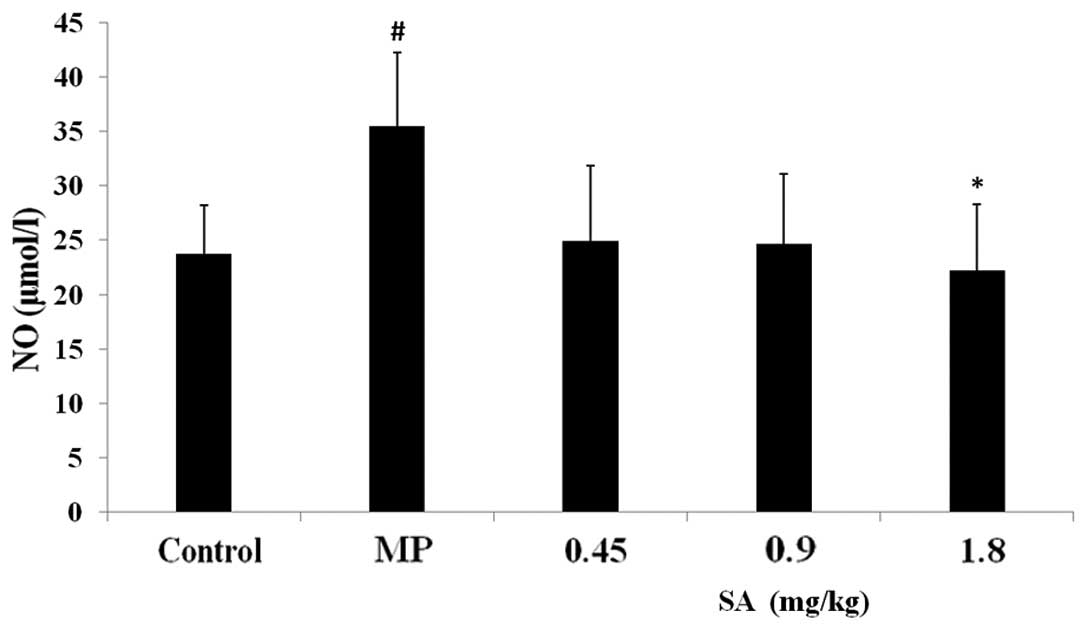
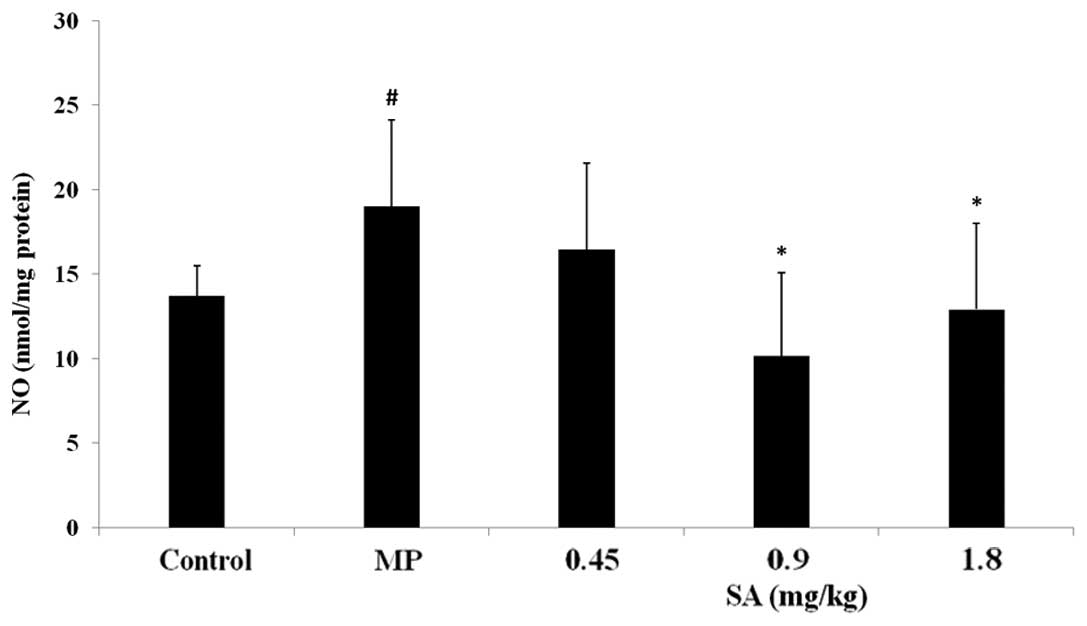
Effect of SA on NO level in the plasma
and liver following methyl parathion poisoning
Compared with the control group, the NO content in
the MP group significantly increased. SA suppressed the elevation
of the NO level in the plasma and liver tissue (Figs. 4 and 5).
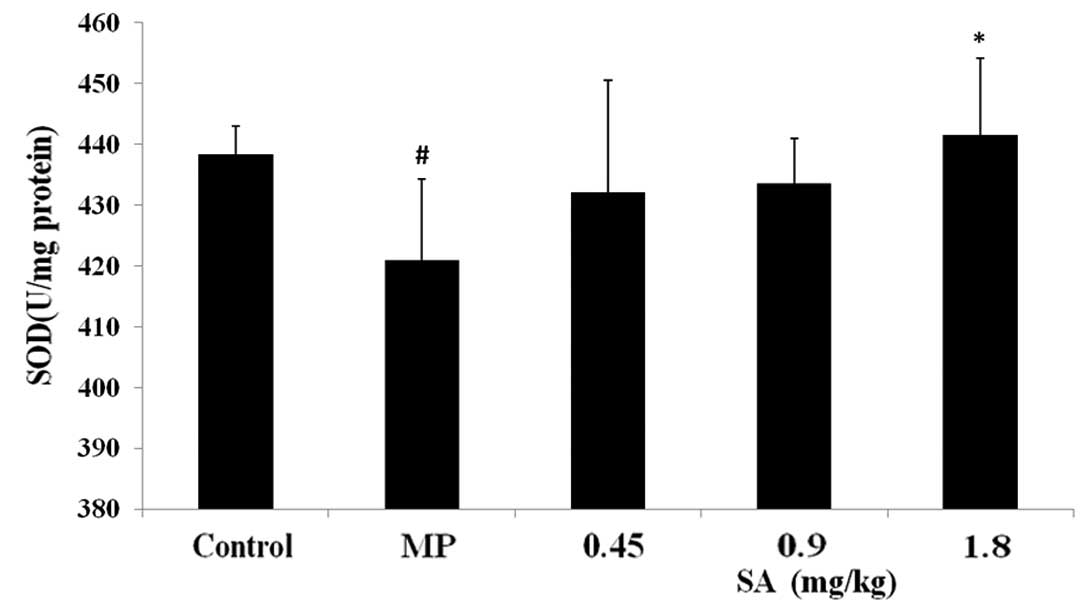
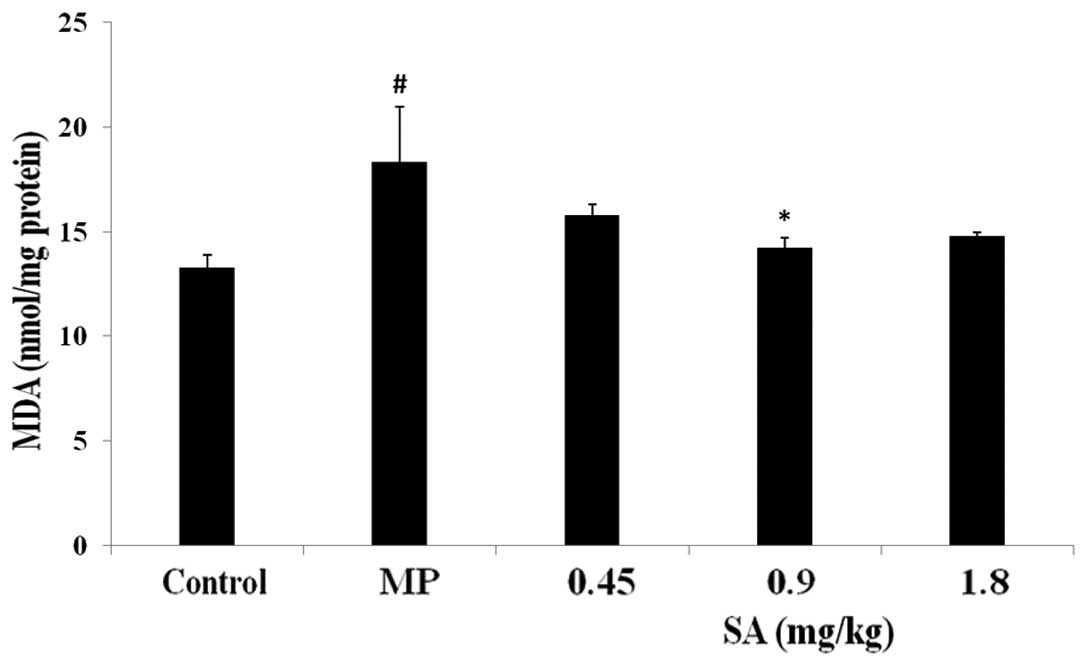
Effect of SA on SOD, GSH-Px activities
and GSH, MDA levels in the liver following methyl parathion
poisoning
Analysis of the antioxidative parameters revealed
that administration of methyl parathion resulted in a significant
decrease in the activities of SOD and GSH-Px, and the level of GSH.
Furthermore, methyl parathion poisoning also led to an increase in
the level of MDA. However, treatment with SA attenuated the changes
in the activities of SOD and GSH-Px, and the levels of GSH and MDA
(Figs. 6–9).
Discussion
The liver plays a pivotal role in a large number of
metabolic and immune processes; therefore, the hepatotoxicity of
the liver as a result of toxic agents, and the potential
therapeutic strategies have attracted numerous studies. The systems
and organs that can be influenced by organophosphate intoxicants
are the immune, urinary and reproductive systems, the pancreas,
liver and the lungs. Certain studies have reported that
organophosphates cause liver damage (7). ALT and AST are significant indicators
of liver damage. These enzymes were revealed to leak out into the
blood following hepatocellular injury (3). Furthermore, other studies have also
indicated that organophosphates lead to serious changes in
hepatocytes and organelles; for example, an increase in the
chromatin content of hepatocyte nuclei and cytoplasmic density. The
involved cells also became vacuolar in appearance as a result of
lysis in the mitochondrial matrices. In certain cells, the lipid
content constituted the majority of the cytoplasm. It was also
revealed that collagen fibers expand to form bands in certain areas
of the liver (8). In our study, MP
caused a significant increase in the activities of the ALT and AST
enzymes. Histological damage in the MP-treated rats was also
observed. However, SA treatment markedly reduced the MP-induced
hepatic dysfunction, as revealed by a significant reduction in the
serum ALT and AST enzyme activities, and an attenuation in the
histological changes in the liver.
NO is a key factor in hepatic injury (9). Increased levels of NO are a natural
sequence to the inhibition of AChE by organophosphates. A number of
studies have demonstrated that NO may promote inflammation-induced
cell and tissue dysfunction. NO-dependent reactions are significant
in modulating the inflammatory response and may account for hepatic
necrosis through specific signaling mechanisms (10,11).
The liver is an organ that is evidently influenced by NO.
Therefore, when a large sustained amount of NO is present, damage
occurs in the liver (12).
Otherwise, the serious liver injury may induce excessive systemic
inflammation (13). In the present
study, we measured the level of NO in plasma and liver tissue. Our
study demonstrated a significant increase in nitrate (measured as
nitrite), the stable end product of NO, in the plasma and the liver
in the MP-treated group. As expected, SA treatment significantly
inhibited MP-induced NO production.
Pesticides have been reported to induce the
generation of reactive oxygen species (ROS) in vitro and
in vivo (14). ROS are
significant in the toxicity of organophosphate compounds (15). Oxidative damage by free radicals or
ROS could result in lipid peroxidation, causing changes in membrane
properties and cell dysfunction (16). Organophosphate pesticides may
induce oxidative stress, leading to the generation of free radicals
and an alteration in antioxidants (17). It was revealed that the lipid
peroxidative substance (MDA) was elevated and SOD was reduced
following organophosphate poisoning (4). Methyl parathion was also able to
deplete GSH in the rat liver by forming GSH conjugates (18). In addition, the activity of GSH-Px
was diminished following organophosphate poisoning (19). Our study revealed that
organophosphate poisoning resulted in a change in oxidative stress.
However, SA administration ameliorated the oxidative damage. These
findings are in accordance with a previous study which reported
that SA improves the antioxidative defense system (20).
The results from the present study demonstrated that
acute organophosphate poisoning causes serious histopathological
changes in the rat liver; however, these changes are reversible
following SA treatment. The pharmacological action of SA is
associated with its antioxidative and anti-inflammation effects.
The protective effects of SA on liver injury induced by
organophosphates require further study.
Acknowledgements
The authors would like to thank
Tongshen Liu for his technical assistance. This study was supported
by the Taishan Scholar Project, the 11th Five-Year Key Programs for
Science and Technology Development of China (grant no.
2008ZX09202-008) and the National Natural Science Foundation of
China (grant no. 30772760).
References
|
1.
|
Atiş S, Cömelekoğlu U, Coşkun B, Ozge A,
Ersöz G and Talas D: Electrophysiological and histopathological
evaluation of respiratory tract, diaphragm, and phrenic nerve after
dichlorvos inhalation in rats. Inhal Toxicol. 14:199–215.
2002.PubMed/NCBI
|
|
2.
|
Zhu H, Rockhold RW, Baker RC, Kramer RE
and Ho IK: Effects of single or repeated dermal exposure to methyl
parathion on behavior and blood cholinesterase activity in rats. J
Biomed Sci. 8:467–474. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Kalender S, Ogutcu A, Uzunhisarcikli M,
Açikgoz F, Durak D, Ulusoy Y and Kalender Y: Diazinon-induced
hepatotoxicity and protective effect of vitamin E on some
biochemical indices and ultrastructural changes. Toxicology.
211:197–206. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Zhang X, Yao W, Jia B, Sun D, Ka W, He D,
Wang X and Wen Z: Acute dichlorvos poisoning induces
hemorheological abnormalities in rabbits via oxidative stress. Clin
Hemorheol Microcirc. 44:207–216. 2010.PubMed/NCBI
|
|
5.
|
Ouyang YH, Li SL, Song W, Zhao N and Ma
ZF: Effect of penehyclidine hydrochloride on tumor necrosis
factor-alpha in liver and spleen in mice with acute
organophosphorus pesticide poisoning. Zhongguo Wei Zhong Bing Ji
Jiu Yi Xue. 22:238–239. 2010.(In Chinese).
|
|
6.
|
Sirtori CR: Aescin: pharmacology,
pharmacokinetics and therapeutic profile. Pharmacol Res.
44:183–193. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Yurumez Y, Ikizceli I, Sozuer EM, Soyuer
I, Yavuz Y, Avsarogullari L and Durukan P: Effect of interleukin-10
on tissue damage caused by organophosphate poisoning. Basic Clin
Pharmacol Toxicol. 100:323–327. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Satar S, Satar D, Tap O, Koseoglu Z and
Kaya M: Ultrastructural changes in rat liver treated with
pralidoxime following acute organophosphate poisoning. Mt Sinai J
Med. 71:405–410. 2004.PubMed/NCBI
|
|
9.
|
Senga F, Yin L, Karasuno H, Ohtaki H,
Nakamachi T, Satoh K and Shioda S: Minus charge stimulation
prevents LPS-induced liver injury by reduction of nitric oxide. J
Clin Biochem Nutr. 42:222–227. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Li J and Billiar TR: Nitric Oxide. IV
Determinants of nitric oxide protection and toxicity in liver. Am J
Physiol. 276:G1069–G1073. 1999.PubMed/NCBI
|
|
11.
|
Grisham MB, Jourd’Heuil D and Wink DA:
Nitric oxide. I. Physiological chemistry of nitric oxide and its
metabolites: implications in inflammation. Am J Physiol. 276:15–21.
1999.PubMed/NCBI
|
|
12.
|
Hon WM, Lee KH and Khoo HE: Nitric oxide
in liver diseases: friend, foe, or just passerby? Ann NY Acad Sci.
962:275–295. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Johnson D and Mayers I: Multiple organ
dysfunction syndrome: a narrative review. Can J Anaesth.
48:502–509. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Abdollahi M, Ranjbar A, Shadnia S, Nikfar
S and Rezaie A: Pesticides and oxidative stress: a review. Med Sci
Monit. 10:141–147. 2004.
|
|
15.
|
Gunay N, Kose B, Demiryurek S, Ocak AR,
Erel O and Demiryurek AT: Effects of a selective Rho-kinase
inhibitor Y-27632 on oxidative stress parameters in acute
dichlorvos poisoning in rats. Cell Biochem Funct. 26:747–754. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Sinclair AJ, Barnett AH and Lunec J: Free
radicals and antioxidant systems in health and disease. Br J Hosp
Med. 43:334–344. 1990.PubMed/NCBI
|
|
17.
|
Agrawal D, Sultana P and Gupta GS:
Oxidative damage and changes in the glutathione redox system in
erythrocytes from rats treated with hexachlorocyclohexane. Food
Chem Toxicol. 29:459–462. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Della Morte R, Villani GR, Di Martino E,
Squillacioti C, De Marco L, Vuotto P, Belisario MA and Staiano N:
Glutathione depletion induced in rat liver fractions by seven
pesticides. Boll Soc Ital Biol Sper. 70:185–192. 1994.PubMed/NCBI
|
|
19.
|
Khan SM, Sobti RC and Kataria L:
Pesticide-induced alteration in mice hepato-oxidative status and
protective effects of black tea extract. Clin Chim Acta.
358:131–138. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Küçükkurt I, Ince S, Keleş H, Akkol EK,
Avci G, Yeşilada E and Bacak E: Beneficial effects of Aesculus
hippocastanum L. seed extract on the body’s own antioxidant
defense system on subacute administration. J Ethnopharmacol.
129:18–22. 2010.
|