Introduction
More than 90% of patients with acute promyelocytic
leukemia (APL) achieve complete remission clinically using
all-trans-retinoic acid (ATRA), a strong differentiation
inducer (1). However, the rapid
development of ATRA resistance brings a new problem to the
treatment of APL. The relapse and refractoriness of APL remains one
of the most difficult problems clinically (2). How to treat APL with ATRA resistance
has become one of the hot spots of APL research.
Interferon (IFN), as an important cytokine, has
broad biological activities. It not only inhibits the growth of
tumor cells, but also reverses the drug resistance of chemotherapy.
As proven by several studies, the mechanisms of ATRA resistance are
probably related to the lack of certain important proteins which
are synthesized by IFN (3–5). In order to solve the problem of ATRA
resistance in APL, we studied the effect and mechanisms of IFN-γ in
combination with ATRA on the proliferation/differentiation of NB4
cells (APL cell line with ATRA sensitivity) and NB4-R1 cells (APL
cell line with ATRA resistance), respectively.
Materials and methods
Reagents
ATRA (Sigma Co.) was dissolved in absolute ethanol
to a concentration of 10−3 mol/l and was stored at
−20°C. IFN-γ (Shanghai Clone Biology Technical Co.) was dissolved
in normal saline, diluted to 4×108 U/l and stored at
−20°C. The rabbit anti-human polyclonal antibody was purchased from
Chemicon Co. The goat anti-rabbit FITC-IgG antibody was purchased
from KPL Co. The CD11b antibody for flow cytometry (FCM) was
purchased from Immunotech Co.
Cell culture
NB4 is an APL cell line established from a patient
with APL by Lanotte. NB4-R1 is an ATRA-resistant APL cell line.
They were all donated by the Hematology Research Institute of
Ruijin Hospital, Shanghai. NB4 and NB4-R1 cells were inoculated at
a density of 1×106/l in RPMI-1640 medium, respectively,
and cultured at 37°C in a humidified 5% CO2
incubator.
Detection of proliferation by MTT assay
(6,7)
NB4 or NB4-R1 cells were inoculated to each well of
96-well culture plates with a concentration of 5×104/l
and 100 μl for each well, respectively. ATRA, IFN-γ and
IFN-γ + ATRA were added respectively to each experimental group.
The final concentration of ATRA was 1×10−6 mol/l and the
final concentration of IFN-γ was 1×106 U/l. RPMI-1640
medium (100 μl) was added to the control group. Each well
volume was kept at 200 μl and made up the residual volume
with RPMI-1640 medium. Cell proliferation was detected by MTT assay
on days 1, 3, 5 and 8, respectively. The results were expressed as
the mean absorbance (A490 nm) value of triplicates.
Observation of cell morphology
NB4 or NB4-R1 cells were cultured in 25-ml culture
flasks at a concentration of 5×104/l and 5 ml for each
flask, respectively. The subgroups and treatment methods were in
accordance with the MTT assay. The cells were harvested on days 1,
3, 5 and 8, respectively. The cell sediments were made into smears
and stained with Wright’s solution, and then observed by light
microscopy.
NBT reduction assay (8,9)
Cell culture and treatment were the same as for
observation of cell morphology. Cells were harvested by
centrifugation and removal of supernatant, and the samples were
blended with 0.5 ml NBT reaction solution (1 mg/ml NBT and 100
ng/ml TPA), incubated at 37°C for 1 h and centrifuged for 5 min.
The cell sediments were made into smears and stained with Wright’s
solution, and then detected under immersion objective. NBT-positive
cell rates were calculated in every 200 cells. The test was
performed three times and the results were expressed as a mean of
triplicates: NBT-positive cell rates = NBT-positive cell
counts/total cell counts × 100%.
Detection of CD11b antigen
Cell culture and treatment were the same as for
observation of cell morphology. Mouse anti-human antibody
FITC-CD11b (10 μl) was added to a 100-μl cell
suspension (total cell counts were ∼5×105) and blended
completely. The samples were stained for 15–30 min at 25°C and
protected from light. After washing with PBS twice, centrifugation
and removal of the supernatant, the samples were fixed with 500
μl 2% paraform solution. The expression of the CD11b antigen
was detected by FCM.
Detection of the promyelocytic leukemia
(PML) protein
Cell culture and treatment were the same as for
observation of cell morphology. The cells were harvested by
centrifugation and removal of supernatant. The cell suspension was
diluted to a concentration of 1×106/l with PBS. The cell
suspension (0.1 ml) was extracted to collect specimens and the
indirect immune fluorescence test was carried out. After being
fixed with 4% paraform solution for 20 min, the slides were washed
with PBS and BSA-PBS twice. Then, the slides were treated with 0.1%
Triton X-100 for 10 min and incubated with anti-PML anti-body
(1:200) for 1 h at 37°C in a waterbath. After washing with BSA-PBS
and PBS twice, 1:10 diluted fluorescence-labeled goat anti-rabbit
IgG was added to the slides for 1 h at 37°C in a waterbath. After
being washed with BSA-PBS and PBS, the slides were investigated
using a fluorescent microscope (wavelength 488 nm) and images were
captured.
Statistical analysis
All data are expressed as the means ± standard
deviation (SD). Statistical analysis of data was carried out using
the Student’s t-test. P<0.05 was considered to denote
statistical significance.
Results
Effect of IFN-γ and/or ATRA on the
proliferation of NB4 and NB4-R1 cells
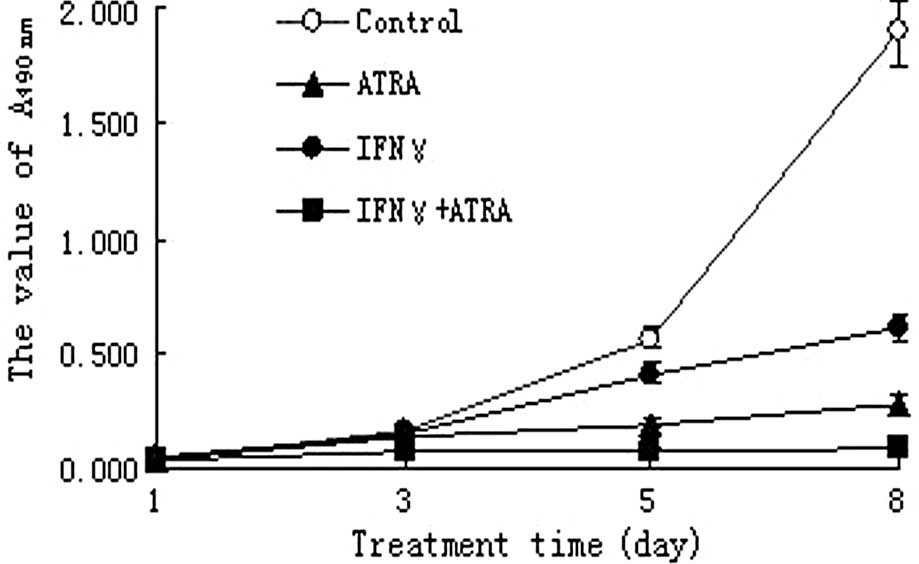
The growth of NB4 cells in the IFN-γ, ATRA and IFN-γ
+ ATRA treatment groups was significantly inhibited after day 5
compared to that in the control group (P<0.05). Meanwhile, the
growth inhibition effect of IFN-γ + ATRA treatment on the NB4 cells
was the strongest among the three experimental groups. The growth
inhibition effect of ATRA was next and last was the IFN-γ treatment
(P<0.05) (Fig. 1).
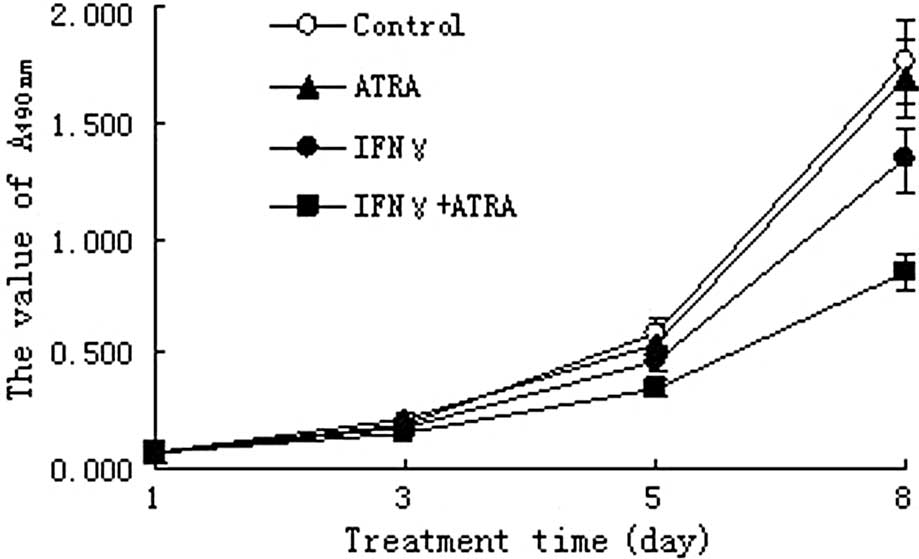
The growth of NB4-R1 cells in the IFN-γ and IFN-γ +
ATRA treatment groups was significantly inhibited after day 5
compared to that in the control group (P<0.05). However, there
were no significant differences in growth inhibition rates between
the ATRA treatment group and the control group (P>0.05).
Meanwhile, the growth inhibition effect of the IFN-γ + ATRA
treatment on NB4-R1 cells was stronger than that of the IFN-γ
treatment (P<0.05) (Fig.
2).
Effect of IFN-γ and/or ATRA on the
differentiation of NB4 and NB4-R1 cells
Morphological observation
Morphological differentiation of NB4 cells was not
observed in the IFN-γ treatment group on day 3. However, it was
observed in both the ATRA and IFN-γ + ATRA treatment groups.
Moreover, the differentiation degree of NB4 cells in the IFN-γ +
ATRA treatment group was higher than that in the ATRA treatment
group. Morphological differentiation of NB4-R1 cells was not
observed in both the IFN-γ and ATRA treatment groups on day 3, but
it was observed in the IFN-γ + ATRA treatment group. Morphological
differentiation of NB4 and NB4-R1 cells was manifested as the
reduction in nuclear size, the increase in cytoplasm and the
disappearance of nucleoli under Wright’s staining (data not shown,
refer to Fig. 3).
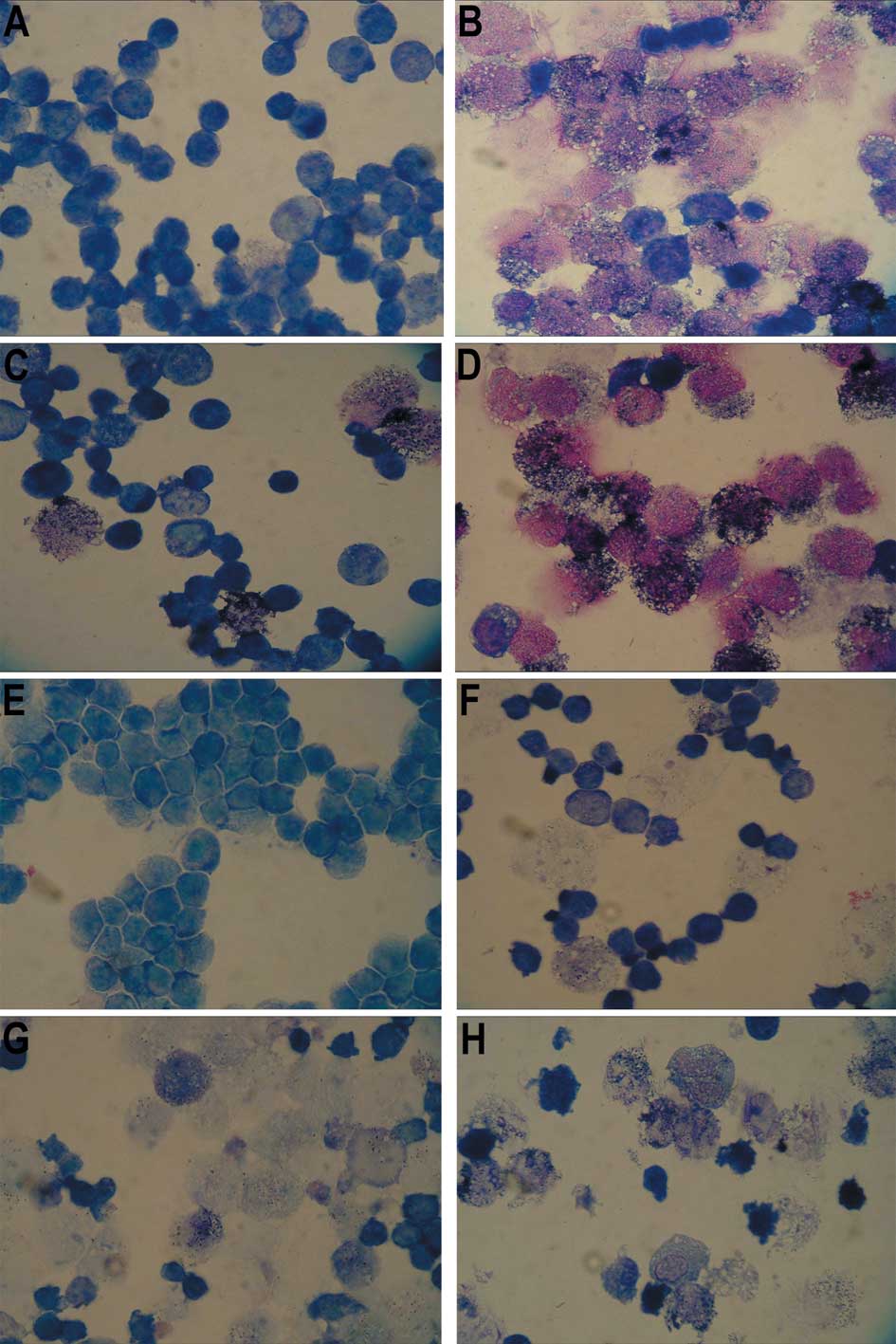 | Figure 3.Photomicrographs of the NBT reduction
assay of NB4 cells in (A) the control group, (B) the ATRA treatment
group, (C) the IFN-γ treatment group, (D) the IFN-γ + ATRA
treatment group, and of NB4-R1 cells in (E) the control group, (F)
the ATRA treatment group, (G) the IFN-γ treatment group and (H) the
IFN-γ + ATRA treatment group (Wright’s stain; magnification,
×1,000). (A and E) There were no black particles (formazan) in the
control group of NB4 and NB4-R1 cells. (B) There were many black
particles (formazan) in the ATRA group of NB4 cells, so-called
NBT-positive cells, which indicated that the NB4 cells had
differentiated. (F) However, almost no black particles (formazan)
appeared in the ATRA group of the NB4-R1 cells. (C and G) There
were a few black particles (formazan) in the IFN-γ group of the NB4
and NB4-R1 cells. (D and H) Notably, in both the NB4 and NB4-R1
cells, the NBT-positive rates in the IFN-γ + ATRA treatment group
were the highest among the four groups, respectively. |
NBT reduction assay
NBT-positive rates of NB4 cells in the ATRA, IFN-γ
and IFN-γ + ATRA treatment groups were significantly higher than
those in the control group (P<0.05). NBT-positive rates of
NB4-R1 cells in the three experimental groups were significantly
higher than those in the control group (P<0.05). Meanwhile, in
both the NB4 and NB4-R1 cells, the NBT-positive rates in the IFN-γ
+ ATRA treatment group were the highest among the three
experimental groups, respectively (P<0.05) (Table I, Fig.
3).
 | Table I.Results of NBT reduction assay of NB4
and NB4-R1 cells in the four treatment groups. |
Table I.
Results of NBT reduction assay of NB4
and NB4-R1 cells in the four treatment groups.
| Treatment group | NB4 | NB4-R1 |
|---|
| Control | 1.1±0.1 | 1.0±0.1 |
| ATRA | 74.7±1.5a | 5.2±0.1a |
| IFN-γ | 19.3±0.5a,b | 16.8±0.3a,b |
| IFN-γ + ATRA | 93.3±2.1a–c | 30.4±0.8a–c |
Detection of the CD11b antigen
Expression of the CD11b antigen on the NB4 cell
surface in the ATRA, IFN-γ and IFN-γ + ATRA treatment groups on day
3 was markedly higher than that in the control group (P<0.05).
Moreover, the expression of the CD11b antigen on the NB4-R1 cell
surface in the IFN-γ and IFN-γ + ATRA treatment groups was
significantly higher than that in the control group (P<0.05).
However, there was no significant difference in the expression of
CD11b antigen on the NB4-R1 cell surface between the ATRA treatment
group and the control group (P<0.05). Meanwhile, both on the
cell surfaces of the NB4 and NB4-R1 cells, the expression of the
CD11b antigen in the IFN-γ + ATRA treatment group was the highest
among the four groups, respectively (P<0.05; Table II).
 | Table II.Expression of the CD11b antigen on the
NB4 and NB4-R1 cell surface in the four treatment groups. |
Table II.
Expression of the CD11b antigen on the
NB4 and NB4-R1 cell surface in the four treatment groups.
| Subgroup | NB4 | NB4-R1 |
|---|
| Control | 2.91±0.06 | 1.58±0.04 |
| ATRA | 61.67±1.12a | 2.19±0.07 |
| IFN-γ |
22.58±0.37a,b |
9.30±0.16a,b |
| IFN-γ + ATRA |
77.14±1.61a–c |
18.45±0.31a–c |
Effect of IFN-γ and/or ATRA on the
expression of PML protein in NB4 and NB4-R1 cells
After the NB4 and NB4-R1 cell control groups were
stained with the rabbit anti-human PML multiple antibody and goat
anti-rabbit FITC-IgG, many diffuse, fine fluorescent particles were
noted in the nuclei of both the NB4 and NB4-R1 cells. These were
called ‘swollen furuncle’ structures and implied that the nuclear
body (NB) structures were destroyed (Fig. 4A and E). After treatment with ATRA
for 3 days, the swollen furuncle structures in the NB4 cell nuclei
disappeared and bulky fused fluorescent particles (NB structures)
appeared, which implied that the NB structures in the NB4 cells
were restored (Fig. 4B). However,
the swollen furuncle structures in the NB4-R1 cell nuclei remained,
which implied that the NB structures in the NB4-R1 cells were not
restored (Fig. 4F). After
treatment with IFN-γ, the size and number of the fluorescent
particles in the NB4 and NB4-R1 cell nuclei were significantly
increased compared to the control group, which implied that the
expression of PML protein in both the NB4 and NB4-R1 cells was
increased. Nevertheless, no bulky fused fluorescent particles (NB
structures) appeared in both the NB4 and NB4-R1 cell nuclei, which
implied that NB structures in both NB4 and NB4-R1 cells were not
restored (Fig. 4C and G). After
treatment with IFN-γ + ATRA, the size and number of the fluorescent
particles were increased and bulky fused fluorescent particles (NB
structures) appeared in the NB4 cell nuclei, implying that the
expression of PML protein was increased and NB structures were
restored (Fig. 4D). Although the
size and number of the fluorescent particles were increased, still
no bulky fused fluorescent particles were noted in the NB4-R1 cell
nuclei, which was similar to the IFN-γ group (Fig. 4H).
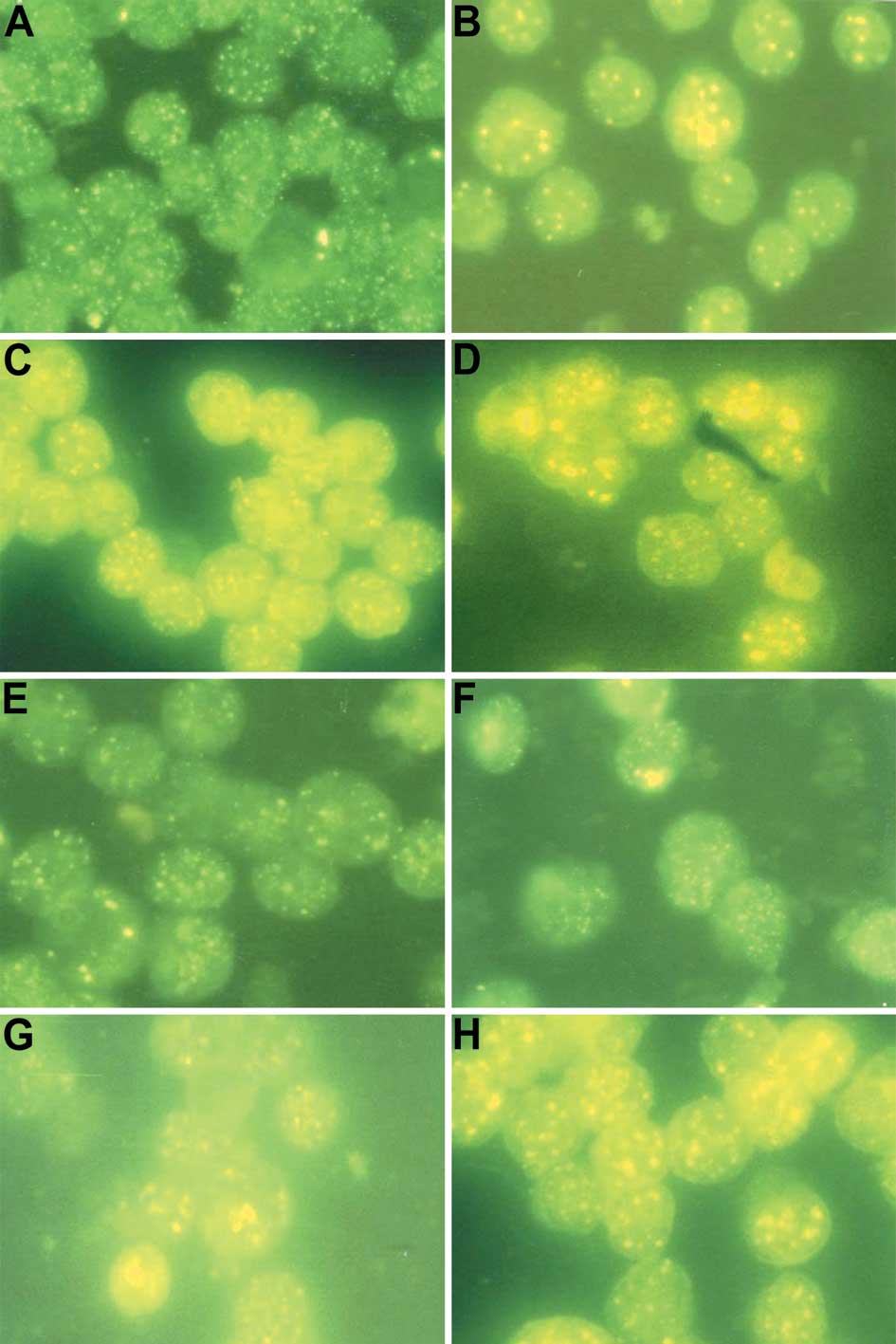 | Figure 4.Photomicrographs of indirect immune
fluorescent test of NB4 cells in (A) the control group, (B) the
ATRA treatment group, (C) the IFN-γ treatment group, (D) the IFN-γ
+ ATRA treatment group, and of NB4-R1 cells in (E) the control
group, (F) the ATRA treatment group, (G) the IFN-γ treatment group
and (H) the IFN-γ + ATRA treatment group (magnification, ×1,000).
(A and E) Many diffused, fine fluorescent particles are noted in
the nuclei of the NB4 and NB4-R1 cells, which implies that nuclear
body (NB) structures were destroyed. (B) Some bulky fused
fluorescent particles (NB structures) appear in the NB4 cell
nuclei, which implies that NB structures were restored. (F)
However, the fluorescent particles in the NB4-R1 cell nuclei
remained diffused and fine, which implies that NB structures were
not restored. (C and G) The size and number of the fluorescent
particles in the NB4 and NB4-R1 cell nuclei were increased compared
to those in the control group, but there were no bulky fused
fluorescent particles, which implies that the expression of PML
protein was increased, but NB structures were not restored. (D) The
size and number of the fluorescent particles were increased and
bulky fused fluorescent particles (NB structures) are noted in the
NB4 cell nuclei, which implies that the expression of PML protein
was increased and NB structures were restored. (H) Although the
size and number of the fluorescent particles were increased, no
bulky fused fluorescent particles (NB structures) are noted in the
NB4-R1 cell nuclei, which is similar to the IFN-γ treatment
group. |
Discussion
As a negative regulating factor of cell growth, IFN
inhibits the growth of many tumor cells (10). IFN has traditionally been applied
to the treatment of many malignant hematologic diseases, such as
chronic myelocytic leukemia (CML), multiple myeloma (MM) and hairy
cell leukemia (HCL) (11–13). ATRA, as a strong differentiation
inductor, also inhibits the proliferation of APL cells (14). In our study, MTT assay showed that
IFN-γ in combination with ATRA significantly enhanced the growth
inhibition effect of ATRA on both NB4 and NB4-R1 cells.
Buonamici et al (15) found that both type I and II IFN
induced the expression of the PML protein. The PML gene promoter
has an IFN-stimulated response element (ISRE) -GAGAATCGAAACT- and
an IFN-γ-activated site (GAS) -TTTACCGTAAG-. IFN combines with ISRE
or GAS of the PML gene to induce transcription and expression of
the PML gene directly (16–19).
In our study, the results of the indirect immune fluorescent test
showed that the size and the number of the fluorescent particles in
the NB4 and NB4-R1 cells were significantly increased compared to
the control group after they were treated with IFN-γ, which implied
that IFN-γ induces the expression of the PML protein. Our findings
are consistent with the reports of Buonamici et al.
As a type of tumor growth inhibitor, the PML protein
inhibits the growth of many types of tumor cells (20,21).
In APL cells, chromosome translocation of t(15,17)
leads to the formation of the PML-RARα fusion gene and the
expression of PML-RARα protein. The latter sequestrates the PML
protein by forming a heterodimer with the PML protein, which
results in the inactivity of PML protein and the occurrence of APL
(22–24). Accordingly, we hypothesized that
there are close relationships between the up-regulation of
expression of the PML protein induced by IFN-γ and the growth
inhibitory effect of IFN-γ on NB4 and NB4-R1 cells.
The NBT reduction assay is an index which is often
used to reflect the differentiation of APL cells functionally. The
CD11b antigen is usually expressed on the surface of mature myeloid
cells, which is also used to represent the maturation degree of APL
cells. In our study, the results of the NBT test and CD11b antigen
detection by FCM suggested that IFN-γ enhances the induction
differentiation effects of ATRA on NB4 cells. Most importantly, it
induces the differentiation of NB4-R1 cells with ATRA resistance
when it cooperates with ATRA. The same results were drawn from the
cell morphological observation (Fig.
3).
Wang et al (25) found that the differentiation of
hematopoietic progenitor cells which was induced by retinoic acid
(RA) required the participation of the PML protein. In
PML+/+ bone marrow cells, RA induced the terminal
differentiation of hematopoietic progenitor cells. On the contrary,
in PML−/− bone marrow cells, RA did not induce the
terminal differentiation of hematopoietic progenitor cells, even at
an extremely high concentration.
Nuclear body (NB) structures are nuclear protein
compounds, which exist in the nucleus of normal cells (26). The breakage of NB structures in APL
cells suggested that the APL cells had lost the abilities of
differentiation or maturation (27). We found that although IFN-γ could
not restore the NB structures in NB4 and NB4-R1 cells, it did
augment the expression of the PML protein and enhance the induction
differentiation effect of ATRA in both NB4 and NB4-R1 cells
(Fig. 4). Therefore, we considered
that IFN-γ in combination with ATRA enhanced the differentiation
effects of ATRA on NB4 cells and induced the maturation of NB4-R1
cells with ATRA-resistant, and this may be related to the
up-regulation of PML protein expression induced by IFN-γ. Many
studies have shown that IFN and ATRA have a synergistic effect on
modulating proliferation and differentiation in many cell lines,
which may be related to the fact that IFN and ATRA can induce
expression of certain genes, such as RIG-G, ISGs and P21WAF1/CIP1,
synergistically (28–31).
Taken together, IFN-γ enhances the growth inhibition
effect of ATRA in both NB4 and NB4-R1 cells. Most importantly,
IFN-γ cooperates with ATRA to induce the maturation of NB4 and
NB4-R1 cells with ATRA resistance, which may relate to the
up-regulation of PML protein expression.
Acknowledgements
The authors would like to thank Ms.
Fang Wang, Dr Qunling Zhang, Ms. Yiping Geng and Dr Ya Zhao for the
technical assistance in this study. The project was supported by
the National Natural Science Foundation of China (No. 30701133),
and the Shaanxi Province Science and Technology Development Fund,
China (2006K09-G5).
References
|
1.
|
Wang Z and Chen Z: Acute promyelocytic
leukemia: from highly fatal to highly curable. Blood.
111:2505–2515. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Zhou GB, Zhang J, Wang ZY, Chen SJ and
Chen Z: Treatment of acute promyelocytic leukaemia with all-trans
retinoic acid and arsenic trioxide: a paradigm of synergistic
molecular targeting therapy. Philos Trans R Soc Lond B Biol Sci.
362:959–971. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Wang X, Yin Y, Ma P, et al: Apoptosis of
human pancreatic carcinoma cells induced by all-trans retinoic acid
and interferon. Chin J Cancer Res. 21:224–228. 2009. View Article : Google Scholar
|
|
4.
|
Park HH, Kim M, Lee BH, et al:
Intracellular IL-4, IL-10, and IFN-gamma levels of leukemic cells
and bone marrow T cells in acute leukemia. Ann Clin Lab Sci.
36:7–15. 2006.PubMed/NCBI
|
|
5.
|
Hoyer KK, Herling M, Bagrintseva K, et al:
T cell leukemia-1 modulates TCR signal strength and IFN-gamma
levels through phosphatidylinositol 3-kinase and protein kinase C
pathway activation. J Immunol. 175:864–873. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Montoro E, Lemus D, Echemendia M, et al:
Comparative evaluation of the nitrate reduction assay, the MTT
test, and the resazurin microtitre assay for drug susceptibility
testing of clinical isolates of Mycobacterium tuberculosis.
J Antimicrob Chemother. 55:500–505. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Huang Y, Liu J, Wang LZ, Zhang WY and Zhu
XZ: Neuroprotective effects of cyclooxygenase-2 inhibitor celecoxib
against toxicity of LPS-stimulated macrophages toward motor
neurons. Acta Pharmacol Sin. 26:952–958. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Owen J, Bates J and Lewis S: Differential
effects of nitroblue tetrazolium on the hemodynamic responses
elicited by activation of alpha(1)-adrenoceptors and 5-HT(2)
receptors in conscious rats. Eur J Pharmacol. 535:248–252. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Choi HS, Kim JW, Cha YN and Kim C: A
quantitative nitroblue tetrazolium assay for determining
intracellular superoxide anion production in phagocytic cells. J
Immunoassay Immunochem. 27:31–44. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Shi J, Zhang Y, Su JY, et al: Cytotoxicity
of IFN-gamma-activated dendritic cells to freshly isolated acute
myeloid leukemia cells. J Exp Hematol. 13:1071–1075.
2005.PubMed/NCBI
|
|
11.
|
Burchert A, Muller MC, Kostrewa P, et al:
Sustained molecular response with interferon alfa maintenance after
induction therapy with imatinib plus interferon alfa in patients
with chronic myeloid leukemia. J Clin Oncol. 28:1429–1435. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Khoo TL, Vangsted AJ, Joshua D and Gibson
J: Interferon-alpha in the treatment of multiple myeloma. Curr Drug
Targets. 12:437–446. 2011.PubMed/NCBI
|
|
13.
|
Benz R, Stussi G and Fehr J: Interferon as
an alternative to purine analogues in the treatment of hairy cell
leukaemia. Brit J Haematol. 148:664–666. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Gupta V, Yi QL, Brandwein J, et al: Role
of all-trans-retinoic acid (ATRA) in the consolidation therapy of
acute promyelocytic leukaemia (APL). Leukemia Res. 29:113–114.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Buonamici S, Li D, Mikhail F, et al: EVI1
abrogates interferon-alpha response by selectively blocking PML
induction. J Biol Chem. 280:428–436. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Reineke EL and Kao HY: Targeting
promyelocytic leukemia protein: a means to regulating PML nuclear
bodies. Int Biol Sci. 5:366–376. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Chelbi-Alix MK, Vidy A, El Bougrini J and
Blondel D: Rabies viral mechanisms to escape the IFN system: the
viral protein P interferes with IRF-3, Stat1, and PML nuclear
bodies. J Interferon Cytokine Res. 26:271–280. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Crowder C, Dahle O, Davis RE, Gabrielsen
OS and Rudikoff S: PML mediates IFN-alpha-induced apoptosis in
myeloma by regulating TRAIL induction. Blood. 105:1280–1287. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Dror N, Rave-Harel N, Burchert A, et al:
Interferon regulatory factor-8 is indispensable for the expression
of promyelocytic leukemia and the formation of nuclear bodies in
myeloid cells. J Biol Chem. 282:5633–5640. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Bernardi R, Papa A and Pandolfi PP:
Regulation of apoptosis by PML and the PML-NBs. Oncogene.
27:6299–6312. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Li L and He DL: Transfection of
promyelocytic leukemia in retrovirus vector inhibits growth of
human bladder cancer cells. Acta Pharmacol Sin. 26:610–615. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Ravindranath Y, Gregory J and Feusner J:
Treatment of acute promyelocytic leukemia by using all-trans
retinoic acid (ATRA). Leukemia. 18:1576–1577. 2004.
|
|
23.
|
Gupta V, Yib QL, Brandwein J, et al:
Clinico-biological features and prognostic significance of
PML/RARalpha isoforms in adult patients with acute promyelocytic
leukemia treated with all trans retinoic acid (ATRA) and
chemotherapy. Leuk Lymphoma. 45:469–480. 2004. View Article : Google Scholar
|
|
24.
|
Zhang X, Yan X, Zhou Z, et al: Arsenic
trioxide controls the fate of the PML-RARα oncoprotein by directly
binding PML. Science. 328:240–243. 2010.PubMed/NCBI
|
|
25.
|
Wang ZG, Delva L, Gaboli M, et al: Role of
PML in cell growth and the retinoic acid pathway. Science.
279:1547–1551. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Abreu ELR, Baruffi MR, de Lima AS, et al:
The co-expression of PML/RAR alpha and AML1/ETO fusion genes is
associated with ATRA resistance. Brit J Haematol. 128:407–409.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Mueller BU, Pabst T, Fos J, et al: ATRA
resolves the differentiation block in t(15;17) acute myeloid
leukemia by restoring PU. 1 expression. Blood. 107:3330–3338. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Andrianifahanana M, Agrawal A, Singh AP,
et al: Synergistic induction of the MUC4 mucin gene by
interferon-gamma and retinoic acid in human pancreatic tumour cells
involves a reprogramming of signalling pathways. Oncogene.
24:6143–6154. 2005. View Article : Google Scholar
|
|
29.
|
Liu CH: Study on the synergic effect of
all-trans retinoic acid combined with IFN-alpha on the
proliferation and differentiation of HL-60 cells. Chin J Cell Mol
Immunol. 21:637–639. 642:2005.PubMed/NCBI
|
|
30.
|
Gu ZM, Wu YL, Zhou MY, et al: Pharicin B
stabilizes retinoic acid receptor-alpha and presents synergistic
differentiation induction with ATRA in myeloid leukemic cells.
Blood. 116:5289–5297. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Wang J, Peng Y, Sun YW, et al: All-trans
retinoic acid induces XAF1 expression through an interferon
regulatory factor-1 element in colon cancer. Gastroenterology.
130:747–758. 2006. View Article : Google Scholar : PubMed/NCBI
|