Introduction
The majority of patients treated with definitive
radiotherapy with or without chemotherapy in recent locally
advanced head and neck cancer trials are oropharyngeal cancer
patients (1–3). The incidence of base of tongue and
tonsil cancer in younger patients has increased dramatically over
the past three decades (4).
Several strategies designed to improve locoregional control for
locally advanced oropharyngeal cancers, including concurrent
chemoradiotherapy, intensity modulated radiation therapy and
altered fractionation radiation therapy, have been extensively
investigated (5–9). However, curing the index cancer
eliminates only one of several mortality risks faced by these
patients (10). Due to competing
risks of second primary cancer and comorbid medical conditions
related to smoking and alcohol use, overall survival remains
consistently lower than disease-free survival (11). Recently, high-risk human papilloma
virus (HPV) infection has emerged as an important risk factor for
oropharyngeal carcinoma (12). HPV
infection is now found in more than 50% of patients with
oropharyngeal cancer and tends to affect younger patients
irrespective of tobacco or alcohol use (13). Preliminary data suggest that these
patients are at lower risk for second primary cancers due to the
focal nature of HPV infection (14). Furthermore, since these patients
tend to be younger and are less likely to be heavy users of
tobacco, they may also be at lower risk for cardiovascular disease
(13,15). To evaluate the different causes of
mortality in patients with oropharyngeal carcinoma treated with
radiotherapy, we conducted an analysis of the SEER database.
Materials and methods
Patient selection
The patient population in this study consisted of
adult patients with stage I–IVb oropharyngeal squamous cell
carcinoma diagnosed between 1988 and 2001 in the Surveillance,
Epidemiology, and End Results (SEER) 17 database, who had been
treated with definitive radiotherapy. The SEER17 database is a
longitudinal database that collects information from 17 cancer
registries covering 26% of the US population. The SEER17 database
is composed of 17 population-based cancer registries from
Connecticut, New Jersey, Atlanta, Kentucky, Louisiana, rural
Georgia, Detroit, Iowa, Hawaii, New Mexico, Seattle-Puget Sound,
Utah, San Francisco-Oakland, San Jose-Monterey, Los Angeles,
greater California and the Alaska Native Tumor Registry. Serial
registry data are de-identified and submitted to the U.S. National
Cancer Institute on a bi-annual basis and these data are publicly
available for investigators (16).
Therefore, approval by an ethics committee was not necessary to
perform the analyses. The population covered by the SEER database
is considered representative of the US population and the case
ascertainment rate is reportedly 97.5% (16).
We identified 3728 eligible patients aged 18 to 85
with squamous cell carcinoma (SCC) of the oropharynx for this
analysis. Patients were excluded if there was no histological
confirmation, missing radiation records or unknown nodal stage.
Additionally, 58% of patients had available size and tumor
extension data allowing for further classification into AJCC 6th
edition T stage. Patients were all subclassified into base of
tongue (C01 or C02.4), tonsil (C09), soft palate (C05.1 or C05.2),
oropharyngeal wall (C10.2 or C10.3) and oropharynx NOS (C10.0,
C10.1, C10.8 or C10.9) using ICD-O-2 codes. Cause of mortality was
determined by SEER site recode codes, since ICD-O-2 codes were not
available. Patients with mortality from oral cavity (20020, 20040
and 20050) and oropharyngeal cancer (20020, 20050, 20070 and 20080)
were classified as mortality from index oropharyngeal cancer.
Additionally, mortality from miscellaneous malignant cancers
(37000) was classified as mortality from index cancer since 88% of
these deaths occurred within 5 years of diagnosis. Patients with
mortality from other types of cancer were classified as second
cancers. Patients with mortality from cardiovascular disease
(50060, 50070, 50080, 50090, 50100 and 50110) and other causes were
scored separately. The most recent follow-up available was December
2006.
Statistical analysis
Categorical variables included age (≤50, 51–60,
61–70 and >70), date of diagnosis (1988–1994 vs. 1995 to 2001),
gender, ethnicity (Caucasian/Asian vs. African descent), primary
site (tonsil vs. base of tongue vs. other), T stage, lymph node
stage (N1, N2a, N2b, N2c, N3), lymph node surgery, tumor size (2
cm, 2.1–4 cm, and >4 cm), tumor grade, marital status and prior
cancer diagnosis (Table I).
Patient age in years was analyzed as a categorical variable on
univariate analysis but as a continuous variable on multivariate
analysis. Information regarding HPV status, use of adjuvant
chemotherapy, performance status and radiotherapy details (dose,
fractionation and 3-dimensional conformal/intensity modulated
radiotherapy) were not available within the SEER database and this
information was not included for analysis. Overall survival was the
primary endpoint and mortality from primary oropharyngeal cancer,
second cancers, cardiovascular and other causes were secondary
endpoints.
 | Table I.Patient and tumor characteristics. |
Table I.
Patient and tumor characteristics.
| Variable | n (%) | 5-year overall
survival (%) | 10-year overall
survival (%) | P-value |
|---|
| Age (years) | median 63 (range
18–85) | | | <0.001 |
| ≤50 | 577 (15) | 49 | 37 | |
| 51–60 | 1033 (28) | 43 | 31 | |
| 61–70 | 1136 (30) | 36 | 21 | |
| >70 | 982 (26) | 26 | 9 | |
| Ethnicity | | | | <0.001 |
| Caucasian | 2797 (75) | 40 | 24 | |
| African
descent | 546 (15) | 24 | 13 | |
| Asian | 161 (4) | 45 | 36 | |
| Hispanic | 207 (6) | 39 | 24 | |
| Other | 18 (0) | 41 | 33 | |
| Gender | | | | 0.18 |
| Male | 2726 (73) | 37 | 22 | |
| Female | 1002 (27) | 39 | 24 | |
| Date of
diagnosis | | | | <0.001 |
| 1988–1994 | 1297 (35) | 32 | 17 | |
| 1995–2001 | 2431 (65) | 41 | 27 | |
| Marital status | | | | <0.001 |
| Married | 1807 (48) | 44 | 28 | |
| Divorced,
separated, widowed | 1140 (31) | 31 | 16 | |
| Single | 643 (17) | 32 | 19 | |
| Unknown | 138 (4) | 36 | 21 | |
| Prior cancer | | | | <0.001 |
| Yes | 553 (15) | 28 | 14 | |
| No | 3175 (85) | 39 | 24 | |
| Primary site | | | | <0.001 |
| Tonsil | 1410 (38) | 41 | 25 | |
| Base of tongue | 1446 (39) | 39 | 26 | |
| Soft palate | 425 (11) | 30 | 15 | |
| Pharyngeal
wall | 96 (3) | 27 | 14 | |
| Other | 351 (9) | 29 | 14 | |
| Grade | | | | |
| 1 | 233 (6) | 36 | 18 | |
| 2 | 1527 (41) | 34 | 20 | |
| 3 | 1274 (34) | 43 | 28 | |
| Unknown | 694 (19) | 37 | 19 | |
| T stage | | | | <0.001 |
| 1 | 471 (13) | 47 | 26 | |
| 2 | 1061 (28) | 42 | 27 | |
| 3 | 470 (13) | 34 | 20 | |
| 4 | 171 (5) | 25 | 17 | |
| Unknown | 1555 (42) | 34 | 20 | |
| Nodal stage | | | | <0.001 |
| 0 | 1392 (37) | 39 | 22 | |
| 1 | 480 (13) | 41 | 25 | |
| 2a | 218 (6) | 43 | 31 | |
| 2b | 1007 (27) | 38 | 24 | |
| 2c | 495 (13) | 30 | 20 | |
| 3 | 136 (4) | 24 | 15 | |
| AJCC stage | | | | <0.001 |
| I | 254 (7) | 43 | 20 | |
| II | 441 (12) | 43 | 29 | |
| III | 606 (16) | 42 | 25 | |
| IVa | 1778 (48) | 36 | 24 | |
| IVb | 136 (4) | 24 | 15 | |
| Missing | 513 (14) | 34 | 16 | |
All analyses were performed using Stata software
(version 9.1; StataCorp, College Station, TX, USA) by importing
data from the SEER (available at URL: www.seer.cancer.gov; accessed on September 14, 2009)
1973–2006 Public Use Data (National Cancer Institute, April 2009
release based on the November 2008 submission) into Stata. Overall
survival was calculated from the time of diagnosis to the time of
mortality or last follow-up using the Kaplan-Meier method.
Cause-specific mortality was calculated from the time of diagnosis
to the time of event or last follow-up. We analyzed the actuarial
rates of cause-specific mortality using the cumulative incidence
method described by Coviello et al using Stata 9.1 (17). When there are competing risks, the
Kaplan-Meier method for estimation of cumulative incidence curves
is considered inaccurate (18).
For overall survival, the stratified log-rank test was utilized to
compute survival estimates that were within specified strata
levels. Results were considered to indicate a statistically
significant difference at P-values <0.05.
Cox proportional hazards regression modeling was
limited to covariates that were found to be statistically
significant on univariate analysis. A multivariate Cox analysis was
developed to calculate the adjusted hazards ratios (HRs) and 95%
confidence intervals (95% CIs) for 1737 patients with complete
datasets. Separate multivariate models were developed for specific
causes of cancer mortality. A formal examination of the
proportional hazards assumption was performed graphically by
plotting −log(log(S(t)) versus log(t) for each covariate. This
confirmed that the covariates are independent with respect to time
and their HRs are constant over the clinically relevant period of
follow-up.
Results
Outcomes
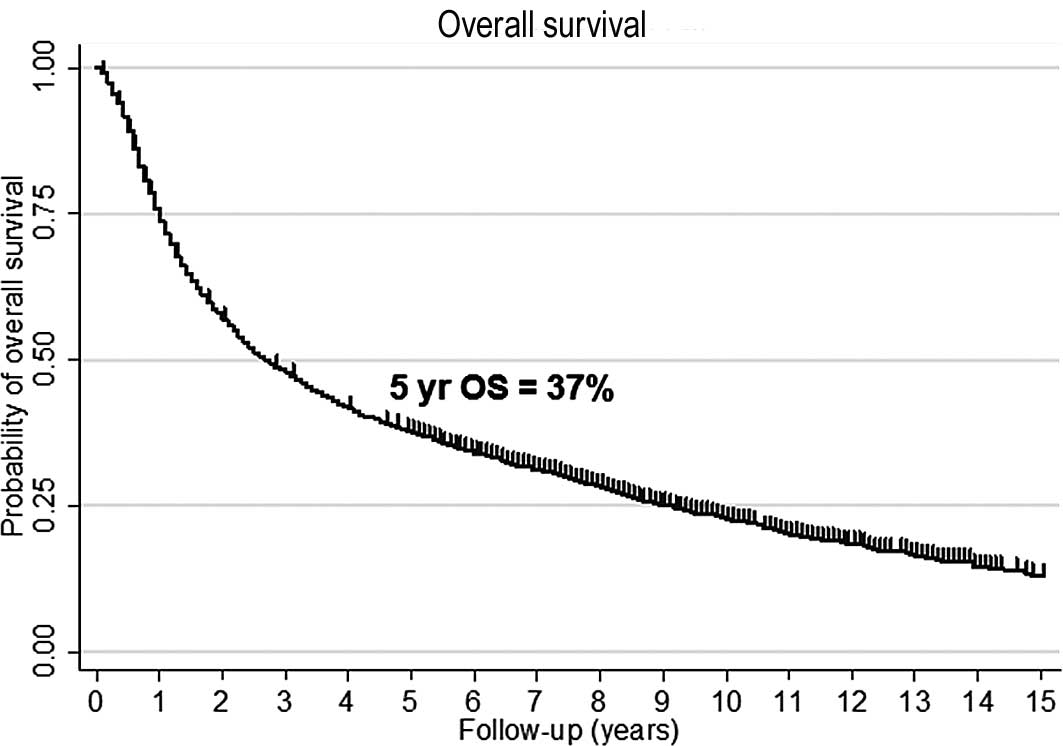
The 5- and 10-year overall survival was 37 and 22%,
respectively (Fig. 1A). Median
follow-up for surviving patients was 6.8 years (range, 0.1–18.8).
Predictors of decreased survival on univariate analysis included
advanced age, African descent, earlier date of diagnosis,
non-married, prior cancer, well or moderately differentiated
disease, subsite other than tonsil or base of tongue, advanced T
stage and advanced nodal disease (Table I). Gender was not significant on
univariate analysis. There was no significant difference by nodal
status of N0 to N2a (p=0.14) or between tonsil vs. base of tongue
(p=0.28).
Causes of mortality
To date, the causes of mortality included none
(n=942, 25%), primary oropharyngeal cancer (n=1428, 38%), second
primary cancer (n=613, 16%), cardiovascular (n=318, 9%) and other
causes (n=427, 11%). Various types of mortality occurred in
different patterns. The 5- and 10-year risk of mortality from
primary cancer was 35 and 37% (Fig.
1B). The 5 and 10-year risk of mortality from second primary
cancers was 16 and 21%, respectively (Fig. 1C). The 5- and 10-year risk of
cardiovascular mortality was 5 and 9% (Fig. 1D). The 5- and 10-year risk of
mortality from other causes was 7 and 11% (Fig. 1E). While 60% of mortalities within
the first 3 years of diagnosis of cancer were classified as oral
cavity or oropharyngeal cancers, 69% of mortalities after 3 years
were related to second primaries, cardiovascular disease or second
primary cancers.
Multivariable analyses
On Cox regression analysis, all available prognostic
factors were predictive of overall survival (Table II). The strongest predictors of
decreased survival in order of significance, were advanced age,
African descent, single marital status, advanced T stage, advanced
N stage and well/moderately differentiated disease. Separate
multivariable analyses of causes of mortality demonstrate that
different prognostic factors are implicated (Table III). Age, advanced nodal disease,
advanced T stage, well/moderately differentiated disease, African
descent and unmarried status were associated with greater risk of
mortality from primary oropharyngeal cancer. Predictors of
mortality from second cancer were non-HPV-associated subsite, prior
cancer diagnosis, advanced age, male gender and well/moderately
differentiated disease. Risk factors associated with mortality from
cardiovascular disease were advanced age, African ethnicity, male
gender and prior diagnosis of cancer. Only advanced age and
unmarried status predicted for mortality from other causes.
 | Table II.Predictors of all cause mortality
using Cox multivariable analysis. |
Table II.
Predictors of all cause mortality
using Cox multivariable analysis.
| HR | P-value |
|---|
| Age
(continuous) | 1.03 | <0.001 |
| African
ethnicity | 1.44 | <0.001 |
| Female gender | 0.82 | 0.004 |
| Date of diagnosis
(dichotomous) | 0.84 | 0.005 |
| Marital status | 0.75 | <0.001 |
| Subsite | 1.15 | 0.04 |
| First cancer
diagnosis | 0.83 | 0.02 |
| T stage
(continuous) | 1.16 | <0.001 |
| N stage
(continuous) | 1.10 | <0.001 |
| Grade
(continuous) | 0.85 | <0.001 |
 | Table III.Predictors of cause-specific
mortality using Cox multivariable analysis. |
Table III.
Predictors of cause-specific
mortality using Cox multivariable analysis.
| Primary cancer
HR | P-value | Second cancer
HR | P-value | CV disease HR | P-value | Other causes
HR | P-value |
|---|
| Age
(continuous) | 1.03 | <0.001 | 1.02 | <0.001 | 1.09 | <0.001 | 1.04 | <0.001 |
| African
ethnicity | 1.48 | <0.001 | 1.12 | 0.55 | 2.02 | 0.004 | 1.39 | 0.14 |
| Female gender | 1.02 | 0.78 | 0.63 | 0.002 | 0.56 | 0.004 | 0.75 | 0.08 |
| Date of diagnosis
(dichotomous) | 0.82 | 0.02 | 0.88 | 0.37 | 0.83 | 0.27 | 0.87 | 0.36 |
| Marital status | 0.75 | <0.001 | 0.83 | 0.16 | 0.89 | 0.50 | 0.60 | 0.001 |
| Subsite | 0.93 | 0.47 | 1.85 | <0.001 | 1.14 | 0.51 | 1.19 | 0.30 |
| First cancer
diagnosis | 1.15 | 0.26 | 0.51 | <0.001 | 0.60 | 0.01 | 0.84 | 0.37 |
| T stage
(continuous) | 1.28 | <0.001 | 1.10 | 0.17 | 0.93 | 0.49 | 1.03 | 0.75 |
| N stage
(continuous) | 1.15 | <0.001 | 1.07 | 0.10 | 1.03 | 0.62 | 1.02 | 0.70 |
| Grade
(continuous) | 0.79 | <0.001 | 0.81 | 0.03 | 1.11 | 0.42 | 0.96 | 0.73 |
Discussion
Treatment of primary oropharyngeal
cancer
Despite advances in non-surgical therapy for
oropharyngeal cancer over the past two decades, patients remain at
significant risk of mortality from primary cancer (1,5–6,8).
Since the majority of deaths occurring in the first 5 years
following diagnosis are attributed to primary cancer, further
improvements in therapy would yield meaningful improvements in
overall survival (19). The
improved prognosis associated with oropharyngeal cancers in recent
years may be related to increased utilization of concurrent
chemoradiation, technological advances in radiation delivery and
the increasing prevalence of HPV-related tumors (5,9,12).
Caucasian ethnicity and high grade tumors have both been associated
with HPV-related tumors and likely account for some of the improved
prognosis observed in those subgroups (13,20).
The 5-year overall survival among patients of African descent was
only 24%. Consistent with previously published work, efforts to
improve outcome in African-American patients by earlier diagnosis
and improved treatment for HPV-negative disease are urgently
required (20).
Treatment of elderly patients remains a challenge
due to the increased difficulty of administering therapy to this
cohort (21). The 5-year overall
survival for the >70 year old population is 26%. Two recent
meta-analyses suggest that neither concurrent chemotherapy nor
altered fractionation radiation therapy were beneficial for
patients older than 70 years of age, at least partly due to the
high risk of mortality from comorbid illness (5,22).
Whether better-tolerated combined modality treatments, such as
concurrent cetuximab and radiotherapy, will improve outcome is
currently unknown (1,23). As expected, advanced T and N stage
(≥N2B) predicted an increased risk of mortality from primary
oropharyngeal cancer (24). Based
on the historically poor survival of patients with T3–4 or N2c-N3
disease, good performance status patients with high-risk disease
are an appropriate population of patients to investigate
intensification of therapy beyond concurrent chemoradiotherapy by
adding induction chemotherapy, altered fractionation radiation and/
or biologically targeted therapy (3,25–26).
The association of unmarried status with mortality from primary
oropharyngeal cancer is largely unexplained (16). Unmarried patients may lack social
support and are at higher risk for pretreatment health behaviors
such as tobacco and alcohol abuse, poor diet and limited physical
activity (27).
Mortality from second primary cancer,
cardiovascular disease and other causes
Between years 5 and 10 following diagnosis, 41% of
5-year survivors succumbed to the disease. During this interval,
73% of mortalities were attributed to diagnoses other than oral
cavity or oropharyngeal cancer. As the long-term survival of head
and neck cancer continues to improve, identifying strategies to
prevent late events may become increasingly important.
In contrast to field cancerization associated with
tobacco-and alcohol-related upper aerodigestive tumors, oral HPV
infection is generally focal (13). Emerging data suggest that
HPV-related patients have a lower risk of second malignancies
(14). Consistent with this
theory, patients more likely to harbor HPV-positive tumors, such as
those of younger age, female gender, tonsil or base of tongue
subsite and high-grade disease, have a lower risk of mortality from
second cancer (13). Given the
increased risk of cancer associated with tobacco use, prior
diagnosis of cancer may be a surrogate for smoking status (28). In this study, we also identified a
cohort of patients at increased risk of cardiovascular disease and
mortality from other causes. Several risk factors overlap with
those associated with second cancers (male gender and prior cancer
diagnosis), indicating that tobacco use is the common risk factor.
African-American patients and the elderly appear to be at
particularly high risk for mortality from cardiovascular disease.
Increased surveillance and optimal medical management appear
appropriate for these subgroups (29).
Limitations
It is important to acknowledge the limitations of
this retrospective analysis of the SEER registry. Several important
factors, including HPV status, performance status, concurrent
administration of chemotherapy and details of radiation delivery,
are not available for analysis. Accurate attribution of cause of
mortality is always a potential source of error. Within the
limitations of diagnosis codes, it is not always possible to
accurately classify mortality from primary or secondary cancer. For
this analysis, we classified mortality from oral cavity or
oropharyngeal cancer as primary disease. This is necessary since in
the SEER site recode schema, base of tongue cancer is subclassified
under oral cavity rather than oropharyngeal cancer. Despite these
limitations, these data provide useful insight into the competing
risks of mortality of patients with oropharyngeal cancer and may
help to assist with future clinical trial design for this patient
population.
In conclusion, although the prognosis of patients
with oropharyngeal cancer treated with radiotherapy has improved in
recent years, the 5-year overall survival remains below 50%.
Earlier diagnosis and more effective cancer therapy is required to
further improve the 5-year survival, while reducing mortality from
other causes may impact the 10-year survival.
References
|
1.
|
Bonner JA, Harari PM, Giralt J, et al:
Radiotherapy plus cetuximab for squamous-cell carcinoma of the head
and neck. N Engl J Med. 354:567–578. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Fu KK, Pajak TF, Trotti A, et al: A
Radiation Therapy Oncology Group (RTOG) phase III randomized study
to compare hyper-fractionation and two variants of accelerated
fractionation to standard fractionation radiotherapy for head and
neck squamous cell carcinomas: first report of RTOG 9003. Int J
Radiat Oncol Biol Phys. 48:7–16. 2000. View Article : Google Scholar
|
|
3.
|
Posner MR, Hershock DM, Blajman CR, et al:
Cisplatin and fluorouracil alone or with docetaxel in head and neck
cancer. N Engl J Med. 357:1705–1715. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Shiboski CH, Schmidt BL and Jordan RC:
Tongue and tonsil carcinoma: increasing trends in the U.S.
population ages 20–44 years. Cancer. 103:1843–1849. 2005.PubMed/NCBI
|
|
5.
|
Bourhis J, Overgaard J, Audry H, et al:
Hyperfractionated or accelerated radiotherapy in head and neck
cancer: a meta-analysis. Lancet. 368:843–854. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Denis F, Garaud P, Bardet E, et al: Final
results of the 94-01 French Head and Neck Oncology and Radiotherapy
Group randomized trial comparing radiotherapy alone with
concomitant radiochemotherapy in advanced-stage oropharynx
carcinoma. J Clin Oncol. 22:69–76. 2004. View Article : Google Scholar
|
|
7.
|
Garden AS, Harris J, Vokes EE, et al:
Preliminary results of Radiation Therapy Oncology Group 97-03: a
randomized phase II trial of concurrent radiation and chemotherapy
for advanced squamous cell carcinomas of the head and neck. J Clin
Oncol. 22:2856–2864. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Kao J, Lavaf A, Teng MS, Huang D and
Genden EM: Adjuvant radiotherapy and survival for patients with
node-positive head and neck cancer: an analysis by primary site and
nodal stage. Int J Radiat Oncol Biol Phys. 71:362–370. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Lee NY, de Arruda FF, Puri DR, et al: A
comparison of intensity-modulated radiation therapy and concomitant
boost radiotherapy in the setting of concurrent chemotherapy for
locally advanced oropharyngeal carcinoma. Int J Radiat Oncol Biol
Phys. 66:966–974. 2006. View Article : Google Scholar
|
|
10.
|
Mell LK, Dignam JJ, Salama JK, et al:
Predictors of competing mortality in advanced head and neck cancer.
J Clin Oncol. 28:15–20. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Argiris A, Brockstein BE, Haraf DJ, et al:
Competing causes of death and second primary tumors in patients
with locoregionally advanced head and neck cancer treated with
chemoradiotherapy. Clin Cancer Res. 10:1956–1962. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Vu HL, Sikora AG, Fu S and Kao J:
HPV-induced oropharyngeal cancer, immune response and response to
therapy. Cancer Lett. 288:149–155. 2009.PubMed/NCBI
|
|
13.
|
Gillison ML: Human papillomavirus and
prognosis of oropharyngeal squamous cell carcinoma: implications
for clinical research in head and neck cancers. J Clin Oncol.
24:5623–5625. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Licitra L, Perrone F, Bossi P, et al:
High-risk human papillomavirus affects prognosis in patients with
surgically treated oropharyngeal squamous cell carcinoma. J Clin
Oncol. 24:5630–5636. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
D’Souza G, Kreimer AR, Viscidi R, et al:
Case-control study of human papillomavirus and oropharyngeal
cancer. N Engl J Med. 356:1944–1956. 2007.PubMed/NCBI
|
|
16.
|
Lavaf A, Genden EM, Cesaretti JA, Packer S
and Kao J: Adjuvant radiotherapy improves overall survival for
patients with lymph node-positive head and neck squamous cell
carcinoma. Cancer. 112:535–543. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Coviello V and Boffess M: Cumulative
incidence estimation in the presence of competing risks. Stata J.
4:103–112. 2004.
|
|
18.
|
Kim HT: Cumulative incidence in competing
risks data and competing risks regression analysis. Clin Cancer
Res. 13:559–565. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Haddad RI and Shin DM: Recent advances in
head and neck cancer. N Engl J Med. 359:1143–1154. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Settle K, Posner MR, Schumaker LM, et al:
Racial survival disparity in head and neck cancer results from low
prevalence of human papillomavirus infection in black oropharyngeal
cancer patients. Cancer Prev Res (Phila Pa). 2:776–781. 2009.
View Article : Google Scholar
|
|
21.
|
Genden EM, Rinaldo A, Shaha AR, et al:
Treatment considerations for head and neck cancer in the elderly. J
Laryngol Otol. 119:169–174. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Pignon JP, le Maitre A, Maillard E and
Bourhis J: Meta-analysis of chemotherapy in head and neck cancer
(MACH-NC): an update on 93 randomised trials and 17,346 patients.
Radiother Oncol. 92:4–14. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Kao J, Sikora AT and Fu S: Dual EGFR and
COX-2 inhibition as a novel approach to targeting head and neck
squamous cell carcinoma. Curr Cancer Drug Targets. 9:931–937. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Greene FL; American Joint Committee on
Cancer. and American Cancer Society: AJCC cancer staging manual.
Springer; New York: 2002, View Article : Google Scholar
|
|
25.
|
Pfister DG, Su YB, Kraus DH, et al:
Concurrent cetuximab, cisplatin, and concomitant boost radiotherapy
for locoregionally advanced, squamous cell head and neck cancer: a
pilot phase II study of a new combined-modality paradigm. J Clin
Oncol. 24:1072–1078. 2006. View Article : Google Scholar
|
|
26.
|
Salama JK, Stenson KM, Kistner EO, et al:
Induction chemotherapy and concurrent chemoradiotherapy for
locoregionally advanced head and neck cancer: a multi-institutional
phase II trial investigating three radiotherapy dose levels. Ann
Oncol. 19:1787–1794. 2008. View Article : Google Scholar
|
|
27.
|
Duffy SA, Ronis DL, McLean S, et al:
Pretreatment health behaviors predict survival among patients with
head and neck squamous cell carcinoma. J Clin Oncol. 27:1969–1975.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Khuri FR, Lee JJ, Lippman SM, et al:
Randomized phase III trial of low-dose isotretinoin for prevention
of second primary tumors in stage I and II head and neck cancer
patients. J Natl Cancer Inst. 98:441–450. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Pearson TA, Blair SN, Daniels SR, et al:
AHA guidelines for primary prevention of cardiovascular disease and
stroke: 2002 update: consensus panel guide to comprehensive risk
reduction for adult patients without coronary or other
atherosclerotic vascular diseases. American Heart Association
Science Advisory and Coordinating Committee. Circulation.
106:388–391. 2002.
|