Introduction
Vascular calcification is highly prevalent and is a
major contributor to cardiovascular disease (CVD) in patients with
chronic kidney disease (CKD). Susceptibility to vascular
calcification is in part genetically determined and actively
regulated by diverse inducers and inhibitors. One of these
inducers, hyperphosphatemia, promotes vascular calcification, and
the control of arterial calcification is now recognized as a means
to prevent CVD events in patients with CKD (1,2).
Vascular calcification is an active, cell-mediated
process that results from an imbalance between the promoters and
inhibitors of mineralization (3,4).
Several molecules that normally regulate osteoblast differentiation
and bone formation have been found in calcifying vessels, such as
osteonectin, osteocalcin, matrix Gla protein and bone morphogenetic
protein 2 (BMP-2) (5–7). Elevated phosphate (Pi) levels also
induce smooth muscle cell (SMC) calcification and osteogenic
phenotypic modulation (8,9).
Bisphosphonates (BPs) are widely used in the
treatment of diseases associated with excessive osteoclast-mediated
bone resorption, such as osteoporosis (10,11).
The classical pharmacological effects of BPs appear to result from
two key properties: their affinity for bone mineral and their
inhibitory effects on osteoclasts. Mineral binding affinities
differ among the clinically used BPs, and this may influence their
differential distribution within bone, their biological potency and
their duration of action (12,13).
It is reported that ibandronate prevents experimentally induced
arterial calcification in uremic rats (14). These findings extend the link
between bone remodeling and vascular calcification of CKD, opening
perspectives toward novel therapeutic strategies. However, whether
zoledronate, a new third generation bisphosphonate, may serve as an
inhibitor of calcification and by what mechanism it may function is
not known. Thus, we designed and completed the present in
vitro study.
Materials and methods
Cell culture and identification
Rat vascular smooth muscle cells (VSMCs) were grown
in Dulbecco’s minimum essential medium (DMEM; Gibco, Carlsbad, CA,
USA). The type and purity of VSMCs were further confirmed using an
α-smooth muscle actin antibody (Sigma-Aldrich, St. Louis, MO, USA),
which indicated >95% positive staining for these cells. VSMCs
(4th to 8th passages) were made quiescent by serum starvation in
0.4% FBS for 24 h for use in all of the experiments in this
study.
Cellular calcification assay
Calcification of VSMCs was induced by 3 mM Pi (but
not by DMEM alone), 1.4 Pi or BMP-2 in our pilot study. Therefore,
VSMC calcification was induced by incubation with calcifying medium
(growth medium supplemented with
NaH2PO4/Na2HPO4 to 3 mM
Pi), and 1.4 mM Pi served as the control. Human recombinant BMP-2
(R&D Systems, Minneapolis, MN, USA) and/or zoledronate
(Novartis Pharmacy AG, Basel, Switzerland) were added every 2 days
during the treatment period. Calcium deposited in the extracellular
matrix was extracted with 0.6 N HCl for 24 h. The calcium content
of the HCl supernatants was determined using the o-cresolphthalein
complex one method (Calcium Assay kit; Bioassays, Hayward, CA, USA)
and normalized relative to the protein concentration of the same
culture well.
Western blot analysis
Protein expression of core binding factor α-1
(Cbfa-1) and osteopontin (OPN) in VSMCs was determined by western
blotting. The specific signal was detected using an enhanced
chemiluminescence system (Cell Signaling Technology, Beverly, MA,
USA).
Real-time PCR
Levels of rat Pit-1 and Pit-2 mRNAs were determined
by quantitative real-time PCR performed using a SYBR GreenER
two-step qRT-PCR kit (Invitrogen, Carlsbad, CA, USA) and an ABI
Prism 7000 sequence detection system (Applied Biosystems, Foster
City, CA, USA). The comparative CT method was used for
quantification, as recommended by the manufacturer, using GAPDH as
the endogenous reference. The primers used for PCR amplification
were: i) Rat Pit-1 forward primer: 5′-CCGTCAGCAACCAGATCAACTC-3′ and
reverse primer: 5′-CCCATGCAGTCTCCCACCTTG-3′, generating an
amplified fragment of 121 bp (NM_031148); ii) Rat Pit-2 forward
primer: 5′-CTATTCCAAGAAGAGGCTCCG-3′ and reverse primer:
5′-TCAGGATCGGTCAGCTCAG-3′, generating an amplified fragment of 126
bp (NM_017223); iii) Rat GAPDH forward primer: 5′-ATGACTCTACCCACG
GCAAG-3′ and reverse primer: 5′-TACTCAGCACCAGC ATCACC-3′,
generating an amplified fragment of 136 bp (NM_017008).
Pi uptake assay
VSMCs were seeded into 24-well plates at
105 cells/well. Transport was initiated by addition of
0.3 ml of the above-mentioned medium containing the labeled
substrate H332PO4 to confluent
VSMCs. The uptake was stopped by washing the cell monolayers three
times with 1 ml of ice-cold Earle’s buffered salt solution (EBSS).
The cells were solubilized with 0.5 ml of 0.1 N NaOH/0.1% SDS, and
the radioactivity of 100-μl aliquots was counted by standard
liquid scintillation techniques (Packard 2500 TR/AB; Packard
Instruments, Meriden, CT, USA). Sodium-dependent Pi uptake was
determined by subtracting the uptake in the presence of EBSS
containing chorine from the uptake in the presence of EBSS
containing sodium. Uptake values were normalized based on the
protein content of the cell culture.
Statistical analysis
Data analyses were conducted using SPSS 13.0
software (SPSS Inc., Chicago, IL, USA). Differences among groups
were determined by analysis of variance (ANOVA), and the Tukey’s
test method was used for post-hoc testing. p<0.05 denoted
statistical significance.
Results
Zoledronate inhibits Pi- and
Pi/BMP-2-induced VSMC calcification
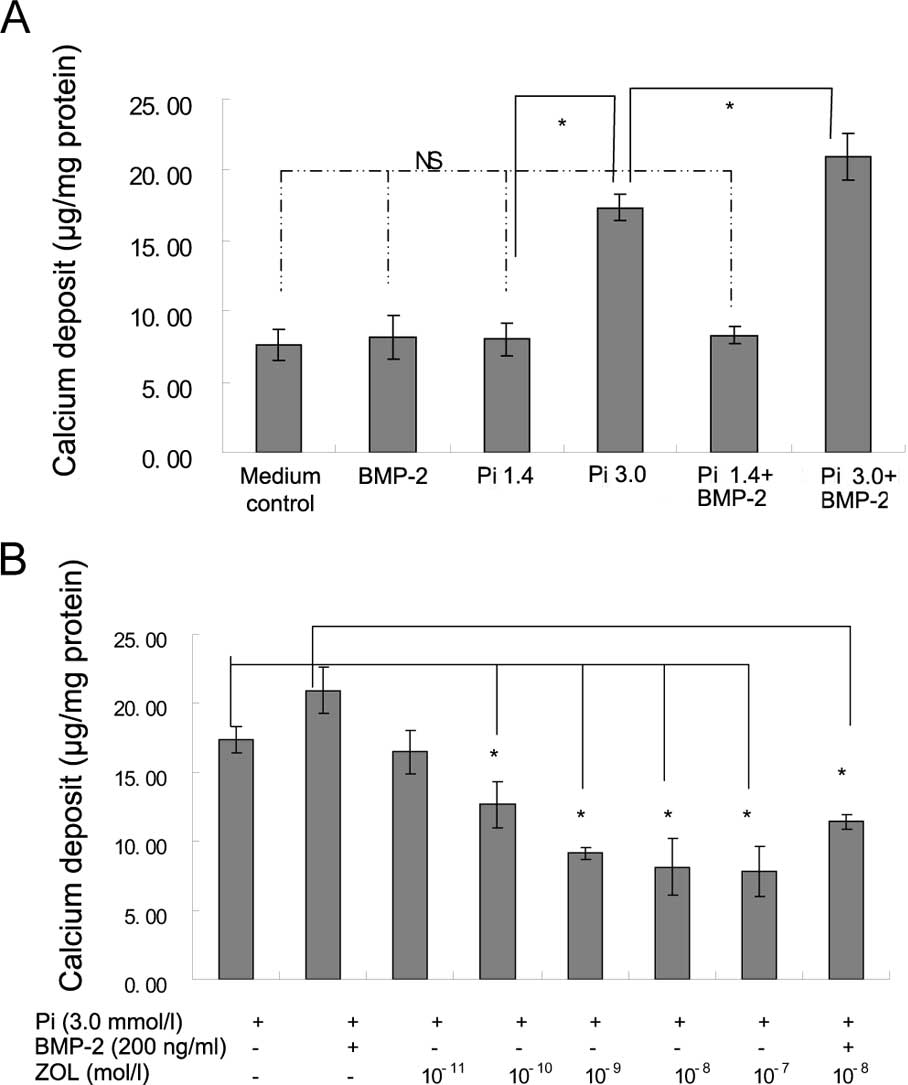
Elevated Pi (3 mM Pi) significantly induced
calcification of VSMCs in comparison to 1.4 mM Pi (p<0.05). The
calcium deposition became more severe after treatment with both
BMP-2 and 3 mM Pi compared to 3 mM Pi alone (p<0.05; Fig. 1A). Zoledronate significantly
inhibited the calcium deposition of VSMCs treated with 3 mM Pi in a
dose-dependent manner (Fig. 1B).
Zoledronate (10−8 mM) also significantly decreased the
calcium deposition of VSMCs induced by the addition of both 3 mM Pi
and 200 ng/ml BMP-2 (Fig. 1B).
Zoledronate inhibits the expression of
Cbfa-1 and OPN upregulated by BMP-2 and elevated Pi
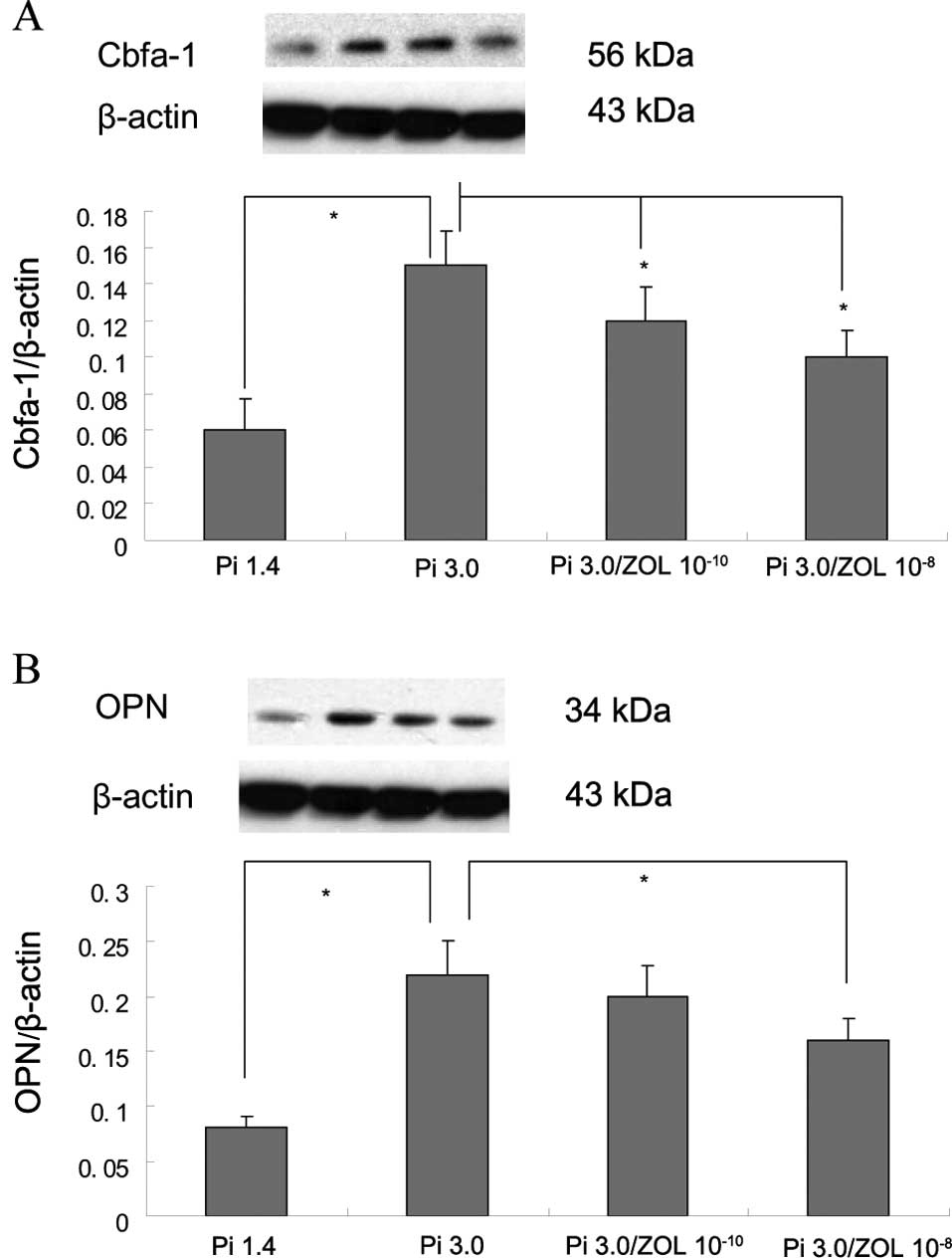
Results demonstrated that 3 mM Pi significantly
upregulated the expression of Cbfa-1 and OPN compared to that of
the control (1.4 Pi; Fig. 2A and
B). Moreover, the expression of Cbfa-1 and OPN was further
increased after treatment with both BMP-2 and 3 mM Pi compared to 3
mM Pi alone (Fig. 2C and D).
Zoledronate significantly suppressed the expression of Cbfa-1 and
OPN upregulated by either 3 mM Pi (Fig. 2A and B) or both 3 mM Pi and BMP-2
(Fig. 2C and D).
Zoledronate suppresses the expression of
Pit-1 mRNA, but not the expression of Pit-2
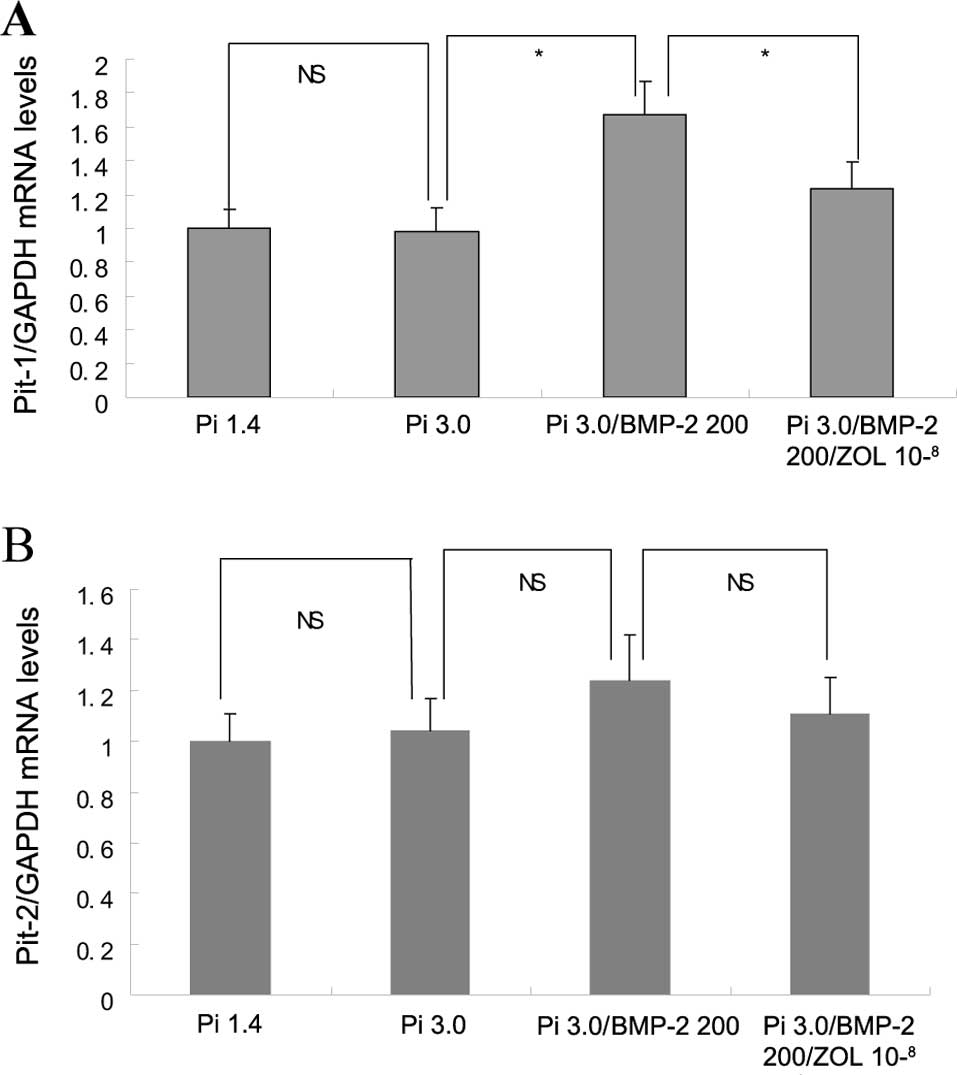
The expression of Pit-1 mRNA in VSMCs increased
significantly after treatment with both BMP-2 and 3 mM Pi compared
to 3 mM Pi alone (p<0.05), and this overexpression of the Pit-1
mRNA was inhibited by the addition of zoledronate (p<0.05;
Fig. 3A). However, the mRNA
expression of Pit-2 was not significantly different among the
elevated Pi, Pi/BMP-2 and zoledronate groups (Fig. 3B).
Zoledronate inhibits Pi uptake of
VSMCs
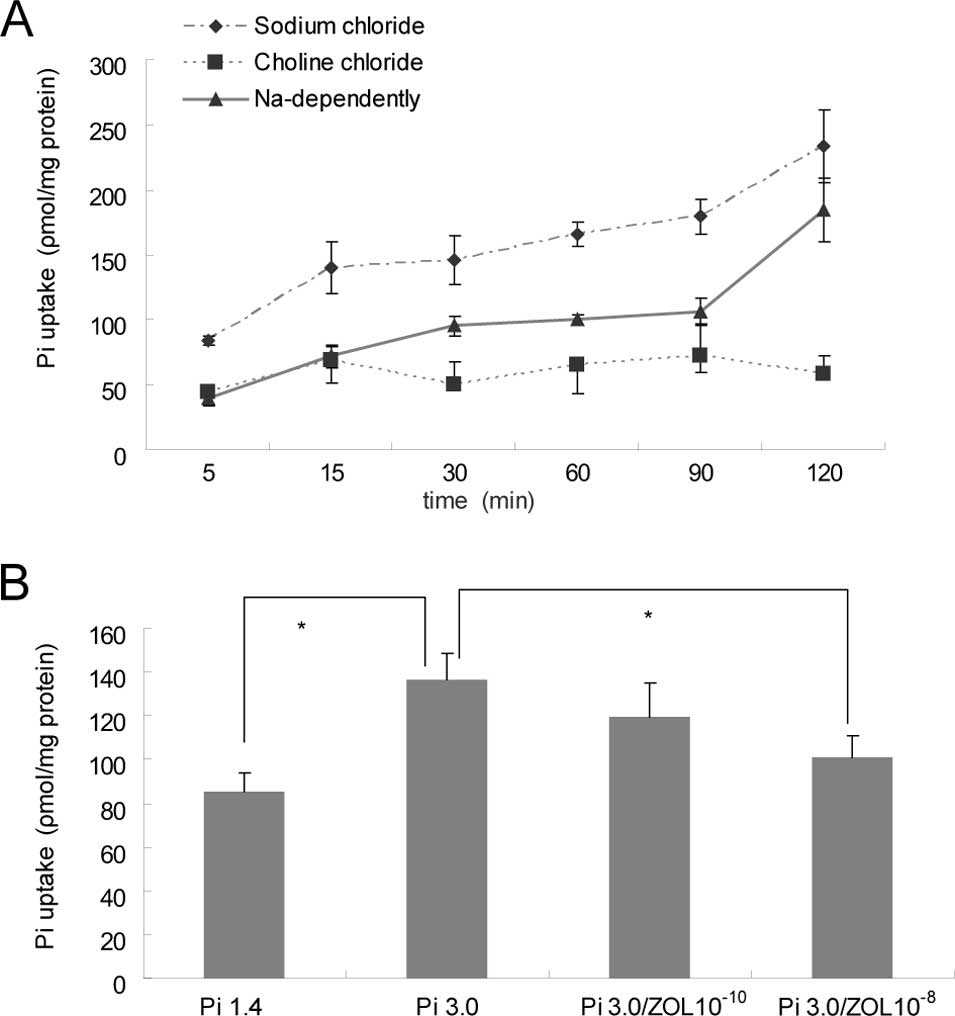
The uptake of Pi by rat VSMCs increased gradually
after treatment with elevated Pi in a time-dependent manner (5–120
min; Fig. 4A). The Pi uptake by
VSMCs was significantly increased in the 3 mM Pi group compared to
that of the 1.4 mM Pi group, and this was significantly inhibited
by the addition of zoledronate (p<0.05; Fig. 4B).
Discussion
In the present study, we demonstrated that an
elevated level of Pi induced calcification of rat VSMCs;
furthermore, we confirmed that this calcification was enhanced by
the addition of BMP-2. This is the first report showing that BMP-2
is involved in the process of calcification induced by elevated Pi
levels. BMPs are part of the TGF-β superfamily, and BMP-2 is
associated with calcific arteriopathy (8). Expression of BMP-2 is also found in
calcified human atherosclerotic lesions (7,8). In
addition, treatment of calcifying vascular or SMCs in vitro
with BMP-2 results in enhanced calcification (15,16).
Thus, BMP-2 may play an important role in the regulation of bone
formation as well as vascular calcification under conditions of
high Pi.
Our results demonstrated that expression of Cbfa-1
and OPN in VSMCs was upregulated after stimulation with elevated Pi
and BMP-2, and this was consistent with the observed calcification,
as the expression of Cbfa-1 and OPN in SMCs usually serve as
markers of osteochondrogenic phenotype transition (16,17).
Thus, elevated Pi and BMP-2 may induce SMCs to transition to an
osteoblast-like phenotype, and this may contribute to cell
calcification. On the other hand, our data showed that addition of
BMP-2 upregulated Pit-1 expression under conditions of elevated Pi,
indicating that BMP-2 may promote vascular calcification via
increased Pi uptake. As such, zoledronate likely inhibited
calcification by means of either inhibition of Pit-1 expression
and/or decreased Pi uptake. However, neither zoledronate nor
inorganic Pi influenced expression of the Pit-2 mRNA in our
experiments. VSMCs appear to respond to elevated Pi levels by
undergoing an osteochondrogenic phenotype change and by
mineralizing their extracellular matrix through a mechanism
requiring sodium-dependent Pi cotransporters (18,19).
It is interesting that zoledronate was found to
effectively inhibit the calcification of VSMCs induced by elevated
Pi and BPM-2. Zoledronate also inhibited expression of Cbfa-1 and
OPN induced by elevated Pi and BMP-2, and this was consistent with
its suppression of calcification. Cbfa-1 and OPN have previously
been described as markers of VSMC transition to osteoblast-like
cells. BPs have been widely used in the treatment of excessive bone
resorption, hypercalcemia and osteoporosis (12,13).
Etidronate has been reported to decrease the intima-media
thickening of carotid arteries (20). Therefore, the above-mentioned data
indicate that zoledronate suppressed calcification induced by
elevated Pi and BMP-2, and the mechanism was likely due to
inhibition of Cbfa-1 and OPN expression in VSMCs. At the same time,
zoledronate also inhibited cell calcification, and this was
probably via the suppression of Pit-1 upregulation and subsequent
decreased Pi transport into cells. However, further studies are
required to confirm these findings.
Abbreviations:
|
ANOVA
|
analysis of variance;
|
|
BPs
|
bisphosphonates;
|
|
BMP-2
|
bone morphogenetic protein 2;
|
|
CVD
|
cardiovascular disease;
|
|
CKD
|
chronic kidney disease;
|
|
Cbfa-1
|
core binding factor α-1;
|
|
DMEM
|
Dulbecco’s minimum essential
medium;
|
|
EBSS
|
Earle’s buffered salt solution;
|
|
OPN
|
osteopontin;
|
|
Pit
|
parathyroid pituitary-specific
transcription factor;
|
|
Pi
|
phosphate;
|
|
SMCs
|
smooth muscle cells;
|
|
VSMCs
|
vascular smooth muscle cells
|
Acknowledgements
The authors thank Xianwu Li, MD, PhD,
of the Department of Bioengineering, University of Washington, for
the technical assistance in this study and Associate Professor
Zhibin Li of the Department of Epidemiology and Statistics at the
First Affiliated Hospital, Sun Yat-sen University, for helping with
the statistics. This study was supported by the Guangdong Natural
Scientific Fund, China (2007B060401040).
References
|
1.
|
Goodman WG, London G, Amann K, Block GA,
Giachelli C, Hruska KA, Ketteler M, Levin A, Massy Z, McCarron DA,
et al: Vascular Calcification Work Group: vascular calcification in
chronic kidney disease. Am J Kidney Dis. 43:572–579. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Jono S, Shioi A, Ikari Y and Nishizawa Y:
Vascular calcification in chronic kidney disease. J Bone Miner
Metab. 24:176–181. 2006. View Article : Google Scholar
|
|
3.
|
Farzaneh-Far A and Shanahan CM: Biology of
vascular calcification in renal disease. Nephron Exp Nephrol.
101:134–138. 2005. View Article : Google Scholar
|
|
4.
|
Giachelli CM: Mechanisms of vascular
calcification in uremia. Semin Nephrol. 24:401–402. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Yao Y, Shahbazian A and Bostrom KI:
Proline and gamma-carboxylated glutamate residues in matrix Gla
protein are critical for binding of bone morphogenetic protein-4.
Circ Res. 102:1065–1074. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Shroff RC and Shanahan CM: The vascular
biology of calcification. Semin Dial. 20:103–109. 2007.PubMed/NCBI
|
|
7.
|
Li X, Yang HY and Giachelli CM: BMP-2
promotes phosphate uptake, phenotypic modulation, and calcification
of human vascular smooth muscle cells. Atherosclerosis.
199:271–277. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Hruska KA, Mathew S and Saab G: Bone
morphogenetic proteins in vascular calcification. Circ Res.
97:105–114. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Giachelli CM: The emerging role of
phosphate in vascular calcification. Kidney Int. 75:890–897. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Coxon FP, Thompson K and Rogers MJ: Recent
advances in understanding the mechanism of action of
bisphosphonates. Curr Opin Pharmacol. 6:307–312. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Mathew S, Tustison KS, Sugatani T,
Chaudhary LR, Rifas L and Hruska KA: The mechanism of phosphorus as
a cardiovascular risk factor in CKD. J Am Soc Nephrol.
19:1092–1095. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Russell RG, Xia Z, Dunford JE, Oppermann
U, Kwaasi A, Hulley PA, Kavanagh KL, Triffitt JT, Lundy MW, Phipps
RJ, et al: Bisphosphonates: an update on mechanisms of action and
how these relate to clinical efficacy. Ann NY Acad Sci.
1117:209–217. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Persy V, De Broe M and Ketteler M:
Bisphosphonates prevent experimental vascular calcification: treat
the bone to cure the vessels? Kidney Int. 70:1537–1538. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Price PA, Roublick AM and Williamson MK:
Artery calcification in uremic rats is increased by a low protein
diet and prevented by treatment with ibandronate. Kidney Int.
70:1577–1583. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Zebboudj AF, Shin V and Bostrom K: Matrix
GLA protein and BMP-2 regulate osteoinduction in calcifying
vascular cells. J Cell Biochem. 90:756–765. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Trion A and van der Laarse A: Vascular
smooth muscle cells and calcification in atherosclerosis. Am Heart
J. 147:808–814. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Steitz SA, Speer MY, Curinga G, Yang HY,
Haynes P and Aebersold R, Schinke T, Karsenty G, Giachelli CM and
Aebersold R: Smooth muscle cell phenotypic transition associated
with calcification: upregulation of Cbfa1 and downregulation of
smooth muscle lineage markers. Circ Res. 89:1147–1154. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Li X and Giachelli CM: Sodium-dependent
phosphate cotransporters and vascular calcification. Curr Opin
Nephrol Hypertens. 16:325–328. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Villa-Bellosta R, Bogaert YE, Levi M and
Sorribas V: Characterization of phosphate transport in rat vascular
smooth muscle cells: implications for vascular calcification.
Arterioscler Thromb Vasc Biol. 27:1030–1036. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Koshiyama H: Antiatherogenic actions of
etidronate on atherosclerosis. Clin Calcium. 12:378–382.
2002.PubMed/NCBI
|