Introduction
Gastric adenocarcinoma is a common gastrointestinal
malignant tumor, accounting for 23.2% of cancer-related deaths in
China (approximately 16 million individuals) (1,2). At
present, the clinical diagnosis of gastric adenocarcinoma mainly
relies on physical and histological examinations, which are
accurate only for middle- or late-stage cases. Therefore, many
patients are diagnosed at a late stage of the disease. Biochemical
markers such as carcinoembryonic antigen, carbohydrate antigen 19-9
and carbohydrate antigen 125 are used as markers for diagnosis;
however they are non-specific and lack adequate sensitivity
(3–6). The present study aimed to design a
new diagnostic system with high sensitivity and specificity for
early-stage gastric cancer detection. We employed a newly emerging
technique, surface-enhanced laser desorption ionization
time-of-flight mass spectrometry (SELDI-TOF-MS) (7–12),
to analyze the serum proteome from healthy volunteers, gastric
cancer patients and gastritis patients, and to screen for specific
protein biomarkers for gastric cancer (13–15).
Materials and methods
Clinical data
One hundred and nine gastric cancer patients (males
41, females 68, age range 32–89 years) (25 cases Dukes’ A, 22 cases
Dukes’ B, 28 cases Dukes’ C and 34 cases of Dukes’ D), and 106
cases of controls (males 50, females 56, age range 26–85 years) (60
healthy volunteers, 16 cases of chronic atrophic gastritis
patients, and 30 cases of chronic superficial gastritis) were
recruited for this study at the First Affiliated Hospital of
Zhejiang University and Zhejiang Taizhou Municipal Hospital. The
subjects were assigned into an experimental group and a
verification group according to Table
I. The peripheral blood samples were collected in the morning
after overnight fasting. The blood samples were then maintained at
4°C for 1–2 h prior to centrifugation at 3,000 rpm at 4°C for 10
min to separate out the serum. The serum samples were frozen at
−80°C in a freezer for storage. All protocols and experiments were
approved by the Taizhou Medical College Ethics Committee for
clinical experiments and use of human samples; written informed
consent was obtained from all subjects participating in this study.
The study complied with the World Medical Association Declaration
of Helsinki regarding ethical conduct of research involving human
subjects.
 | Table I.Clinical subjects involved in the
study. |
Table I.
Clinical subjects involved in the
study.
| Groups | Experimental | Verification | Total |
|---|
| Gastric
adenocarcinoma | | | |
| Dukes’ A | 15 | 10 | 25 |
| Dukes’ B | 12 | 10 | 22 |
| Dukes’ C | 20 | 8 | 28 |
| Dukes’ D | 18 | 16 | 34 |
| Control | | | |
| Chronic superficial
gastritis | 15 | 15 | 30 |
| Chronic atrophic
gastritis | 8 | 8 | 16 |
| Healthy
controls | 30 | 30 | 60 |
| Total | 118 | 97 | 215 |
Protein chip analysis
After thawing and 10 min of centrifugation (10,000
rpm), a 20-μl serum sample without fraction treatment (which does
not affect the protein mining efficiency as shown by the authors)
was added to 30 μl 0.5% U9 (9 mol/l urea, 2% CHAPS
(3[(3-cholamidopropyl)dimethylammonio]-l-propanesulfonate), 1% DTT
(DL-dithiothreitol)) in a 96-well plate and incubated for 20 min at
4°C with 600 rpm vigorous agitation. The ProteinChip array cassette
was put into a 96-well bioprocessor and 100 μl U1 buffer (50 mmol/l
Tris-HCL diluted 10% U9 buffer) was added into each well, and
incubated for 10 min at 4°C with 600 rpm vigorous agitation. Q10
buffer (200 μl) (100 mM Tris-HCl buffer pH 9.0) was then added and
a 5-min incubation was carried out 2 times with agitation. All
experimental reagents were obtained from Shanghai Shenggong
Company, Shanghai, China.
Fifty microliters of the protein-denatured serum
samples were removed to a new tube, and 200 μl Q10 buffer was added
to dilute the samples before being applied onto the Q10 chip
Bioprocessor (Ciphergen) for 60 min. Then each plate of the Q10
chip was added together with 200 μl Q10 buffer, incubation was
carried out for 5 min two times with agitation, and finally 20 mm/l
HEPES (pH 7.4) buffer was added for washing before drying. SPA (0.5
μl) was added 2 times into each plate with drying between each
addition. Mass spectrometry was set as laser intensity 185,
sensitivity 8, 2,000–20,000 m/z. Inter-chip CV was <10%.
All-in-one control chip was used to adjust the system with a
systemic error <0.1%. All of the data were processed with
ProteinChip 3.0 software, then the Biomarker Wizard software 3.1
and Biomarker Wizard software 4.0.1. P<0.01 was determined to
indicate statistically significant differences in the comparison
between two protein peaks.
Purification and identification of
specific protein peaks
Serum samples (100 μl) with 300 μl water and 700 μl
acetonitrile were mixed and maintained at −20°C in a freezer for 30
min prior to centrifugation at 3000 rpm for 10 min. The supernatant
was freeze-dried for 20 min before collection for HPLC. The
purified solutions were collected at different time periods,
freeze-dried to obtain a 20-μl volume solution. Solution (0.5 μl)
was mixed with 1.5 μl matrix solution (10 mg/ ml CHCA) to be placed
on chip points, with crystallization for MALDI-TOF MS detection.
The conditions included pulsed nitrogen laser (337 nm),
accelerating voltage 20 KV, linear analysis mode, mass range
3,000–20,000 m/z. The samples corresponding to the specific protein
peaks in SELDI-TOF MS were subsequently identified.
LC-MS/MS analysis
Purified target protein of 20 μl was mixed with 60
μl 8M urea (final concentration of urea 6 M) and was agitated at
room temperature for 20 min. Then 0.8 μl 1 M DTT (final
concentration 10 mM) was added and mixed at room temperature for 1
h, and 3.2 μl 1 M iodine acetyl amine (final concentration 40 mM)
was added and maintained for 45 min in the dark. DTT (3.2 μl 1 M)
(final concentration 40 mM) was added for 20 min, and then 400 μl
50 mM NH4HCO3 was added to dilute the
solution, with urea concentration at 1 M and pH 8.0. Subsequently,
0.1 μg protease in a 37°C water bath for 1 h, and formic acid was
adjusted to a pH <3 to terminate the reaction. The hydrolysates
were subjected to LC-MS/MS analysis. Sample solutions were put in a
self-made C18 capillary column for liquid chromatography: inner
diameter 100 μm, filled part 100 mm, filled particles with diameter
5 μm. The flow phase A was water and 0.1% formic acid; the flow
phase B was acetonitrile and 0.1% formic acid. The washout followed
the sequences below: 100% A (0 min) - 100% A (5 min) - 5% B (5.1
min) - 65% B (60 min) - 100% B (75 min) - 100% B (85 min). The flow
speed was 200–800 nl/min. The data-dependent mode was used; the
scanning ranges were from 400 to 2,000 m/z; the five strongest
signal peaks of each full scan were selected for secondary MS (MS2)
analysis.
The data retrieval used the XCalibur program
components BioWorks 3.2 (Thermo Finnigan) and the peptide sequences
were searched for in the NCBI human protein database (human.ref)
according to the mass spectrum. The following parameters were used:
enzyme split site at random site; fixed modification at cysteine
amine formylation modification; variable modification at methionine
oxidation; retrieval parameters ΔCN >0.1; Sp >500; Rsp ≥5;
Xcorr vs. Charge: Xcorr (+1) >1.9, Xcorr (+2)>2.5, Xcorr (+3)
>3.75.
Results
The serum protein fingerprint
spectrum
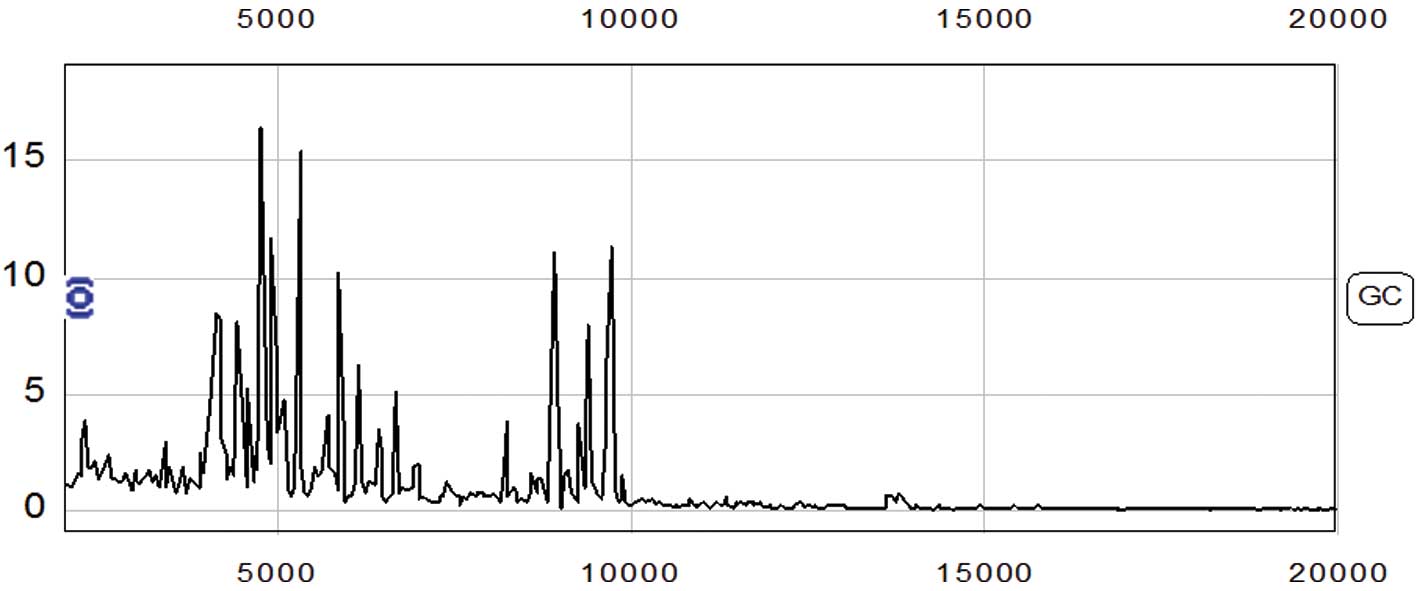
The protein fingerprint spectrum from 65 cases of
gastric adenocarcinoma and 53 cases of control were normalized
first, then analyzed using Biomarker wizard software. Two hundred
and twenty-seven protein peaks were found in the m/z range from
2,000–50,000 (Fig. 1).
Data analysis of the gastric
adenocarcinoma protein fingerprint spectrum and the diagnostic
model
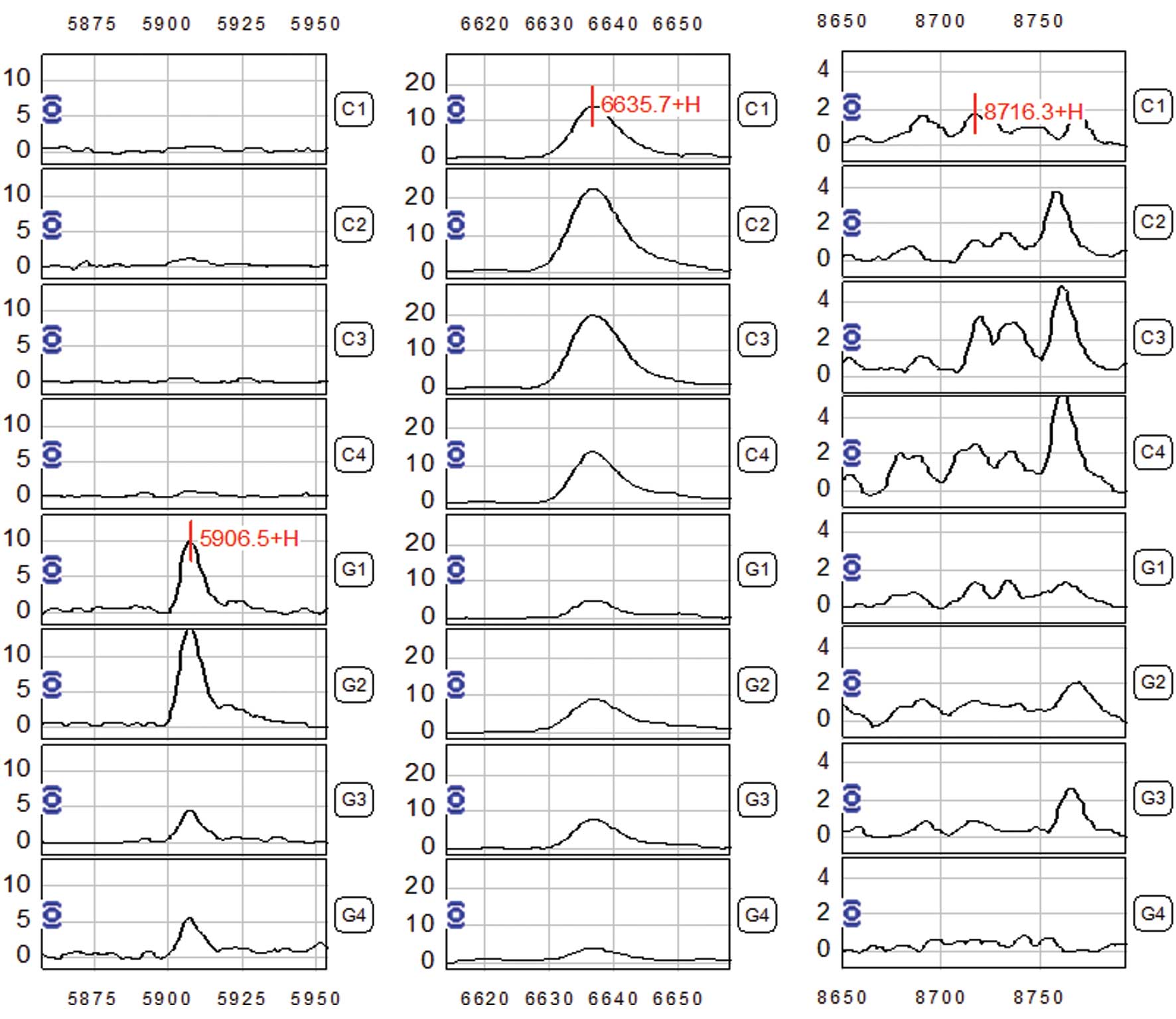
The Biomarker Wizard software analysis revealed
three differentially expressed protein peaks in serum samples from
gastric adenocarcinoma patients, with m/z at 5,906.5, 6,635.7 and
8,716.3 respectively (P<0.01) (Fig.
2 and Table II), which were
considered as potential biomarkers for gastric adenocarcinoma. The
5,906.5 peak showed increased expression in the gastric
adenocarcinoma cases; while the 6,635.7 and 8,716.3 peaks showed a
decreased expression level in the gastric adenocarcinoma serum
samples (Table II). The combined
analysis with the three peaks as the basis for the diagnostic model
showed a sensitivity of 93.85 (61/65) and a specificity of 94.34%
(50/53) in analyzing the mass spectrometry data from the 65 gastric
adenocarcinoma patients and 53 cases of control subjects (Table III).
 | Table II.Intensity of the three differential
peaks in gastric adenocarcinoma and control groups. |
Table II.
Intensity of the three differential
peaks in gastric adenocarcinoma and control groups.
| (m/z) | Gastric
adenocarcinoma | Control | P-values |
|---|
| 5,907.5 | 8.53±4.33 | 0.88±0.31 |
2.8×10−7 |
| 6,636.7 | 6.54±2.44 | 17.56±4.43 |
4.5×10−6 |
| 8,716.3 | 0.93±0.29 | 2.16±0.98 |
8.4×10−4 |
 | Table III.Characteristics of the gastric
adenocarcinoma diagnostic model. |
Table III.
Characteristics of the gastric
adenocarcinoma diagnostic model.
| Set | Groups | Case number | Correct cases | Diagnosis rate
(%) |
|---|
| Experimental | Gastric
adenocarcinoma | 65 | 61 | 93.85 |
| Control | 53 | 50 | 94.34 |
| Verification | Gastric
adenocarcinoma | 44 | 40 | 90.91 |
| Control | 53 | 48 | 90.57 |
Purification of the protein peaks and the
MS analysis
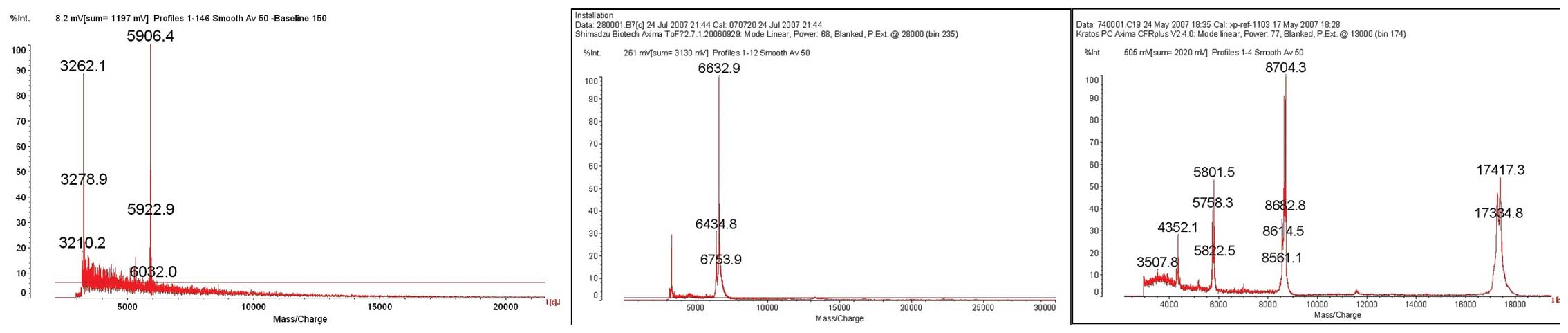
The protein peaks of the three biomarkers (m/z
5,906.5, 6,635.7 and 8,716.3) were isolated and purified with HPLC,
and then collected into PCR tubes for MALDI-TOF-MS examination. The
results showed that the three peaks were proteins with molecular
weights of 5,906.4, 6,632.9 and 8,704.3, respectively (Fig. 3).
Following LC-MS/MS measurement of the digested
proteins and the data screening from NCBI human protein database,
the 5,906.4 peak was found to correspond to the fibrinogen α chain
(100% match), the 6,632.9 peak corresponded to the apolipoprotein
A-II (96% match), and the 8,704.3 peak corresponded to the
lipid-laden protein C-I (60% match) (Table IV).
 | Table IV.Amino acid sequence of the
differential protein peaks in the gastric adenocarcinoma cases. |
Table IV.
Amino acid sequence of the
differential protein peaks in the gastric adenocarcinoma cases.
| (m/z) | Protein | Molecular weight | Amino acid
sequence | Match rate (%) |
|---|
| 5,906.5 | Fibrinogen
α-chain | 5,904.0 | SSSYSKQFTS STSYNRGDST
FESKSYKMAD | 100 |
| | | EAGSEADHEG THSTKRGHAK
SRPV | |
| 6,635.7 | Apolipoprotein
A-II | 6,630.0 | TPDY SSALDKLKEF
GNTLEDKARE | 96 |
| | | LISRIKQSEL SAKMREWFSE
TFQKVKEKLK IDS | |
| 8,716.3 | Apolipoprotein
C-I | 8,707.8 | QAK EPCV ESLVSQYFQT
VTDYGKDLME | 60 |
| | | KVKSPELQAE
AKSYFEKSKE | |
| | | QLTPLIKKAG TELVNFLSYF
VELGTQPATQ | |
Discussion
Gastric cancer is a cancer without clear symptoms at
onset, and metastasis and recurrence are common (16,17).
The prognosis of this disease is poor as well. Early diagnosis of
gastric cancer urgently requires novel techniques with high
sensitivity and specificity. Since the onset and progression of
cancer lead to characteristic changes in the serum proteome, it is
possible to employ proteomic techniques to screen for potential
biomarkers of gastric cancer (11,18,19).
The present study utilized SELDI-TOF MS, and successfully
identified a new diagnostic model for gastric cancer, including
suitability for early-stage patients.
SELDI-TOF MS is an ideal platform for proteomic
studies with several advantages. i) A small amount of sample is
required. The scan is fast and suitable for clinical diagnosis and
high throughput-screening analysis. ii) The technique can identify
the specific spectrum including several biomarkers at the same
time. iii) Crude samples without prior purification can be used.
iv) The technique can be combined with many genomic techniques. v)
The technique is of high reliability and can be reproduced in
repeated tests. vi) The technique is applicable to proteins that
are not suitable for 2D-PAGE analysis, such as those with extremely
small molecular weights, or hydrophobic, transmembrane, as well as
isoelectric point (8–10). The technique currently shows
progressive results in biomarker screening of autoimmune diseases,
inflammation disorders and many types of malignant cancers
(9,10,20–24),
providing the basis for diagnosis and treatment of these diseases
clinically.
The present study described three potential
biomarkers for gastric cancer with high sensitivity and specificity
in both the experimental and verification set, as mentioned in
Results. The three identified markers were fibrinogen α-chain,
apolipoprotein A-II, and lipid-laden proteins C-I. They may play
different roles in the onset and progression of gastric cancer.
Fibrinogen participates in blood coagulation processes, and it may
mediate the interaction of cancer cells and platelet, which occurs
during cancer metastasis (25,26).
Apolipoprotein participates in lipid transport and may be involved
in cell proliferation/apoptosis regulation (27,28).
C-I is mainly synthesized in the liver, and is less well-known in
cancer biology. Several previous studies have shown a lower
expression of C-I in serum from cancer patients (29,30),
which was consistent with the present study. However the detailed
mechanism requires future studies.
Taken together, the present study proved the
efficiency of SELDI-TOF MS in screening for biomarkers of gastric
cancer in a serum proteome-based manner. The three discovered
biomarkers could be effectively used for gastric cancer diagnosis.
Due to the limited number of patients, we did not perform a
correlation analysis between the stage of cancer progression and
the biomarker profiles. It is necessary to recruit more patients
with early-stage disease to identify various biomarkers for the
diagnosis of patients as early as possible.
Acknowledgements
The study was supported by the
Zhejiang Medicine and Health Science and Technology Program grant
2010KYB127, and the Zhejiang Gongyi Applied Technology Research
Program grant 2011C33045.
References
|
1.
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
2.
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar
|
|
3.
|
Tseng CW, Yang JC, Chen CN, et al:
Identification of 14-3-3beta in human gastric cancer cells and its
potency as a diagnostic and prognostic biomarker. Proteomics.
11:2423–2439. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Kobayashi Y, Niwa Y, Tajika M, et al:
Serum tumor antigen REG4 as a useful diagnostic biomarker in
gastric cancer. Hepatogastroenterology. 57:1631–1634.
2010.PubMed/NCBI
|
|
5.
|
Yang S and Chung HC: Novel biomarker
candidates for gastric cancer. Oncol Rep. 19:675–680.
2008.PubMed/NCBI
|
|
6.
|
Chan DC, Chen CJ, Chu HC, et al:
Evaluation of serum amyloid A as a biomarker for gastric cancer.
Ann Surg Oncol. 14:84–93. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Poon TC: Opportunities and limitations of
SELDI-TOF-MS in biomedical research: practical advices. Expert Rev
Proteomics. 4:51–65. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Cho WC: Research progress in SELDI-TOF MS
and its clinical applications. Sheng Wu Gong Cheng Xue Bao.
22:871–876. 2006.(In Chinese).
|
|
9.
|
Clarke CH, Buckley JA and Fung ET:
SELDI-TOF-MS proteomics of breast cancer. Clin Chem Lab Med.
43:1314–1320. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Liu C: The application of SELDI-TOF-MS in
clinical diagnosis of cancers. J Biomed Biotechnol.
2011:2458212011.PubMed/NCBI
|
|
11.
|
Caffrey RE: A review of experimental
design best practices for proteomics based biomarker discovery:
focus on SELDI-TOF. Methods Mol Biol. 641:167–183. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Kristina G, Radomir P, Eva B, et al: When
one chip is not enough: augmenting the validity of SELDI-TOF
proteomic profiles of clinical specimens. Lab Chip. 9:1014–1017.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Liu W, Gao X, Cai Q, et al: Identification
of novel serum biomarkers for gastric cancer by magnetic bead.
Front Biosci (Elite Ed). 2:961–971. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Huang Q, Chen W, Wang L, Lin W, Lin J and
Lin X: Identification of transgelin as a potential novel biomarker
for gastric adenocarcinoma based on proteomics technology. J Cancer
Res Clin Oncol. 134:1219–1227. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Umemura H, Togawa A, Sogawa K, et al:
Identification of a high molecular weight kininogen fragment as a
marker for early gastric cancer by serum proteome analysis. J
Gastroenterol. 46:577–585. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Wang J, Yu JC, Kang WM and Ma ZQ:
Treatment strategy for early gastric cancer. Surg Oncol. Jan
21–2011.(Epub ahead of print).
|
|
17.
|
Saka M, Morita S, Fukagawa T and Katai H:
Present and future status of gastric cancer surgery. Jpn J Clin
Oncol. 41:307–313. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Cho WC: Proteomics technologies and
challenges. Genomics Proteomics Bioinformatics. 5:77–85. 2007.
View Article : Google Scholar
|
|
19.
|
Cho WC: Contribution of oncoproteomics to
cancer biomarker discovery. Mol Cancer. 6:252007. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Lei L, Wang XJ, Zheng ZG, et al:
Identification of serum protein markers for breast cancer relapse
with SELDI-TOF MS. Anat Rec (Hoboken). 294:941–944. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Felix K, Fakelman F, Hartmann D, et al:
Identification of serum proteins involved in pancreatic cancer
cachexia. Life Sci. 88:218–225. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Liu L, Liu J, Wang Y, et al: A combined
biomarker pattern improves the discrimination of lung cancer.
Biomarkers. 16:20–30. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Hogdall E, Fung ET, Christensen IJ, et al:
Proteomic biomarkers for overall and progression-free survival in
ovarian cancer patients. Proteomics Clin Appl. 4:940–952. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Gemoll T, Roblick UJ, Auer G, Jornvall H
and Habermann JK: SELDI-TOF serum proteomics and colorectal cancer:
a current overview. Arch Physiol Biochem. 116:188–196. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Konstantopoulos K and Thomas SN: Cancer
cells in transit: the vascular interactions of tumor cells. Annu
Rev Biomed Eng. 11:177–202. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Costantini V and Zacharski LR: The role of
fibrin in tumor metastasis. Cancer Metastasis Rev. 11:283–290.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Vanhollebeke B and Pays E: The function of
apolipoproteins L. Cell Mol Life Sci. 63:1937–1944. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Ashe PC and Berry MD: Apoptotic signaling
cascades. Prog Neuropsychopharmacol Biol Psychiatry. 27:199–214.
2003. View Article : Google Scholar
|
|
29.
|
Engwegen JY, Helgason HH, Cats A, et al:
Identification of serum proteins discriminating colorectal cancer
patients and healthy controls using surface-enhanced laser
desorption ionisation-time of flight mass spectrometry. World J
Gastroenterol. 12:1536–1544. 2006.
|
|
30.
|
Fan Y, Wang J, Yang Y, et al: Detection
and identification of potential biomarkers of breast cancer. J
Cancer Res Clin Oncol. 136:1243–1254. 2010. View Article : Google Scholar : PubMed/NCBI
|