Introduction
Bone is one of the most preferential metastatic
locations in patients with breast cancer. Although bone metastasis
itself rarely becomes a life-threatening lesion, it often impairs
the quality of a patient’s life due to pathological fracture and/or
severe pain (1). To develop bone
metastasis, cancer cells have to invade the surrounding tissues,
escape from the primary site, survive within the blood stream,
adhere to the target, and migrate out of the vessel into the bone
marrow. Subsequent to going through these common stages of
metastasis, certain special features of the cancer cells in
cooperation with the microenvironment of the bone marrow are
considered to be prerequisite in establishing a metastatic bone
lesion. One of the most important capacities required is the
potential to produce parathyroid hormone-related protein (PTHrP)
(2,3).
PTHrP is a cytokine, which was originally identified
as a causative factor of malignant humoral hypercalcemia (4). The bone matrix contains a variety of
growth factors, such as insulin-like growth factor (IGF),
transforming growth factor β (TGF-β), fibroblast growth factor
(FGF), platelet-derived growth factor (PDGF), and Ca++.
These factors are released by bone resorption. Among these, TGF-β
and Ca++ bind to the TGF-β receptor and calcium-sensing
receptor of the cancer cells, respectively, to stimulate PTHrP
production (5,6). PTHrP then stimulates the osteoblasts
to upregulate the ligand of receptor activator of nuclear factor κ
β (RANKL) expression. RANKL bound with RANK is expressed in
premature osteoclasts, and promotes differentiation into mature
osteoclasts (7). Finally, bone
resorption is activated by mature osteoclasts, completing a
‘vicious cycle’ that accelerates bone resorption, coinciding with
the growth of a metastatic bone lesion stimulated by other growth
factors.
PTHrP is reported to be produced in various
malignant tumors such as breast, prostate and lung cancer. These
are the cancers that frequently result in bone metastasis (2,8).
More than 60% of cancerous lesions of the breast were reported to
secrete PTHrP (9–16). In several studies, the patients who
subsequently develop bone metastasis showed higher or more frequent
PTHrP expression than those without bone metastasis (9,11,14,17–19).
Several previous studies have, thus, suggested that PTHrP
expression contributes to the formation and development of bone
metastasis in breast cancer patients (2,5,18,20,21).
However, controversy still exists over the clinical or prognostic
significance of PTHrP expression in cancer cells in the primary
site. Several studies have reported that PTHrP expression in the
primary lesion correlates with the decreased risk of developing
bone metastasis (13) or the
favorable prognosis in breast cancer patients (9,15,16).
Herein, we investigated the expression of PTHrP in
primary lesions of breast cancer, and attempted to reveal the role
of its expression by analyzing its relationship to
clinicopathological characteristics by enrolling patients at
various stages of primary breast cancer, treated in a university
hospital, whose long-term prognosis was available. We found a
potential role of PTHrP expression in the formation of bone
metastasis according to the status of local disease.
Materials and methods
Clinical materials
One hundred and twenty-five surgical specimens from
patients with primary breast cancer who underwent surgery at the
Department of Surgical Oncology, Osaka City University Hospital,
between 1996 and 1999 were investigated. All the patients were
women. Their ages ranged from 31 to 88 years (mean 57.4) at the
time of surgery. Cases who received either preoperative
chemotherapy or endocrine therapy were excluded from this study.
The patients were followed up for 5 to 243 months. The median
follow-up period was 97 months. The patients were followed up with
ultrasonography for determining local recurrence, computed
tomography for evaluating metastatic lesions in the lung and the
liver, and bone scintigraphy to detect bone metastasis annually, as
well as physical examination every six months. The patient
clinicopathological features are presented in Table I. Postoperative adjuvant therapies,
including chemotherapy, hormonal therapy, and radiation therapy
were conducted according to the status of the disease and the
condition of the patient. No patient had received elective
administration of bisphosphonates. Thirty-five patients were
premenopausal and 90 patients were postmenopausal. Thirty-four
patients had a tumor without skin or chest wall invasion equal to
or less than 2 cm in diameter (T1), 63 patients had a tumor of 2.1
to 5 cm (T2) and 14 patients had a tumor of more than 5 cm (T3).
Fourteen patients had a tumor with skin or chest wall invasion of
any size (T4). Seventy patients had lymph node metastasis and 9 had
distant metastasis at the time of initial surgery. Estrogen
receptor (ER) was expressed in 61 patients. Twenty cases (16.0%)
developed bone metastasis, 13 developed visceral metastasis, 9
developed local recurrence and 25 succumbed to breast cancer during
follow-up.
 | Table I.Relationship between PTHrP expression
and clinicopathological findings. |
Table I.
Relationship between PTHrP expression
and clinicopathological findings.
| | PTHrP expression
| |
|---|
| Factor | No. of patients | Negative, n (%) | Positive, n (%) | P-value |
|---|
| Menopausal
status | | | | |
| Premenopausal | 35 | 7 (20.0) | 28 (80.0) | 0.015 |
| Postmenopausal | 90 | 39 (53.3) | 51 (56.7) | |
| T category | | | | |
| T1 | 34 | 17 (50.0) | 17 (50.0) | n.s. |
| T2 | 63 | 20 (31.7) | 43 (68.3) | |
| T3 | 14 | 5 (35.7) | 9 (64.3) | |
| T4 | 14 | 4 (18.6) | 10 (71.4) | |
| Nodal status | | | | |
| (−) | 55 | 22 (40.0) | 33 (60.0) | n.s. |
| (+) | 70 | 24 (34.3) | 46 (65.7) | |
| Distant
metastasis | | | | |
| M0 | 116 | 46 (39.7) | 70 (60.3) | 0.044 |
| M1 | 9 | 0 (0) | 9 (100) | |
| Estrogen
receptor | | | | |
| (−) | 64 | 22 (34.4) | 42 (65.6) | n.s. |
| (+) | 61 | 24 (39.3) | 37 (60.7) | |
| Lymphatic
infiltrationa | | | | |
| (−) | 59 | 24 (41.7) | 35 (59.3) | n.s. |
| (+) | 61 | 19 (31.1) | 42 (68.9) | |
| Vascular
invasiona | | | | |
| (−) | 116 | 39 (42.7) | 67 (57.3) | n.s. |
| (+) | 14 | 4 (28.6) | 10 (71.4) | |
| Total | 125 | 46 (36.8) | 79 (63.2) | |
Immunohistochemical techniques
To study PTHrP expression in the tissues,
immunohistochemical staining was used (21). Formalin-fixed, paraffin-embedded
tissue blocks were obtained from each patient including normal
mammary glands and cancerous lesions. Sections were dewaxed, and
antigen retrieval was performed by autoclaving in Target Retrieval
Solution (Dako Cytomation, Carpinteria, CA, USA) at 105°C for 15
min, followed by incubation with 0.3% hydrogen peroxide in methanol
for 30 min. Following blocking with 10% normal rabbit serum to
reduce nonspecific antibody binding, mouse monoclonal antibody
against PTHrP (100 μg/ ml) (Oncogene Science Inc., Uniondale, NY,
USA) was reacted with tissue sections overnight at 4°C followed by
three washes with PBS. The sections were incubated with
biotinylated rabbit anti-mouse immunoglobulin G (IgG), then reacted
with streptoavidin-biotin peroxidase reagent (Histofine Kit;
Nichirei Co. Tokyo, Japan). Finally, diaminobenzidine and 1%
hydrogen peroxide were applied as a chromogen, and counterstaining
was carried out with hematoxylin. Normal mouse IgG was substituted
for the primary antibody as the negative control. The sections were
then assessed independently by two investigators without knowledge
of the clinical outcome of the patient. Tumors were classified as
being PTHrP-positive when more than 10% of the cancer cell was
positively stained.
Statistics
Mann-Whitney’s U test was used to define statistical
difference. Survival curves for patients were calculated using the
Kaplan-Meier method and analyzed using the log-rank test. The
multivariate analysis concerning bone metastasis was evaluated by
logistic regression analysis, and multiple factors concerning
overall survival were calculated using the Cox regression test.
Statistical significance was defined as P<0.05. All analyses
were carried out using the analytical software SPSS (SPSS Inc.,
Chicago, IL, USA).
Results
Expression of PTHrP
PTHrP expression was observed in 79 out of 125 cases
(63.2%) and 46 (36.8%) were negative for PTHrP expression in tumor
cells. The relationship between PTHrP expression and
clinicopathological findings is presented in Table I. No relationship was demonstrated
between PTHrP expression and tumor size, lymph node metastasis,
estrogen receptor status, lymphatic infiltration, or vascular
invasion. PTHrP was statistically more frequently expressed in the
tumors of premenopausal women (80%) compared with those of
postmenopausal women (57%). There were three cases that had bone
metastasis at the initial diagnosis. PTHrP expression in the
primary lesion was observed in each of the three cases.
Factors related to bone metastasis
The relationship between initial (at the time of
surgery) and late (during the postoperative follow-up period) bone
metastasis and clinicopathological findings is presented in
Table II. There was no factor that
significantly related to the occurrence of initial bone metastasis.
However, patients with a T4 tumor, or with positive nodal status
significantly more commonly developed late bone metastasis.
Patients with a PTHrP-expressing tumor tended to develop late bone
metastasis more often than those with a PTHrP-negative tumor, and
positive PTHrP expression in the tumor was significantly related to
the development of bone metastasis at any time.
 | Table II.Relationship between bone metastasis
and clinicopathological findings. |
Table II.
Relationship between bone metastasis
and clinicopathological findings.
| Factors at the time
of surgery | No. of
patients | Bone metastasis
(BM)
| P-value
|
|---|
| Initial [n
(%)] | Late [n (%)] | Total [n (%)] | Late vs. no BM | Total vs. no
BM |
|---|
| Menopausal
status | | | | | | |
|
Premenopausal | 35 | 1 (2.9) | 6 (17.1) | 7 (20.0) | n.s. | n.s. |
|
Postmenopausal | 90 | 2 (2.2) | 14 (15.6) | 16 (17.8) | | |
| T category | | | | | | |
| T1 | 34 | 0 (-) | 3 (8.8) | 3 (8.8) | 0.014b | 0.004b |
| T2 | 63 | 2 (3.2) | 11 (17.5) | 13 (20.6) | | |
| T3 | 14 | 0 (-) | 1 (7.1) | 1 (7.1) | | |
| T4 | 14 | 1 (7.1) | 5 (35.6) | 6 (42.9) | | |
| Nodal status | | | | | | |
| (-) | 55 | 1 (1.8) | 4 (7.3) | 5 (9.1) | 0.018 | 0.017 |
| (+) | 70 | 2 (2.9) | 16 (22.9) | 18 (25.7) | | |
| Distant
metastasis | | | | | | |
| M0 | 116 | 0 (-) | 15 (12.9) | 15 (12.9) | 0.004 | 0.000 |
| M1 | 9 | 3 (33.3) | 5 (55.6) | 8 (88.9) | | |
| Estrogen
receptor | | | | | | |
| (-) | 64 | 3 (4.7) | 7 (10.9) | 10 (15.6) | n.s. | n.s. |
| (+) | 61 | 0 (-) | 13 (21.3) | 13 (21.3) | | |
| Lymphatic
infiltrationa | | | | | | |
| (-) | 59 | 1 (1.7) | 10 (17.0) | 11 (18.6) | n.s. | n.s. |
| (+) | 61 | 2 (3.3) | 8 (13.1) | 10 (16.4) | | |
| Vascular
invasiona | | | | | | |
| (-) | 116 | 2 (1.7) | 16 (13.8) | 18 (15.5) | n.s. | n.s. |
| (+) | 14 | 1 (7.1) | 2 (14.3) | 3 (21.4) | | |
| PTHrP
expression | | | | | | |
| (-) | 46 | 0 (-) | 3 (6.5) | 3 (6.5) | 0.051 | 0.018 |
| (+) | 79 | 3 (3.8) | 17 (21.5) | 20 (25.3) | | |
| Total | 125 | 3 (2.4) | 20 (16.0) | 23 (18.4) | | |
Multivariate logistic regression
analysis
Multivariate logistic regression analysis between
bone metastasis and clinicopathological findings is presented in
Table III. PTHrP status was an
independent risk factor for bone metastasis (HR, 7.104; 95% CI,
1.782–48.110; P=0.0037). Having a T4-tumor was also an independent
risk factor for bone metastasis (HR, 5.124; 95% CI, 1.169–24.243;
P=0.0303). Menopausal status, lymph node metastasis, estrogen
receptor status, lymphatic involvement and venous involvement did
not affect bone metastasis.
 | Table III.Multivariate logistic regression
analysis between bone metastasis and clinicopathological
findings. |
Table III.
Multivariate logistic regression
analysis between bone metastasis and clinicopathological
findings.
| Factor | Odds ratio | 95% CI | P-value |
|---|
| PTHrP | 7.104 | 1.7815–48.1101 | 0.0037 |
| Menopausal
status | 1.244 | 0.4059–4.1746 | 0.7083 |
| T stage | 5.123 | 1.1690–24.2426 | 0.0303a |
| Nodal status | 3.229 | 0.9912–12.1161 | 0.0518 |
| Estrogen
receptor | 1.577 | 0.5323–4.8187 | 0.4097 |
| Lymphatic
infiltration | 0.331 | 0.0870–1.1042 | 0.0728 |
| Vascular
invasion | 0.557 | 0.0704–2.8288 | 0.5024 |
Nodal involvement status and bone
metastasis
The relationship between bone metastasis and PTHrP
expression in combination with nodal involvement status is
presented in Table IV. Patients
were divided into four groups according to the status of PTHrP
expression and lymph node metastasis. Initial bone metastasis was
not observed in patients of the double-negative group, on the other
hand the highest rate (4.3%) of bone metastasis was shown in those
of the double-positive group. This tendency was pronounced when the
relationship between late bone metastasis was examined. In the
double-positive group, the risk of late bone metastasis (28.3%) was
significantly higher than that in the double-negative group (0%).
The occurrence of bone metastasis was increased by adding nodal and
menopausal status to the PTHrP expression status, and as high as
32% of the cases with PTHrP-positive tumor, node-positive, and
postmenopausal status developed late bone metastasis.
 | Table IV.Relationship between bone metastasis
and PTHrP expression in combination with nodal involvement
status. |
Table IV.
Relationship between bone metastasis
and PTHrP expression in combination with nodal involvement
status.
| PTHrP | Nodal status | No. of
patients | Initial bone
metastasis (%) | Late bone
metastasis (%) | P-value |
|---|
| (-) | (-) | 22 | 0 (0.0) | 0 (0.0) | |
| (+) | 24 | 0 (0.0) | 3 (12.5) | |
| (+) | (-) | 33 | 1 (3.0) | 4 (12.1) | |
| (+) | 46 | 2 (4.3) | 13 (28.3) | |
| Total | | 125 | 3 (2.4) | 20 (16.0) | <0.01a |
Overall survival
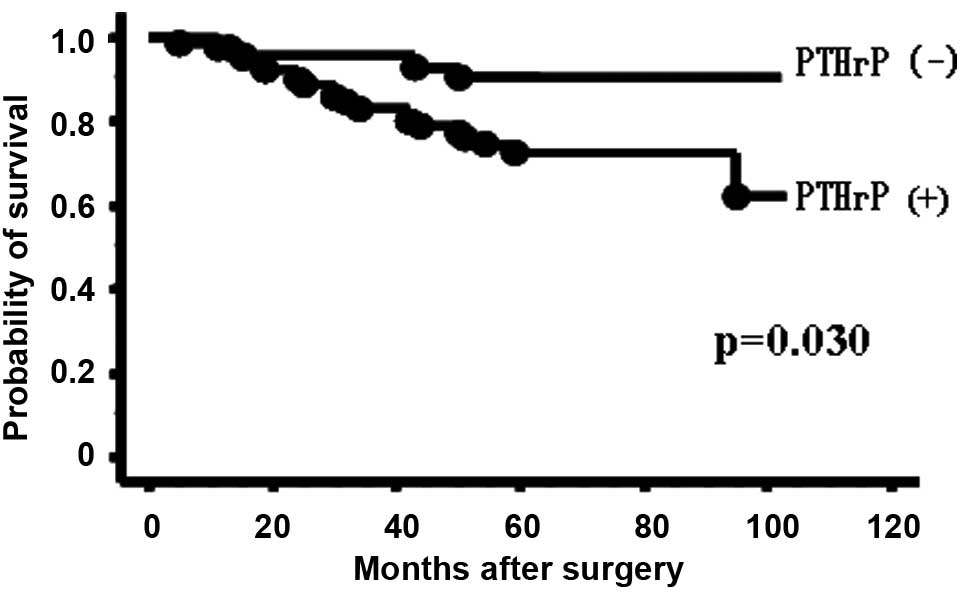
Fig. 1 presents the
overall survival of the 116 patients without initial metastatic
disease according to PTHrP status. The patients with PTHrP
expression had significantly poorer outcomes than those without
PTHrP expression (P=0.030). Five-year survival rate was 94.0% in
the patients without PTHrP expression, falling to 77.3% in those
with PTHrP expression. Multivariate analysis for overall survival
(Table V) showed that PTHrP
expression (RR, 3.644; 95% CI, 1.182–15.880; P=0.022) and tumor
size (RR, 5.028; 95% CI, 1.665–15.589; P=0.005) were independently
correlated with shorter overall survival.
 | Table V.Multivariate analysis for overall
survival was performed using Cox regression analysis. |
Table V.
Multivariate analysis for overall
survival was performed using Cox regression analysis.
| Factor | Relative Risk | 95% CI | P-value |
|---|
| PTHrP | 3.644 | 1.1821–15.8803 | 0.0226 |
| Menopausal
status | 0.753 | 0.3200–1.8149 | 0.5188 |
| T stage | 5.028 | 1.6654–15.5891 | 0.0046a |
| Nodal status | 1.506 | 0.5759–4.1956 | 0.4058 |
| Estrogen
receptor | 0.807 | 0.3134–2.0563 | 0.6509 |
| Lymphatic
infiltration | 0.828 | 0.2969–2.2623 | 0.7108 |
| Vascular
invasion | 0.683 | 0.1039–2.5606 | 0.6083 |
Discussion
Previous studies have demonstrated that the
expression of PTHrP in a primary breast tumor had a negative impact
on the prognosis of the patient (14), as demonstrated by the positive
correlation with advanced clinical stages (12,17)
and with developing bone metastasis (11,12).
These observations were in line with our results. PTHrP expression
in the primary breast lesion in cases who had already developed
bone metastasis has also been frequently demonstrated (12,23),
as in the present series. Moreover, it has been clearly described
that PTHrP was almost always expressed in the metastatic lesions in
the bone (9,11,14,18,23).
These investigations clearly demonstrate the essential role of
PTHrP expression in the formation of bone metastasis. However,
several studies have suggested PTHrP expression in the tumor might
act against its progression, and be associated with better
prognosis during early stage (15,23),
and non-invasive breast cancer (16). Experimental analyses have also
suggested that PTHrP has little involvement in the initial stages
of metastasis (24,25). These observations suggest that
PTHrP expression plays a role in preventing breast cancer
progression while the disease remains in situ. Taken
together, these findings suggest that PTHrP expression in breast
cancer has dual roles according to the disease stage; inhibiting
progression in the primary site and promoting it in the bone
marrow.
Similarly to previous studies (11,12,14,17),
we were able to demonstrate that PTHrP expression in the primary
lesion was indicative of poorer outcome. A significantly worse
survival was also demonstrated when patients with stage IV disease
were excluded. However, one previous study involving 526 patients
suggested the opposite result (23). There were certain unique points to
their study, compared with the others. Tumors were judged positive
for PTHrP when staining was observed in any cell. This criterion
led to a positive rate of as high as 80% in their study. As a
consequence the representative figures might be altered
significantly by a small number of highly malignant patients in the
PTHrP-negative group. The occurrence of bone metastasis was more
than 30% and the survival rate was 51% at 10 years in the
PTHrP-negative cases, which differed from our observation. We also
assumed there were differences in the nature of the tumor according
to the race or the volume of therapeutic intervention following
surgery.
In the present study, we found that a combination
analysis of PTHrP expression with nodal status clearly indicated
the risk of bone metastasis during the post-operative follow-up
period. In breast cancer, lymph node involvement is known as the
most reliable clinical indicator for prognosis. Nodal status may
also be practically used as a decision maker in performing adjuvant
chemotherapy. Cancer cells found in the regional lymph node may be
interpreted as the disease having already spread to the whole body.
Braun et al reported that patients with lymph node
involvement significantly more often had bone marrow
micrometastasis than node-negative patients (26). Furthermore, Wulf et al
demonstrated the evidence of disseminated PTHrP-expressing breast
cancer cells to the peripheral blood and also bone marrow (27). It is easy to imagine that, when
disseminated cancer cells have the ability to express PTHrP in
response to the microenvironment of the bone marrow, forming
metastatic lesions in the bone might confer an advantage to these
cases. Therefore, by combining positive nodal status as an
indicator of cancer cell dissemination to the circulation, the
potential value of PTHrP expression in the primary lesion is
pronounced in predicting bone metastasis, as shown in this
study.
Aromatase inhibitors and LH-RH agonists are the key
drugs for patients with hormone receptor-positive breast cancer and
they reduce the recurrence rate significantly by regulating
estrogen production. At the same time, they have the adverse
effects on the bone of decreasing bone mineral density. Early
menopause induced by adjuvant chemotherapy may also have a similar
effect on bone. Bone resorption caused by these anti-cancer
therapies results in the release of bone growth factors, which may
unintentionally activate the formation of bone metastases by
beginning the ‘vicious cycle’ (28). In the present study, we
demonstrated that menopausal status might have a role in increasing
the risk of late bone metastasis, especially in node-positive
patients with a PTHrP-positive tumor. Thus, not only avoiding
osteoporosis or fracture, but also to protect against bone
metastasis, careful surveillance of bone resorption might be
advisable.
Several strategies have been devised to prevent bone
metastasis during breast cancer treatments. Bisphosphonate is able
to protect against resorption of the bone by downregulating the
function of osteoclasts (29),
preventing cancer cell activation by reducing cytokine release from
the bone marrow and resulting in protection against skeletal bone
events (30). Recent studies have
also demonstrated the possible impact of bisphosphonates in
protecting against bone metastasis in breast cancer patients
(31). Experimental evidence shows
that humanized monoclonal antibody against PTHrP suppresses
osteolytic bone metastasis of human breast cancer cells (32). Anti-RANKL antibody is also a
promising drug being investigated for protection against the
progression of bone metastasis (33). These novel agents could be useful
in preventing development or progression of bone metastasis in high
risk cases.
In conclusion, our results indicate that patients
with PTHrP-producing tumors potentially risk development of bone
metastasis. The risk increased predominantly in patients with lymph
node metastasis who may already have systemic micrometastasis,
especially when undergoing hormonal changes associated with
postmenopausal status. Investigation of PTHrP expression of the
primary lesion, in combination with lymph node metastasis is, thus,
suggested to be a novel useful marker for predicting the risk of
bone metastasis in breast cancer patients.
References
|
1.
|
Coleman RE: Skeletal complications of
malignancy. Cancer. 80:1588–1594. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Mundy GR: Metastasis to bone: causes,
consequences and therapeutic opportunities. Nat Rev Cancer.
2:584–593. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Yoneda T and Hiraga T: Crosstalk between
cancer cells and bone microenvironment in bone metastasis. Biochem
Biophys Res Commun. 328:679–687. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Suva LJ, Winslow GA, Wettenhall RE, et al:
A parathyroid hormone-related protein implicated in malignant
hypercalcemia: cloning and expression. Science. 237:893–896. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Yin JJ, Selander K, Chirgwin JM, et al:
TGF-β signaling blockade inhibits PTHrP secretion by breast cancer
cells and bone metastases development. J Clin Invest. 103:197–206.
1999.
|
|
6.
|
Sanders JL, Chattopadhyay N, Kifor O,
Yamaguchi T, Butters RR and Brown EM: Extracellular calcium-sensing
receptor expression and its potential role in regulating
parathyroid hormone-related peptide secretion in human breast
cancer cell lines. Endocrinology. 141:4357–4364. 2000.
|
|
7.
|
Suda T and Takahashi N: Modulation of
osteoclast differentiation and function by the new members of the
tumor necrosis factor receptor and ligand families. Endocr Rev.
20:345–357. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Rubens RD: Clinical aspects of bone
metastases. Tumor Bone Diseases and Osteoporosis in Cancer
Patients. Body JJ: Marcel Dekker, Inc.; Brussels: 85. 2000
|
|
9.
|
Southby J, Kissin MW, Danks JA, et al:
Immunohistochemical localization of parathyroid hormone-related
protein in human breast cancer. Cancer Res. 50:7710–7716.
1990.PubMed/NCBI
|
|
10.
|
Liapis H, Crouch EC, Grosso LE, Kitazawa S
and Wick MR: Expression of parathyroidlike protein in normal,
proliferative, and neoplastic human breast tissues. Am J Pathol.
143:1169–1178. 1993.PubMed/NCBI
|
|
11.
|
Kohno N, Kitazawa S, Fukase M, et al: The
expression of parathyroid hormone-related protein in human breast
cancer with skeletal metastases. Surg Today. 24:215–220. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Downey SE, Hoyland J, Freemont AJ, Knox F,
Walls J and Bundred NJ: Expression of the receptor for parathyroid
hormone-related protein in normal and malignant breast tissue. J
Pathol. 183:212–217. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Henderson M, Danks J, Moseley J, et al:
Parathyroid hormone-related protein production by breast cancers,
improved survival, and reduced bone metastases. J Natl Cancer Inst.
93:234–237. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Linforth R, Anderson N, Hoey R, et al:
Coexpression of parathyroid hormone related protein and its
receptor in early breast cancer predicts poor patient survival.
Clin Cancer Res. 8:3172–3177. 2002.PubMed/NCBI
|
|
15.
|
Surowiak P, Dziegiel P, Matkowski R, Sopel
M, Wojnar A, Kornafel J and Zabel M: Prognostic value of
immunocytochemical determination of parathyroid hormone-related
peptide expression in cells of mammary ductal carcinoma. Analysis
of 7 years of the disease course. Virchows Arch. 442:245–251.
2003.
|
|
16.
|
Fleming NI, Trivett MK, George J, et al:
Parathyroid hormone-related protein protects against mammary tumor
emergence and is associated with monocyte infiltration in ductal
carcinoma in situ. Cancer Res. 69:7473–7479. 2009. View Article : Google Scholar
|
|
17.
|
Bouizar Z, Spyratos F, Deytieux S, de
Vernejoul MC and Jullienne A: Polymerase chain reaction analysis of
parathyroid hormone-related protein gene expression in breast
cancer patients and occurrence of bone metastasis. Cancer Res.
53:5076–5078. 1993.PubMed/NCBI
|
|
18.
|
Powell GJ, Southby J, Danks JA, et al:
Localization of parathyroid hormone-related protein in breast
cancer metastases: increased incidence in bone compared with other
sites. Cancer Res. 51:3059–3061. 1991.PubMed/NCBI
|
|
19.
|
Kohno N, Kitazawa S, Sakoda Y, Kanbara Y,
Furuya Y, Ohashi O and Kitazawa R: Parathyroid hormone-related
protein in breast cancer tissues: relationship between primary and
metastatic sites. Breast Cancer. 1:43–49. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Guise TA, Yin JJ, Taylor SD, et al:
Evidence for a causal role of parathyroid hormone-related protein
in the pathogenesis of human breast cancer-mediated osteolysis. J
Clin Invest. 98:1544–1549. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Thomas RJ, Guise TA, Yin JJ, Elliot J,
Horwood NJ, Martin TJ and Gillespie MT: Breast cancer cells
interact with osteoblasts to support osteoclast formation.
Endocrinology. 140:4451–4458. 1999.PubMed/NCBI
|
|
22.
|
Tezuka K, Onoda N, Takashima T, Ishikawa
T, Wakasa T, Wakasa K and Hirakawa K: Clinical significance of
intra-tumoral sinusoidal structures showing lympho-endothelial
immunoreactivity in breast cancer. Oncol Rep. 20:25–32. 2008.
|
|
23.
|
Henderson MA, Danks JA, Slavin JL, et al:
Parathyroid hormone-related protein localization in breast cancers
predict improved prognosis. Cancer Res. 66:2250–2256. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Torricelli C, Fortino V, Capurro E, et al:
Role of PTHrp and PTHrp-engaged pathways in MCF-7 cells
migration/invasion. Matrix Biol. 25:104–111. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Dittmer A, Vetter M, Schunke D, Span PN,
Sweep F, Thomssen C and Dittmer J: Parathyroid hormone-related
protein regulates tumor-relevant genes in breast cancer cells. J
Biol Chem. 281:14563–14572. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Braun S, Vogl FD, Naume B, et al: A pooled
analysis of bone marrow micrometastasis in breast cancer. N Engl J
Med. 353:793–802. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Wulf GG, Jürgens B, Liersch T, et al:
Reverse transcriptase/polymerase chain reaction analysis of
parathyroid hormone-related protein for the detection of tumor cell
dissemination in the peripheral blood and bone marrow of patients
with breast cancer. J Cancer Res Clin Oncol. 123:514–521. 1997.
View Article : Google Scholar
|
|
28.
|
Guise TA, Kozlow WM, Heras-Herzig A,
Padalecki SS, Yin JJ and Cirgwin JM: Molecular mechanisms of breast
cancer metastases to bone. Clin Breast Cancer. 5(Suppl 2): 46–53.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Russell RG, Rogers MJ, Frith JC, et al:
The pharmacology of bisphosphonates and new insights into their
mechanisms of action. J Bone Miner Res. 14(Suppl 2): 53–65. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Gnant M: Bisphosphonates in the prevention
of disease recurrence: current results and ongoing trials. Curr
Cancer Drug Targets. 9:824–833. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Aft R, Naughton M, Trinkaus K, et al:
Effect of zoledronic acid on disseminated tumour cells in women
with locally advanced breast cancer: an open label, randomised,
phase 2 trial. Lancet Oncol. 11:421–428. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Saito H, Tsunenari T, Onuma E, Sato K,
Ogata E and Yamada-Okabe H: Humanized monoclonal antibody against
parathyroid hormone-related protein suppresses osteolytic bone
metastasis of human breast cancer cells derived from MDA-MB-231.
Anticancer Res. 25:3817–3824. 2005.
|
|
33.
|
Body JJ, Facon T, Coleman RE, et al: A
study of the biological receptor activator of nuclear factor-kappaB
ligand inhibitor, denosumab, in patients with multiple myeloma or
bone metastases from breast cancer. Clin Cancer Res. 12:1221–1228.
2006. View Article : Google Scholar : PubMed/NCBI
|