Introduction
Double-stranded breaks (DSBs) in genomic DNA are
potentially fatal for all cells, and damage-induced repair
mechanisms are necessary in order to ensure cell survival (1). There are at least two fundamentally
different repair mechanisms: Homologous recombination (HR) and
non-homologous end-joining (NHEJ) (1). The activation of cell-cycle
checkpoints by DNA damage leads to mitotic arrest until the damage
is repaired. Once the damage is repaired, cells will re-enter
mitosis regardless of whether the repair process introduced errors
in the DNA sequences (2,3). DNA damage that is too severe to be
successfully repaired instead triggers programmed cell death
(4,5). Thus, DNA damage and the fidelity of
the repair process are critical in determining whether cells
survive with a normal phenotype, or become malignant with an
abberant one.
Cancer stem cells (CSCs) are the major cause of
resistance to therapy, disease recurrence and metastasis to other
organs (6–8). As with the stem cells that underlie
homeostasis in normal tissues, such as skin, intestine and the
haematopoietic system (9), CSCs
may be either dormant (dCSCs) or actively dividing (aCSCs). In
hepatocellular carcinoma, CD13+ dCSCs residing in
hypoxic areas of the tumour in the liver margin are capable of
surviving chemotherapy or radiotherapy (10). The incidence of DSBs in
CD13+ dCSCs is low following chemotherapy, most likely
due to the scavenging of reactive oxygen species (ROS) by CD13
(aminopeptidase N) (10). This is
in contrast to CD13−CD90+/− dCSCs, which
undergo ROS-induced cell death following chemotherapy (11). Significantly, chemotherapy results
in a shift in the CSC population from aCSCs to dCSCs, with dormancy
acting as a refuge for the survival of malignant cells. CD13 is
critical in suppressing ROS-dependent cell death during the
epithelial-mesenchymal transition of CSCs during metastasis
(12). Exposure to the
CD13-specific inhibitor, ubenimex, results in considerable
eradication of malignant cells in vivo, indicating the
likely benefit of the combination of conventional chemotherapy with
the CD13-specific inhibitor.
In this study, we show that DSBs in dCSCs result
predominantly in the activation of DNA repair via the NHEJ pathway,
whereas DNA repair by HR is the predominant response in aCSCs.
Based on these data, we propose that dCSCs should be the major
target of therapy in order to completely eradicate the malignant
cell population, although hibernation therapy (the induction to
dormancy) may be a suitable rationale dependent on the medical
condition of patients.
Materials and methods
Cell culture
Cell lines were maintained in Dulbecco's modified
Eagle's medium (DMEM; Nacalai Tesque, Kyoto, Japan) supplemented
with 10% foetal bovine serum (FBS) in a humidified incubator at
37°C in 5% CO2. All plasmids were purchased from Addgene
(Cambridge, MA, USA).
RNA
Total RNA was extracted from tissues using TRIzol
reagent (Invitrogen, Tokyo, Japan), and cDNA was synthesised using
SuperScript III RT kits (Invitrogen). Quantitative real-time
polymerase chain reaction (qRT-PCR) for specific genes and GAPDH
was performed using a LightCycler TaqMan Master kit with specific
primers provided by the manufacturer (Roche Diagnostics, Tokyo,
Japan). The amplification protocol consisted of 35 cycles of
denaturation at 95°C for 10 sec and annealing and elongation at
60°C for 30 sec. The products were then subjected to a temperature
gradient of 55–95°C with continuous fluorescence monitoring to
generate a melting curve. mRNA expression levels were normalised in
comparison to GAPDH mRNA.
Protein
Cell surface phenotypes were characterised by flow
cytometry on a FACSAria (BD, Tokyo, Japan). Cells were incubated
with primary antibodies in cold PBS containing 0.1% FBS for 1 h on
ice, washed twice, incubated with fluorescent-labelled secondary
antibodies for 30 min on ice and analyzed by flow cytometry.
Statistical analysis
For continuous variables, the results were expressed
as the means ± standard error. The correlation between the gene
expression level and cell count was analysed by Chi-square and
Wilcoxon rank tests. All data were analysed using JMP software (SAS
Institute, Cary, NC, USA). A value of p<0.05 was considered to
indicate a statistically significant difference.
Results
In hepatocellular carcinoma, CD13 is expressed in
CSCs (10) and is involved in the
scavenging of ROS via the glutathione reductase pathway. CD90 has
been proposed as a marker of stem cells, and its expression is
correlated with tumourigenicity in mouse in vivo tumour
models and the clinical outcome in human patients (13). In hepatocellular carcinoma, the
CD13+CD90− cell population is predominantly
dormant, CD13+CD90+ cells are predominantly
in the S phase and CD13−CD90+ cells are in
the G2/M phase (10). Following
exposure to genotoxic insults, such as chemotherapy or radiation
therapy, the proportions of these cell populations shift;
CD13− cells become less common, whereas the dormant
CD13+ population increases. We were interested in the
mechanism of DNA repair in the various CSC populations; therefore,
we used fluorescence-activated cell sorting (FACS) to separate
dormant CSCs (CD13+) from non-dormant CSCs
(CD13−) and analysed the expression of proteins involved
in the various DNA repair pathways. Rad51, Rad54, Rpa, Smc6, Xrcc2
and Xrcc3 are involved in HR, whereas Ku80, Ku70 and Prkdc are
indicative of NHEJ. We found that the expression ratio of
NHEJ-associated proteins to HR-associated proteins increased in
dormant CSCs, indicating that DSBs are repaired predominantly
through NHEJ in these cells. DNA repair by NHEJ is also the
dominant mechanism in quiescent hematopoietic stem cells (16). Conversely, in non-dormant CSCs,
HR-associated repair proteins increased relative to NHEJ-associated
proteins (Figs. 1–3).
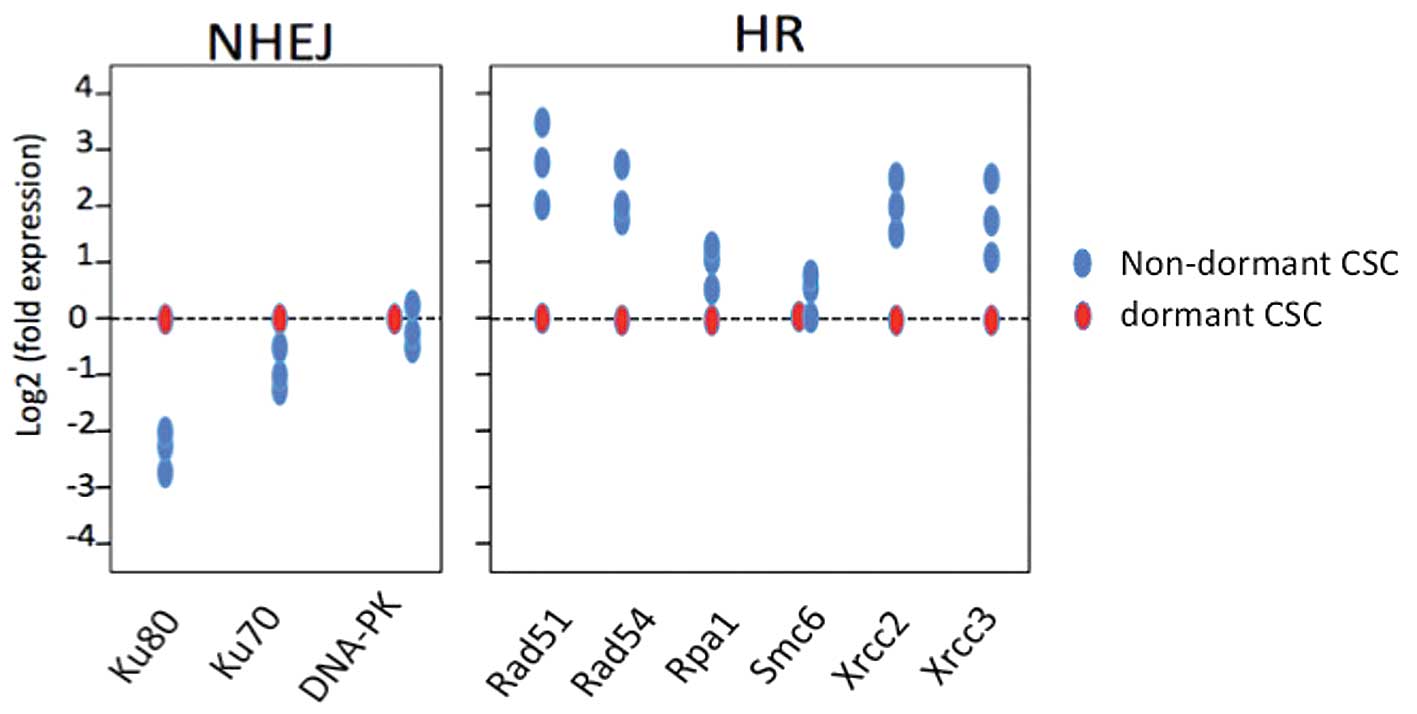 | Figure 1.Quantitative analysis of mRNAs
encoding proteins involved in HR and NHEJ. A total of 24 h after
exposure to 7 Gy radiation, cells were separated by FACS into pools
of dormant CD13+ CSCs and actively dividing
(non-dormant) CD13-CSCs. The RNAs were extracted and subjected to
quantitative analysis of PCR by utilising specific primers. For HR,
Rad51, Rad54, Rpa, Smc6, Xrcc2 and Xrcc3 were evaluated, whereas
Ku80, Ku70 and Prkdc were assessed for NHEJ. HR, homologous
recombination; NHEJ, non-homologous end-joining; CSCs, cancer stem
cells; PCR, polymerase chain reaction. |
Discussion
In proliferating CSCs, either in whole tissues or
tissue culture, exposure to genotoxic insults, such as chemotherapy
and radiation therapy, causes DNA damage during the S phase of the
cell cycle, followed by apoptosis of tumour cells (4). In a fraction of the actively dividing
tumour cells that survive therapy, the DNA damage is repaired by
HR, consistent with the data shown in this study. DNA repair by HR
is typically a high-fidelity, low-error mechanism. By contrast,
dormant cells are more likely to utilise NHEJ for DNA repair. NHEJ
may occur at any stage of the cell cycle but has only a limited
requirement for sequence homology and, importantly, is more prone
to error than repair by HR (1).
Thus, NHEJ is associated with the misrepair of DSBs, and may result
in chromosomal deletions, insertions or translocations and
subsequent genomic instability (14). These genomic alterations may lead
to the inactivation of tumour suppressor genes or activation of
oncogenes, which lead to the development of primary and metastatic
tumours. In our study, we found that DNA repair by NHEJ is
characteristic of dormant CSCs. Due to the mutagenic effects of
NHEJ, dormant CSCs that survive chemoradiation therapy may acquire
new mutations and become more likely to cause tumour recurrence
and/or metastasis (15). It is
therefore critical to eradicate dCSCs in order to improve patient
survival.
Quiescence is widely accepted to be an essential
protective mechanism for stem cells that minimises the endogenous
stress caused by cellular respiration and DNA replication in normal
cells (16). However, it is also
important in tumour evolution after therapy, since quiescent cells
that survive therapy are likely to have acquired mutations during
DNA repair by NHEJ (8). Thus,
genotoxic therapies, such as chemoradiation therapy, that suppress
genotoxic effects are a double-edged sword, removing primary tumour
bulk, but also contributing to tumour recurrence after therapy.
References
|
1.
|
Sancar A, Lindsey-Boltz LA, Unsal-Kacmaz K
and Linn S: Molecular mechanisms of mammalian DNA repair and the
DNA damage checkpoints. Annu Rev Biochem. 73:39–85. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Bartek J and Lukas J: Chk1 and Chk2
kinases in checkpoint control and cancer. Cancer Cell. 3:421–429.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Bartek J, Lukas C and Lukas J: Checking on
DNA damage in Sphase. Nat Rev Mol Cell Biol. 5:792–804. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Lowe SW and Lin AW: Apoptosis in cancer.
Carcinogenesis. 21:485–495. 2000. View Article : Google Scholar
|
|
5.
|
Kunkel TA: DNA replication fidelity. J
Biol Chem. 279:16895–16898. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Reya T, Morrison SJ, Clarke MF and
Weissman IL: Stem cells, cancer, and cancer stem cells. Nature.
414:105–111. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Visvader JE and Lindeman GJ: Cancer stem
cells in solid tumours: accumulating evidence and unresolved
questions. Nat Rev Cancer. 10:755–768. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Dewi DL, Ishii H, Kano Y, Nishikawa S,
Haraguchi N, Sakai D, Satoh T, Doki Y and Mori M: Cancer stem cell
theory in gastrointestinal malignancies: recent progress and
upcoming challenges. J Gastroenterol. 46:1145–1157. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Li L and Clevers H: Coexistence of
quiescent and active adult stem cells in mammals. Science.
327:542–545. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Haraguchi N, Ishii H, Mimori K, Tanaka F,
Ohkuma M, Kim HM, Akita H, Takiuchi D, Hatano H, Nagano H, Barnard
G, Doki Y and Mori M: CD13 is a therapeutic target in human liver
cancer stem cells. J Clin Invest. 120:3326–33239. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Haraguchi N, Ishii H, Nagano H, Doki Y and
Mori M: The future prospects and subject of the liver cancer stem
cells study for the clinical application. Gastroenterology. Feb
23–2011, (Epub ahead of print).
|
|
12.
|
Kim HM, Haraguchi N, Ishii H, Ohkuma M,
Okano M, Mimori K, Eguchi H, Yamamoto H, Nagano H, Sekimoto M, Doki
Y and Mori M: Increased CD13 expression reduces reactive oxygen
species, promoting survival of liver cancer stem cells via an
epithelial-mesenchymal transition-like phenomenon. Ann Surg Oncol.
Aug 31–2011, (Epub ahead of print).
|
|
13.
|
Yang ZF, Ho DW, Ng MN, Lau CK, Yu WC, Ngai
P, Chu PW, Lam CT, Poon RT and Fan ST: Significance of
CD90+ cancer stem cells in human liver cancer. Cancer
Cell. 13:153–166. 2008.
|
|
14.
|
Weinstock DM, Richardson CA, Elliott B and
Jasin M: Modeling oncogenic translocations: distinct roles for
double-strand break repair pathways in translocation formation in
mammalian cells. DNA Repair (Amst). 5:1065–1074. 2006. View Article : Google Scholar
|
|
15.
|
Ishii H, Iwatsuki M, Ieta K, Ohta D,
Haraguchi N, Mimori K and Mori M: Cancer stem cells and
chemoradiation resistance. Cancer Sci. 99:1871–1877. 2008.
View Article : Google Scholar
|
|
16.
|
Mohrin M, Bourke E, Alexander D, Warr MR,
Barry-Holson K, Le Beau MM, Morrison CG and Passegué E:
Hematopoietic stem cell quiescence promotes error-prone dna repair
and mutagenesis. Cell Stem Cell. 7:174–185. 2010. View Article : Google Scholar : PubMed/NCBI
|
















