Introduction
Colon cancer is one of the three leading causes of
cancer-related mortality worldwide and survival is affected by
local recurrence and lymphatic and hematogenous dissemination
(1). This neoplasm frequently
metastasizes to the lymph nodes at early stages of the disease. In
advanced disease, the majority of patients develop extra lymph node
metastases, most often in the liver, lungs and peritoneum. An
understanding of the factors involved in colon cancer metastasis is
largely lacking. At present, the growth and metastasis of tumors
are considered to be due to the dysregulation of molecular
processes. This also gives rise to several features of tumor cells,
including resistance to apoptosis, migration, invasion and the
ability to escape the immune system. The results of previous
studies indicate that chemokine receptors direct the lymphatic and
hematogenous spread of tumors and may also influence the site of
metastasis (2–5).
CXCR4 is a chemokine receptor that was first
identified as a regulator of the homing of lymphocytes in
inflammatory tissues (6). Stromal
cell-derived factor (SDF)-1α is a ligand of CXCR4 and has high
levels of expression in sites of metastasis, including the lung,
liver and lymph nodes, where it attracts lymphocytes (7). It has been shown that CXCR4 is
critical for the adhesion and/or migration of tumor cells,
indicating that CXCR4 is involved in tumor invasion and metastasis
(8). Numerous authors have
reported on the involvement of the CXCR4/SDF-1α axis in promoting a
metastatic phenotype in tumors (9–18).
For example, high CXCR4 expression has been shown to be associated
with lymph node metastasis in breast cancer and oral squamous cell
carcinoma (19,20).
An increasing amount of experimental evidence
supports the hypothesis that stem cells play a significant role in
the progression of cancer. Cancer stem cells are able to self-renew
and are believed to drive tumor growth (21,22).
Thus far, cancer stem cells have been identified in a great number
of solid tumors (23,24). CD133 is a well-studied cancer stem
cell marker that has been implicated in numerous types of solid
tumors, including colorectal cancer (25–27).
Preliminary evidence suggests that CD133 is involved in
bioenergetic stress, resistance to apoptosis and the activation of
a stemness-related signaling pathway, although its function is
unknown (28–33). CD133 was first described in
hematopoietic stem cells and is now established as a cancer stem
cell marker in a number of types of solid tumors, including those
of the brain, breast, lung, liver, colon and prostate as well as in
pancreatic carcinomas, medulloblastoma and melanoma (34–38).
The cancer stem cell compartment is increasingly being recognized
as a necessary target for the effective treatment of cancers
(39) and supporting data from
in vitro and murine tumor models have underlined the key
roles of CXCR4 and CD133 in tumor cell malignancy. However, no data
are presently available concerning the co-expression of the two
proteins in human colon cancer and the impact of these proteins on
disease progression and prognosis. Therefore, we evaluated the
expression of CXCR4 and CD133 in colon cancer specimens and
correlated the results with the clinicopathological parameters and
survival of the patients.
Materials and methods
Patients and follow-up
A total of 125 pathologically confirmed specimens
were obtained from colon cancer patients with TNM stage II or III
tumors that were subjected to radical resection between January
2001 and July 2005 in The First Affiliated Hospital, Sun Yat-Sen
University (Guangzhou, China). None of the patients had undergone
either chemotherapy or radiotherapy prior to the collection of the
samples. Postoperative therapeutic strategies were applied
according to the stage of the disease and the presumed risk of
relapse. Patients with stage II disease underwent follow-up based
on history, physical examination, complete blood count, liver
function tests, ultrasound scan of the abdomen and carcinoembryonic
antigen monitoring every three months. Total body computed
tomography scan and colonoscopy were performed once a year.
Patients with stage II high-risk disease (pT4 and/or
gross volume tumors, perforation, obstruction, poorly
differentiated histology, long-lasting symptoms, preoperative
elevated carcinoembryonic antigen levels or blood or lymphatic
vessel invasion) were encouraged to undergo adjuvant chemotherapy.
If no contraindications were present, patients with stage III
disease underwent six months of fluorouracil (Fu)-based adjuvant
chemotherapy and were then followed up. A total of 90 patients
received Fu-based adjuvant chemotherapy and 35 stage II patients
did not receive adjuvant interventions. The patients were observed
once every three months during the first year, once every six
months in the second year and by telephone or mail communication
once every year thereafter for a total of 5 years. If recurrence or
metastasis occurred, 5-Fu-based chemotherapy was administered
according to the National Comprehensive Cancer Network (NCCN)
guidelines. Overall survival was defined as the time from surgery
to mortality or was censored at the last known date alive.
Histopathological characteristics were confirmed by a blinded
review of the original pathology slides. The TNM classification was
used for pathological staging and the World Health Organization
classification was used for pathological grading.
Immunohistochemical assay
The expression of CXCR4 and CD133 in primary tumors
and lymph node metastasis was examined using an immunohistochemical
assay. The immunohistochemical assay was performed within seven
days of section preparation. To prevent antigen degradation, the
sections were stored at 4°C prior to the assay. Briefly,
formalin-fixed, paraffin-embedded archived tissues were sectioned
at a thickness of 4 μm. The sections were then dewaxed, rehydrated
and blocked with hydrogen peroxide. The slides were then immersed
in 10 mmol/l sodium citrate buffer (pH 6.0) for CXCR4 staining or
in 10 mmol/l citrate buffer (pH 6.0) for CD133 staining, incubated
for 10 min on a hot plate (95–99°C) or boiled and allowed to cool
for 20 min. For CXCR4 staining, following blocking with 1% goat
serum albumin, the sections were incubated with mouse monoclonal
antibodies against human CXCR4 at a dilution of 1:150 (Abcam,
Cambridge, UK) for 2 h at room temperature, followed by a
biotinylated secondary antibody and streptavidin-biotinylated
horseradish peroxidase complex. For CD133 staining, following
blocking with 1% goat serum albumin, the sections were incubated
with mouse monoclonal antibodies against human CD133 at a dilution
of 1:150 (Novus, Littleton, CO, USA) overnight at 4°C, followed by
the biotinylated secondary antibody and streptavidin-biotinylated
horseradish peroxidase complex. The slides were stained for 5 min
with 0.05% diaminobenzidine tetrahydrochloride (DAB) freshly
prepared in 0.05 mol/l Tris-HCl buffer (pH 7.6) containing 0.024%
hydrogen peroxidase and then counterstained with hematoxylin,
dehydrated and mounted. All series included positive controls
(melanoma and glioma samples). Negative controls were obtained by
substituting the primary antibody with a mouse myeloma protein of
the same subclass at the same concentration as the monoclonal
antibody. All controls yielded satisfactory results.
The specimens were evaluated by two authors (Y.L.
and W.-T.L.) who had no knowledge of the prognosis or other
clinicopathological variables. Following Matsumoto's method, each
image was analyzed for immunoreactivity using a 0 to 3
semi-quantitation system for the intensity of staining and the
percentage of positive cells (labeling frequency percentage)
(40). The samples were grouped
into the following four categories based on the intensity of
membrane staining: 0, no staining/background equal to the negative
controls; 1, weak staining detectable above background; 2, moderate
staining; 3, intense staining. The labeling frequency was scored as
0 (0%), 1 (1–33%), 2 (34–66%) or 3 (67–100%). The index sum was
obtained by totaling the scores of intensity and percentages. If
the final score was equal to or greater than four, the result was
considered positive; otherwise, the result was considered
negative.
Statistical analysis
The associations between immunohistochemical scores
and clinicopathological variables of the tissue specimens were
evaluated using the χ2 test. When the subset sample size
was small, the corresponding test was performed using Fisher's
exact test. Univariate analysis was performed using the log-rank
test. The Cox proportional hazards regression was used to analyze
the effect of several risk factors on overall survival. The
following factors were assessed using univariate and multivariate
analyses for their influence on overall survival: gender, age
(<60 vs. ≥60 years), location of the primary mass (left vs.
right hemicolon), pathological grades [well-differentiated (G1),
moderately differentiated (G2) or poorly differentiated (G3)],
tumor size (<2, 2–5 or >5 cm), American Joint Committee on
Cancer (AJCC) stage (stage II vs. III), tumor invasion (pT1–T2, pT3
or pT4), lymph node status (pN0, pN1 or pN2), CXCR4 expression
(positive vs. negative), CD133 expression (positive vs. negative)
and the combined expression of CXCR4 and CD133 (both positive vs.
other combinations). Kaplan-Meier curves were used to estimate the
contributions of the clinicopathological characteristics to
survival. All analyses were performed using the Statistical Package
for Social Sciences (SPSS) software, version 13.0, for Windows
(SPSS Inc., Chicago, IL, USA). P<0.05 was considered to indicate
a statistically significant result. All reported P-values are
two-sided.
Results
Characteristics of patients and
tumors
A total of 125 patients seen between January 2001
and July 2005 were studied. The characteristics of all patients are
summarized in Table I. The median
age was 61.8 years; 68 patients were ≥60 years old. The genders
were equally represented. A total of 71 tumors originated in the
left hemicolon. The majority of the patients (102/125, 81.6%) had
moderate-grade disease. Approximately 62% of the patients had
tumors between 2 and 5 cm in size, 61 patients presented with stage
II disease and 64 with stage III. The majority of the lesions
presented with a pT3 or pT4 extent of invasion and 64 patients
presented with pN+ disease (Table
I).
 | Table I.Clinicopathological features of the
patients with colon cancer (n=125). |
Table I.
Clinicopathological features of the
patients with colon cancer (n=125).
| Parameters | No. of patients
(%) |
|---|
| Age (years) | |
| <60 | 57 (45.6) |
| ≥60 | 68 (54.4) |
| Gender | |
| Male | 78 (62.4) |
| Female | 47 (37.6) |
| Location | |
| Left
hemicolon | 71 (56.8) |
| Right
hemicolon | 54 (43.2) |
| Pathological
grade | |
| G1 | 14 (11.2) |
| G2 | 102 (81.6) |
| G3 | 9 (7.2) |
| Tumor size
(cm) | |
| <2 | 14 (11.2) |
| 2–5 | 77 (61.6) |
| >5 | 34 (27.2) |
| AJCC stage | |
| II | 61 (48.8) |
| III | 64 (51.2) |
| Tumor invasion | |
| pT1–T2a | 16 (12.8) |
| pT3 | 60 (48.0) |
| pT4 | 49 (39.2) |
| Lymph node
status | |
| pN0 | 61 (48.8) |
| pN1 | 38 (30.4) |
| pN2 | 26 (20.8) |
Expression of CXCR4 in colon epithelium
and colon cancer tissues
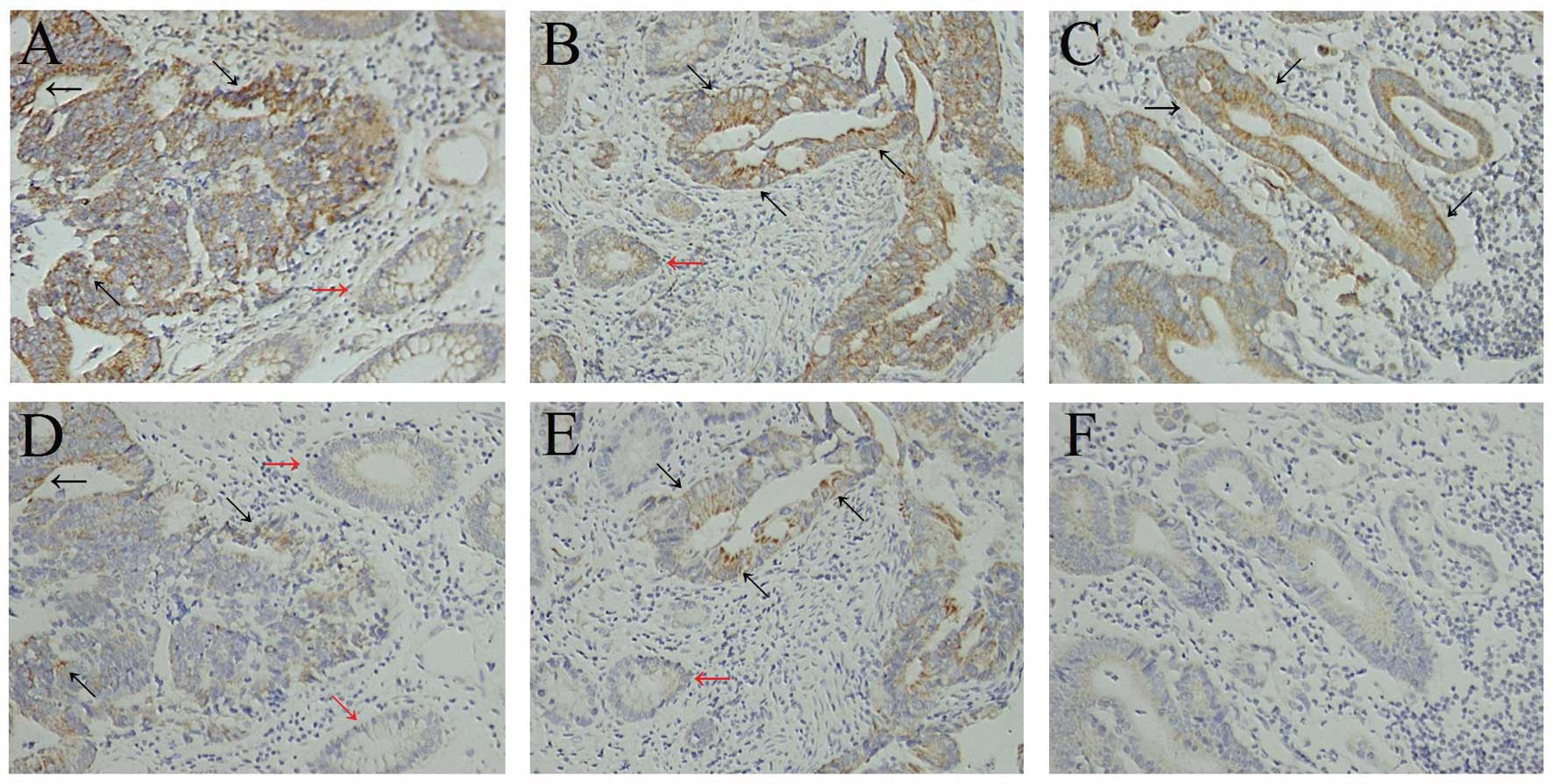
In the normal colon epithelium adjacent to the
tumor, weak immunoreactivity for CXCR4 was detected in the
cytoplasm and plasma membrane of non-neoplastic epithelial cells
(Fig. 1A and B). Paratumorous
normal colon epithelium was observed in 102 of the 125 specimens
used for this study and, of those 102, expression of CXCR4 in the
colon epithelium was found in only 11 cases.
Of the 125 colon cancer tissues, 74 (59.2%) were
positive for CXCR4 expression. Staining for CXCR4 revealed a
predominantly cytoplasmic and, in a few specimens, an additional
weak membranous location of CXCR4. Nuclear staining of CXCR4 was
not observed. Fig. 1 shows
examples of CXCR4 staining of colon cancer tissues (Fig. 1A and B). In addition, some
tumor-infiltrating lymphocytes and fibroblastic cells exhibited
positive cytoplasmic staining for CXCR4.
Among the 125 stage II or III colon cancer patients,
no significant correlation existed between CXCR4 expression and
gender, age, location of primary mass, pathological grade, tumor
size, AJCC stage, tumor invasion or lymph node status (Table II).
 | Table II.Correlation between CXCR4 and CD133
expression and clinicopathological features in colon cancer
patients. |
Table II.
Correlation between CXCR4 and CD133
expression and clinicopathological features in colon cancer
patients.
| CXCR4
| | CD133
| | CD133 and CXCR4
| |
|---|
| Positive | Negative | P-value | Positive | Negative | P-value | Both positive | Others | P-value |
|---|
| Age (years) | | | | | | | | | |
| <60 | 34 | 23 | | 16 | 41 | | 9 | 48 | |
| ≥60 | 40 | 28 | 0.925 | 29 | 39 | 0.091 | 20 | 48 | 0.072 |
| Gender | | | | | | | | | |
| Male | 49 | 29 | | 31 | 47 | | 17 | 61 | |
| Female | 25 | 22 | 0.289 | 14 | 33 | 0.261 | 12 | 35 | 0.632 |
| Location | | | | | | | | | |
| Left
hemicolon | 43 | 28 | | 28 | 43 | | 19 | 52 | |
| Right
hemicolon | 31 | 23 | 0.722 | 17 | 37 | 0.359 | 10 | 44 | 0.28 |
| Pathological
grade | | | | | | | | | |
| G1 | 5 | 9 | | 2 | 12 | | 0 | 14 | |
| G2 | 63 | 39 | | 39 | 63 | | 27 | 75 | |
| G3 | 6 | 3 | 0.159 | 4 | 5 | 0.186 | 2 | 7 | 0.089 |
| Tumor size
(cm) | | | | | | | | | |
| <2 | 6 | 8 | | 2 | 12 | | 1 | 13 | |
| 2–5 | 50 | 27 | | 30 | 47 | | 18 | 59 | |
| >5 | 18 | 16 | 0.207 | 13 | 21 | 0.199 | 10 | 24 | 0.251 |
| AJCC stage | | | | | | | | | |
| II | 33 | 28 | | 18 | 43 | | 9 | 52 | |
| III | 41 | 23 | 0.257 | 27 | 37 | 0.140 | 20 | 44 | 0.029 |
| Tumor invasion | | | | | | | | | |
| pT1–T2a | 7 | 9 | | 4 | 12 | | 2 | 14 | |
| pT3 | 33 | 27 | | 26 | 34 | | 16 | 44 | |
| pT4 | 34 | 15 | 0.127 | 15 | 34 | 0.240 | 11 | 38 | 0.485 |
| Lymph node
status | | | | | | | | | |
| pN0 | 33 | 28 | | 18 | 43 | | 9 | 52 | |
| pN1 | 22 | 16 | | 15 | 23 | | 9 | 29 | |
| pN2 | 19 | 7 | 0.252 | 12 | 14 | 0.290 | 11 | 15 | 0.020 |
Expression of CD133 in colon epithelium
and colon cancer tissues
In the normal colon epithelium adjacent to the
tumor, CD133 expression was absent in the majority of cases and was
mild and focal in intensity in the remainder (Fig. 1D and E). Paratumorous normal colon
epithelium was observed in 102 of the 125 specimens used for this
study. Expression of CD133 in the colon epithelium was found in
only 8 of the 102 specimens.
CD133 expression was detected in 45 of the 125
tumors (36.0%). CD133 expression in colon cancer was polar and
confined to the apical luminal surface of colon cancer cells with
glandular differentiation, which is in accordance with previous
results. We observed staining of CD133 on the luminal cell surface
of colon cancer glands (Fig. 1D and
E) and only tumor cells in direct contact with these luminal
surfaces were CD133+. CD133+ tumor cells were
mostly observed in groups, with some glands being completely
positive. In addition, tumor-infiltrating lymphocytes and
fibroblastic cells did not exhibit positive cytoplasmic staining
for CD133.
Among the 125 stage II or III colon cancer patients,
no significant correlation existed between CD133 expression and
gender, age, location of primary mass, pathological grade, tumor
size, AJCC stage, tumor invasion or lymph node status (Table II).
Correlation between CXCR4 and CD133
expression in colon cancer
Among the 125 stage II or III colon cancer patients,
no significant correlation was found between CXCR4 or CD133
expression and gender, age, location of primary mass, pathological
grade, tumor size, AJCC stage, tumor invasion or lymph node status
(Table II). CXCR4-positive colon
cancer cases showed stronger CD133 expression than negative cases,
but no significant difference was observed (Table III; P= 0.371). By combining the
expression of CXCR4 and CD133, we obtained the following four
combinations: CXCR4-positive and CD133-positive, CXCR4-positive and
CD133-negative, CXCR4-negative and CD133-positive and
CXCR4-negative and CD133-negative. Notably, co-expression (CXCR4-
and CD133-positive) was detected in 29 of the 125 tumors (23.2%)
and, compared with the other combinations, the co-expression of the
CXCR4 and CD133 proteins was significantly associated with the AJCC
stage (P=0.029) and the lymph node status (Table II; P=0.020).
 | Table III.Correlation between CXCR4 expression
and CD133 expression in primary cancer. |
Table III.
Correlation between CXCR4 expression
and CD133 expression in primary cancer.
| CD133 expression n
(%)
| |
|---|
| CXCR4
expression | Positive | Negative | P-value |
|---|
| Positive | 29 (39.2) | 45 (60.8) | |
| Negative | 16 (31.4) | 35 (68.6) | 0.371 |
Expression of CXCR4 and CD133 in
metastasized tumors in lymph nodes (MTLNs)
A total of 64 stage III colon cancer cases showed
metastasis to their related lymph nodes. Among these primary cancer
cases, 41 (64.1%) showed a marked expression of CXCR4. Of the
MTLNs, 43 (67.2%) showed a marked expression of CXCR4. Thus, most
of the stage III colon cancer cases (n=41, 64.1%) showed strong
CXCR4 expression, not only in the primary lesions but also in their
MTLNs (Fig. 1B and C; Table IV; P=0.710).
 | Table IV.Correlation between CXCR4 and CD133
expression in primary cancer and in lymph node metastasis of stage
III colon cancer patients. |
Table IV.
Correlation between CXCR4 and CD133
expression in primary cancer and in lymph node metastasis of stage
III colon cancer patients.
| CXCR4
| | CD133
| | CD133 and CXCR4
| |
|---|
| Positive | Negative | P-value | Positive | Negative | P-value | Both positive | Others | P-value |
|---|
| Primary cancer | 41 | 23 | | 27 | 37 | | 20 | 44 | |
| Lymph node
metastases | 43 | 21 | 0.710 | 15 | 49 | 0.024 | 9 | 55 | 0.020 |
Of the 64 stage III colon cancer cases, 27 (42.2%)
were positive for CD133 expression, but only 15 (23.4%) of the
MTLNs were positive for CD133 expression. These findings suggest
that, compared with their primary lesions, MTLNs are significantly
more likely to show decreased CD133 expression (Fig. 1E and F; Table IV; P=0.024).
Upon combining the expression of CXCR4 and CD133,
the co-expression of the CXCR4 and CD133 proteins was significantly
different between the primary cancers and MTLNs. Notably, the
primary cancers showed a marked co-expression of the CXCR4 and
CD133 proteins (31.3%), whereas in MTLNs, the co-expression was
observed in only 14.1% of cases (Table
IV; P=0.020).
Survival analysis
All 125 patients were followed up for survival to
assess CXCR4 and CD133 expression as a prognostic factor. The
median follow-up period was 6.5 years. Of the 125 patients, 39 died
during the follow-up period and the 5-year survival rate was 68.8%.
The analysis of prognostic factors for survival is summarized in
Table V. Log-rank analysis
revealed that AJCC stage, lymph node status, CXCR4 expression,
CD133 expression and the co-expression of the CXCR4 and CD133
proteins were significant prognostic indicators for overall patient
survival (Table V). The predictive
ability of the AJCC stage, lymph node status, CXCR4 expression,
CD133 expression and co-expression of the CXCR4 and CD133 proteins
was confirmed by multivariate analysis (Table V). No significant correlation was
observed between the prognosis and the other clinicopathological
features.
 | Table V.Univariate and multivariate analyses
of overall survival in the 125 colon cancer patients. |
Table V.
Univariate and multivariate analyses
of overall survival in the 125 colon cancer patients.
| Clinicopathological
characteristics | n (n=125) | 5-year survival
rate | Kaplan-Meier
analysis (P-value) | Cox regression
model analysis (P-value) |
|---|
| Age (years) | | | | |
| <60 | 57 | 42/57 (73.7%) | | |
| ≥60 | 68 | 44/68 (64.7%) | 0.236 | 0.253 |
| Gender | | | | |
| Male | 78 | 57/78 (73.1%) | | |
| Female | 47 | 29/47 (61.7%) | 0.124 | 0.162 |
| Location | | | | |
| Left
hemicolon | 71 | 45/71 (63.4%) | | |
| Right
hemicolon | 54 | 41/54 (75.9%) | 0.186 | 0.154 |
| Pathological
grade | | | | |
| G1 | 14 | 10/14 (71.4%) | | |
| G2 | 102 | 72/102 (70.6%) | | |
| G3 | 9 | 4/9 (44.4%) | 0.168 | 0.113 |
| Tumor size
(cm) | | | | |
| <2 | 14 | 9/14 (64.3%) | | |
| 2–5 | 77 | 57/77 (74.0%) | | |
| >5 | 34 | 20/34 (58.8%) | 0.192 | 0.383 |
| AJCC stage | | | | |
| II | 61 | 48/61 (78.7%) | | |
| III | 64 | 38/64 (59.4%) | 0.014 | 0.032 |
| Tumor invasion | | | | |
| pT1–T2a | 16 | 12/16 (73.7%) | | |
| pT3 | 60 | 44/60 (73.7%) | | |
| pT4 | 49 | 30/49 (64.7%) | 0.237 | 0.289 |
| Lymph node
status | | | | |
| pN0 | 61 | 48/61 (78.7%) | | |
| pN1 | 38 | 25/38 (65.8%) | | |
| pN2 | 26 | 13/26 (50.0%) | 0.011 | 0.023 |
| CXCR4
expression | | | | |
| Positive | 74 | 45/74 (60.8%) | | |
| Negative | 51 | 41/51 (80.4%) | 0.023 | 0.036 |
| CD133
expression | | | | |
| Positive | 45 | 26/45 (57.8%) | | |
| Negative | 80 | 60/80 (75.0%) | 0.034 | 0.021 |
| CXCR4 and
CD133 | | | | |
| Both
positive | 29 | 14/29 (48.3%) | | |
| Other
combinations | 96 | 72/96 (75.0%) | 0.003 | 0.001 |
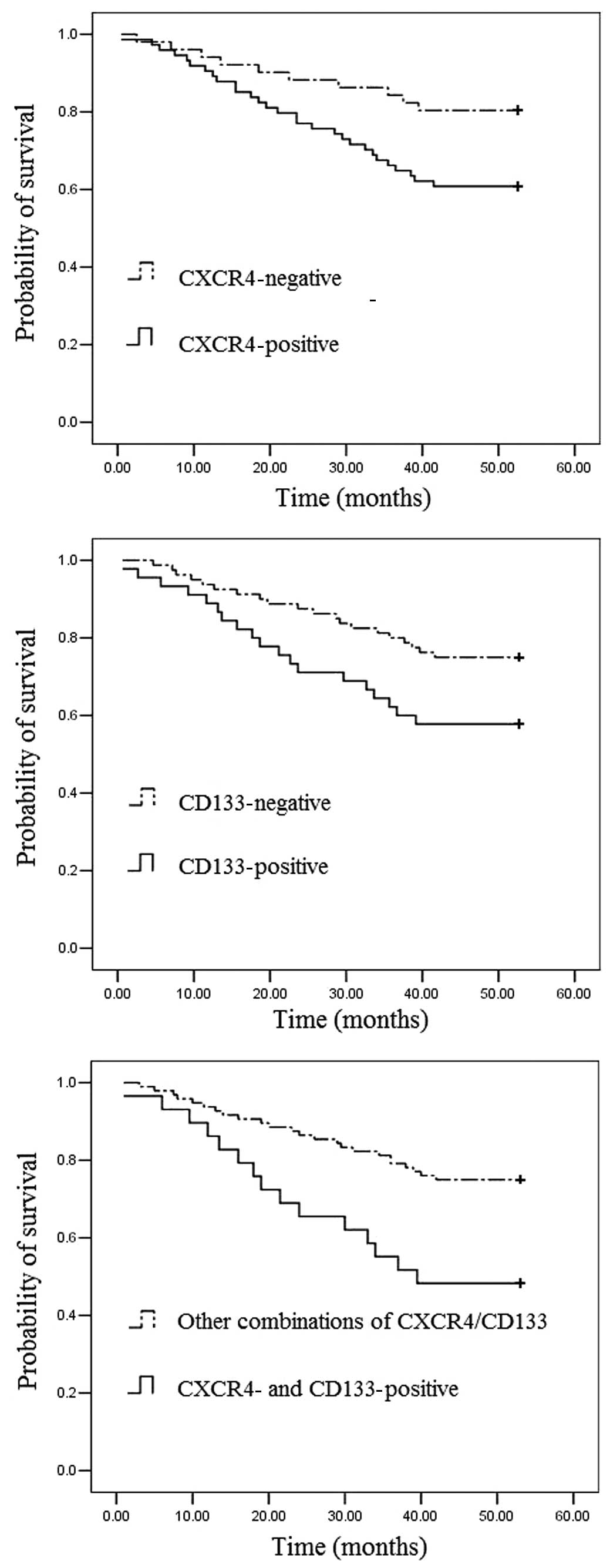
To assess the prognostic significance of CXCR4 and
CD133 expression, Kaplan-Meier survival curves were constructed.
The prognosis of colon cancer patients with positive CXCR4
expression was significantly worse than that of colon cancer
patients with negative CXCR4 expression (Fig. 2A). Patients with CD133-positive
colon cancers also had significantly poorer outcomes than those
with CD133-negative tumors (Fig.
2B). Furthermore, colon cancer patients with co-expression of
CXCR4 and CD133 proteins tended to have poorer outcomes than those
with other combinations (Fig. 2C).
The Kaplan-Meier estimated curves suggest that the prognosis is
particularly unfavorable for patients whose primary tumors express
both CXCR4 and CD133. Of the patients with co-expression of the
CXCR4 and CD133 proteins, the 5-year survival rate was only 48.3%;
by contrast, the survival rate was 75.0% in patients with tumors
that expressed the other combinations (log-rank test, P=0.003).
Discussion
It has been shown that CXCR4 is critical for the
adhesion and/or migration of tumor cells, indicating that CXCR4 is
involved in tumor invasion and metastasis (8). Previous studies have suggested the
existence of a cancer stem cell compartment that is able to
self-renew and differentiate into mature and diverse cancer cells
that are capable of tumor initiation, growth, invasion and
metastasis (21,22). CD133 is currently one of the best
markers for the detection of colon cancer stem cells (28–38).
As CXCR4 and CD133 have been implicated in the metastatic process
of malignant tumors (27,41), we investigated CXCR4 and CD133
expression in a collection of colon cancer cases that was
stratified towards patient outcome.
A number of functional studies investigating the
influence of CXCR4 expression and activation by its ligand SDF-1α
(CXCL12) have recently been performed, revealing that CXCR4 is
crucial for the adhesion, migration and invasion of tumor cells.
The expression of CXCR4 has been shown to be associated with the
dissemination of ovarian cancer (42), lymph node metastasis in breast
cancer (19) and oral squamous
cell carcinoma (20. The results of microarray analyses of cDNA have
shown that CXCR4 is also overexpressed in renal (43), esophageal (44) and pancreatic (13) cancer, indicating that CXCR4
expression plays a role in the tumorigenesis of these types of
cancer. A previous study used microarray screening of tissues from
patients with stage I or II colon cancer to identify CXCR4 as a
significant chemokine receptor. Of the 30 patients with tissues
showing a high level of expression of CXCR4, two and four
experienced local and distant recurrence, respectively. By
contrast, during a median follow-up period of 28 months, none of
the 27 patients with tissues showing a low level of CXCR4
expression experienced recurrence. CXCR4 expression was found to be
a significant prognostic factor for disease-free survival in
patients with stage I or II disease and for overall survival in
patients with stage IV by multivariate analysis adjusted by
clinicopathological characteristics (45). Schimanski et al reported
that, although the levels of expression of CXCR4 and CCR7 vary in
human colon carcinoma tissues and cell lines, only the expression
of CXCR4 has a significant correlation with stage of the disease
and lymph node and distant metastasis and an association with a
reduced 3-year survival rate (46). Ottaiano et al showed that
CXCR4 is expressed in 77.8% of stage II–III colorectal cancer
patients and that CXCR4 expression has a significant prognostic
value for disease-free survival using univariate analysis. The
authors also reported a significant association between CXCR4
expression and lymph node status (47).
Supporting these data, our clinicopathological study
revealed that patients with CXCR4-positive colon cancer had a
significantly poorer outcome than those with CXCR4-negative colon
cancer, suggesting that CXCR4 is a significant prognostic marker in
colon cancer patients. Of the 125 stage II or III colon cancer
patients, no significant correlation was observed between CXCR4
expression and gender, age, location of primary mass, pathological
grade, tumor size, AJCC stage, tumor invasion or lymph node status
(Table II). Ottaiano et al
reported that a significant association exists between CXCR4
expression and lymph nodal status (47), but our results demonstrated that no
significant correlation existed between CXCR4 expression and lymph
node status in stage II or III colon cancer patients. This
discrepancy may be due to inadequate patient numbers and the mixed
tumor stage.
CD133 is recognized as a marker of cancer and
organ-specific stem cells (21–39).
A number of previous studies have demonstrated that
CD133+, and not CD133−, cells derived from
human colon cancer tissues are the only cells that are able to
initiate tumors in immunodeficient mice. Certain studies isolated
cells from colorectal cancer that were capable of initiating tumors
and reported that the cells expressed CD133 and exhibited stem
cell-like properties (26,27,48).
Horst et al reported that the expression of CD133 in
colorectal cancer is an independent prognostic marker that was
correlated with poor survival in a stratified patient population
(49). Kojima et al
reported that CD133 expression was detected in only 29 of 189
tumors (15.3%) and that patients with CD133 overexpression had a
significantly poorer overall survival. However, CD133 expression
was not found to be an independent risk factor associated with
patient survival in multivariate analysis (50). With regard to stage IIIB colon
carcinoma patients, Li et al found that a higher percentage
of CD133+ cells was associated with poor prognosis in
patients with stage IIIB tumors (51). These contradicting results seem not
to provide enough evidence of the malignant potential of
CD133-positive cells. As cancer stem cells divide slowly, they are
considered to be resistant to most of the current chemotherapies
that target differentiated or highly proliferating tumor cells. Ong
et al provided evidence that the expression of CD133 is
associated with the poor response of colorectal cancer to
5-Fu-based chemotherapy, as well as with poor prognosis (52).
Our results showed that CD133 expression was
detected in 45 of the 125 tumors (36.0%) and that CD133+
cancer cells contributed to the progression of stage II and III
colon cancer, suggesting that CD133 is a significant prognostic
marker in colon cancer patients. Moreover, we found the CD133
antigen at the luminal surface of epithelial tumor glands with
shedding into the lumina. This result is in agreement with those of
previous studies in which CD133 was detected in embryonal tissue
and on the apical surface of the cultured colon cancer cell line
Caco-2 (53). Among the 125 stage
II or III colon cancer patients, no significant correlation was
found between CD133 expression and gender, age, location of primary
mass, pathological grade, tumor size, AJCC stage, tumor invasion or
lymph node status (Table II).
These contradicting results seem not to provide enough evidence of
the malignant potential of CD133 positive cells. A larger study
with a greater number of CD133-positive cases may show a more
marked association between CD133 expression and clinicopathological
features. Although the function of CD133 has not yet been
elucidated, the chemoresistance of CD133-positive cells may result
in the poor survival rate of patients with CD133-positive tumors
(54).
One possibility is that the number of CD133-positive
cells is increased during cancer progression, rather than having a
biological function associated with the malignancy of colon cancer.
In addition, we found that the tumor cells positive for CD133 also
tended to be positive for CXCR4. In the present study, we showed
that neither CXCR4 nor CD133 expression was able to predict lymph
node metastases in stage II–III colon cancer, but the combination
of the expression of CXCR4 and CD133 was able to predict lymph node
metastasis in stage II–III colon cancer; these data confirm that
CXCR4- and CD133-positive cells are more prone to metastasize to
the draining lymph nodes. Although only 29 (23.2%) CXCR4- and
CD133-positive cases were identified in our series and further
investigation in a larger series of CXCR4- and CD133-positive cases
may be necessary, cases which were positive for both CXCR4 and
CD133 expression had a poorer outcome than the other combinations
of CXCR4 and CD133 expression. In addition, CXCR4 and CD133
expression was observed more frequently in advanced cancers. The
chemoresistance of CXCR4- and CD133-positive cells may also be
associated with the poor survival of patients whose tumors are
positive for CXCR4 and CD133.
Furthermore, we analyzed the expression of CXCR4 and
CD133 in the related lymph nodes. The results suggested that,
compared with their primary lesions, the co-expression of the CXCR4
and CD133 proteins was significantly decreased in the MTLNs.
Specifically, there was no difference in the expression of CXCR4 in
the primary lesions and MTLNs, but the expression of CD133 was
downregulated in the subset of tumor cells following the lymph node
metastatic transition. Colon cancer may also encompass
heterogeneous subtypes that include CD133-positive cells with stem
cell-like properties and CD133-negative cells and several studies
have suggested that CD133 expression is not limited to
organ-specific stem cells (55–57).
One explanation for this phenomenon is that all the cancerous cells
in primary colon tumors are CD133+, so the cancer stem
cells are included in this population. CD133− cells may
then appear at the epithelial-mesenchymal transition and result in
metastasis (58). Shmelkov et
al proposed that the expression of CD133 is not limited to
tumor-initiating or intestinal stem cells and that, during
metastasis, CD133+ tumor cells generate
CD133− cells, which are more aggressive and also able to
initiate tumors in nude mice. The authors reported that 40% of
metastatic tumors in their study were CD133-negative, indicating
that CD133+ cells are not the cause of the tumors
(58).
Our results showed that, compared with the
expression of CD133 in the primary lesions, CD133 expression was
significantly decreased in the MTLNs. This result is consistent
with those of previous studies concerning CD133 expression in
metastatic tumors.
In conclusion, we demonstrated that the
co-expression of the CXCR4 and CD133 proteins is closely associated
with lymph node metastasis and poor prognosis in patients with
colon cancer. We also provide further evidence that the
co-expression of the CXCR4 and CD133 proteins in the tumor cell
population of colon cancer is specifically, although not
exclusively, significant in colon cancer progression. Furthermore,
the concomitant positive expression of CXCR4 and CD133 is an
independent predictor of survival in stage II–III colon cancer
patients. Confirmation of this observation in independent colon
cancer cohorts could lead to the improved targeting of conventional
cytotoxic therapies to the patient subgroups that are most likely
to benefit.
Acknowledgements
This study was supported by the
Guangdong Natural Science Foundation (grant no.
10251051501000008).
References
|
1.
|
Weir HK, Thun MJ, Hankey BF, et al: Annual
report to the nation on the status of cancer, 1975–2000, featuring
the uses of surveillance data for cancer prevention and control. J
Natl Cancer Inst. 95:1276–1299. 2003.
|
|
2.
|
Singh R, Lillard JW Jr and Singh S:
Chemokines: key players in cancer progression and metastasis. Front
Biosci (Schol Ed). 3:1569–1582. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Liekens S, Schols D and Hatse S:
CXCL12-CXCR4 axis in angiogenesis, metastasis and stem cell
mobilization. Curr Pharm Des. 16:3903–3920. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Arya M, Patel HR and Williamson M:
Chemokines: key players in cancer. Curr Med Res Opin. 19:557–564.
2003. View Article : Google Scholar
|
|
5.
|
Ali S and Lazennec G: Chemokines: novel
targets for breast cancer metastasis. Cancer Metastasis Rev.
26:401–420. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Murdoch C: CXCR4: chemokine receptor
extraordinaire. Immunol Rev. 177:175–184. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Phillips RJ, Burdick MD, Lutz M, Belperio
JA, Keane MP and Strieter RM: The stromal derived
factor-1/CXCL12-CXC chemokine receptor 4 biological axis in
non-small cell lung cancer metastases. Am J Respir Crit Care Med.
167:1676–1686. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Kawamata H, Furihata T, Omotehara F, et
al: Identification of genes differentially expressed in a newly
isolated human metastasizing esophageal cancer cell line, T.Tn-AT1,
by cDNA microarray. Cancer Sci. 94:699–706. 2003. View Article : Google Scholar
|
|
9.
|
Bachelder RE, Wendt MA and Mercurio AM:
Vascular endothelial growth factor promotes breast carcinoma
invasion in an autocrine manner by regulating the chemokine
receptor CXCR4. Cancer Res. 62:7203–7206. 2002.
|
|
10.
|
Kijima T, Maulik G, Ma PC, et al:
Regulation of cellular proliferation, cytoskeletal function, and
signal transduction through CXCR4 and c-Kit in small cell lung
cancer cells. Cancer Res. 62:6304–6311. 2002.PubMed/NCBI
|
|
11.
|
Gao Z, Wang X, Wu K, Zhao Y and Hu G:
Pancreatic stellate cells increase the invasion of human pancreatic
cancer cells through the stromal cell-derived factor-1/CXCR4 axis.
Pancreatology. 10:186–193. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Guleng B, Tateishi K, Ohta M, et al:
Blockade of the stromal cell-derived factor-1/CXCR4 axis attenuates
in vivo tumor growth by inhibiting angiogenesis in a vascular
endothelial growth factor-independent manner. Cancer Res.
65:5864–5871. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Koshiba T, Hosotani R, Miyamoto Y, et al:
Expression of stromal cell-derived factor 1 and CXCR4 ligand
receptor system in pancreatic cancer: a possible role for tumor
progression. Clin Cancer Res. 6:3530–3535. 2000.PubMed/NCBI
|
|
14.
|
Van der Meulen AA, Biber K, Lukovac S, et
al: The role of CXC chemokine ligand (CXCL)12-CXC chemokine
receptor (CXCR)4 signalling in the migration of neural stem cells
towards a brain tumour. Neuropathol Appl Neurobiol. 35:579–591.
2009.PubMed/NCBI
|
|
15.
|
Li W, Gomez E and Zhang Z:
Immunohistochemical expression of stromal cell-derived factor-1
(SDF-1) and CXCR4 ligand receptor system in hepatocellular
carcinoma. J Exp Clin Cancer Res. 26:527–533. 2007.PubMed/NCBI
|
|
16.
|
Chen GS, Yu HS, Lan CC, et al: CXC
chemokine receptor CXCR4 expression enhances tumorigenesis and
angiogenesis of basal cell carcinoma. Br J Dermatol. 154:910–918.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Barbero S, Bonavia R, Bajetto A, et al:
Stromal cell-derived factor 1alpha stimulates human glioblastoma
cell growth through the activation of both extracellular
signal-regulated kinases 1/2 and Akt. Cancer Res. 63:1969–1974.
2003.PubMed/NCBI
|
|
18.
|
Zeelenberg IS, Ruuls-Van Stalle L and Roos
E: The chemokine receptor CXCR4 is required for outgrowth of colon
carcinoma micrometastases. Cancer Res. 63:3833–3839.
2003.PubMed/NCBI
|
|
19.
|
Kato M, Kitayama J, Kazama S and Nagawa H:
Expression pattern of CXC chemokine receptor-4 is correlated with
lymph node metastasis in human invasive ductal carcinoma. Breast
Cancer Res. 5:144–150. 2003. View
Article : Google Scholar
|
|
20.
|
Uchida D, Begum NM, Almofti A, et al:
Possible role of stromal-cell-derived factor-1/CXCR4 signaling on
lymph node metastasis of oral squamous cell carcinoma. Exp Cell
Res. 290:289–302. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Singh A and Settleman J: EMT, cancer stem
cells and drug resistance: an emerging axis of evil in the war on
cancer. Oncogene. 29:4741–4751. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Todaro M, Francipane MG, Medema JP and
Stassi G: Colon cancer stem cells: promise of targeted therapy.
Gastroenterology. 138:2151–2162. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Boman BM and Wicha MS: Cancer stem cells:
a step toward the cure. J Clin Oncol. 26:2795–2799. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Wang J, Guo LP, Chen LZ, Zeng YX and Lu
SH: Identification of cancer stem cell-like side population cells
in human nasopharyngeal carcinoma cell line. Cancer Res.
67:3716–3724. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Horst D, Kriegl L, Engel J, Kirchner T and
Jung A: Prognostic significance of the cancer stem cell markers
CD133, CD44, and CD166 in colorectal cancer. Cancer Invest.
27:844–850. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
O’Brien CA, Pollett A, Gallinger S and
Dick JE: A human colon cancer cell capable of initiating tumour
growth in immunodeficient mice. Nature. 445:106–110.
2007.PubMed/NCBI
|
|
27.
|
Ricci-Vitiani L, Lombardi DG, Pilozzi E,
et al: Identification and expansion of human
colon-cancer-initiating cells. Nature. 445:111–115. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Murat A, Migliavacca E, Gorlia T, et al:
Stem cell-related ‘self-renewal’ signature and high epidermal
growth factor receptor expression associated with resistance to
concomitant chemoradiotherapy in glioblastoma. J Clin Oncol.
26:3015–3024. 2008.
|
|
29.
|
Hambardzumyan D, Squatrito M and Holland
EC: Radiation resistance and stem-like cells in brain tumors.
Cancer Cell. 10:454–456. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Clement V, Sanchez P, de Tribolet N,
Radovanovic I and Ruiz i Altaba A: HEDGEHOG-GLI1 signaling
regulates human glioma growth, cancer stem cell self-renewal, and
tumorigenicity. Curr Biol. 17:165–172. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Hambardzumyan D, Becher OJ and Holland EC:
Cancer stem cells and survival pathways. Cell Cycle. 7:1371–1378.
2008. View Article : Google Scholar
|
|
32.
|
Bao S, Wu Q, McLendon RE, et al: Glioma
stem cells promote radioresistance by preferential activation of
the DNA damage response. Nature. 444:756–760. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Griguer CE, Oliva CR, Gobin E, et al:
CD133 is a marker of bioenergetic stress in human glioma. PLoS One.
3:e36552008. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Eramo A, Lotti F, Sette G, et al:
Identification and expansion of the tumorigenic lung cancer stem
cell population. Cell Death Differ. 15:504–514. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Ma S, Chan KW, Hu L, et al: Identification
and characterization of tumorigenic liver cancer stem/progenitor
cells. Gastroenterology. 132:2542–2556. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Yin AH, Miraglia S, Zanjani ED, et al:
AC133, a novel marker for human hematopoietic stem and progenitor
cells. Blood. 90:5002–5012. 1997.PubMed/NCBI
|
|
37.
|
Monzani E, Facchetti F, Galmozzi E, et al:
Melanoma contains CD133 and ABCG2 positive cells with enhanced
tumourigenic potential. Eur J Cancer. 43:935–946. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Wright MH, Calcagno AM, Salcido CD,
Carlson MD, Ambudkar SV and Varticovski L: Brca1 breast tumors
contain distinct CD44+/CD24− and
CD133+ cells with cancer stem cell characteristics.
Breast Cancer Res. 10:R102008. View Article : Google Scholar : PubMed/NCBI
|
|
39.
|
Boman BM and Huang E: Human colon cancer
stem cells: a new paradigm in gastrointestinal oncology. J Clin
Oncol. 26:2828–2838. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40.
|
Matsumoto K, Shariat SF, Ayala GE, Rauen
KA and Lerner SP: Loss of coxsackie and adenovirus receptor
expression is associated with features of aggressive bladder
cancer. Urology. 66:441–446. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41.
|
Brabletz T, Jung A, Spaderna S, Hlubek F
and Kirchner T: Opinion: migrating cancer stem cells - an
integrated concept of malignant tumour progression. Nat Rev Cancer.
5:744–749. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
42.
|
Kajiyama H, Shibata K, Terauchi M, Ino K,
Nawa A and Kikkawa F: Involvement of SDF-1alpha/CXCR4 axis in the
enhanced peritoneal metastasis of epithelial ovarian carcinoma. Int
J Cancer. 122:91–99. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43.
|
Schrader AJ, Lechner O, Templin M, et al:
CXCR4/CXCL12 expression and signalling in kidney cancer. Br J
Cancer. 86:1250–1256. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
44.
|
Sasaki K, Natsugoe S, Ishigami S, et al:
Expression of CXCL12 and its receptor CXCR4 correlates with lymph
node metastasis in submucosal esophageal cancer. J Surg Oncol.
97:433–438. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
45.
|
Kim J, Takeuchi H, Lam ST, et al:
Chemokine receptor CXCR4 expression in colorectal cancer patients
increases the risk for recurrence and for poor survival. J Clin
Oncol. 23:2744–2753. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
46.
|
Schimanski CC, Schwald S, Simiantonaki N,
et al: Effect of chemokine receptors CXCR4 and CCR7 on the
metastatic behavior of human colorectal cancer. Clin Cancer Res.
11:1743–1750. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
47.
|
Ottaiano A, Franco R, Aiello Talamanca A,
et al: Overexpression of both CXC chemokine receptor 4 and vascular
endothelial growth factor proteins predicts early distant relapse
in stage II–III colorectal cancer patients. Clin Cancer Res.
12:2795–2803. 2006.PubMed/NCBI
|
|
48.
|
Todaro M, Alea MP, Di Stefano AB, et al:
Colon cancer stem cells dictate tumor growth and resist cell death
by production of interleukin-4. Cell Stem Cell. 1:389–402. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
49.
|
Horst D, Kriegl L, Engel J, Kirchner T and
Jung A: CD133 expression is an independent prognostic marker for
low survival in colorectal cancer. Br J Cancer. 99:1285–1289. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
50.
|
Kojima M, Ishii G, Atsumi N, Fujii S,
Saito N and Ochiai A: Immunohistochemical detection of CD133
expression in colorectal cancer: a clinicopathological study.
Cancer Sci. 99:1578–1583. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
51.
|
Li CY, Li BX, Liang Y, et al: Higher
percentage of CD133+ cells is associated with poor
prognosis in colon carcinoma patients with stage IIIB. J Transl
Med. 7:562009.PubMed/NCBI
|
|
52.
|
Ong CW, Kim LG, Kong HH, et al: CD133
expression predicts for non-response to chemotherapy in colorectal
cancer. Mod Pathol. 23:450–457. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
53.
|
Corbeil D, Röper K, Hellwig A, et al: The
human AC133 hematopoietic stem cell antigen is also expressed in
epithelial cells and targeted to plasma membrane protrusions. J
Biol Chem. 275:5512–5520. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
54.
|
Liu G, Yuan X, Zeng Z, et al: Analysis of
gene expression and chemoresistance of CD133+ cancer
stem cells in glioblastoma. Mol Cancer. 5:672006. View Article : Google Scholar : PubMed/NCBI
|
|
55.
|
Florek M, Haase M, Marzesco AM, et al:
Prominin-1/CD133, a neural and hematopoietic stem cell marker, is
expressed in adult human differentiated cells and certain types of
kidney cancer. Cell Tissue Res. 319:15–26. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
56.
|
Pfenninger CV, Roschupkina T, Hertwig F,
et al: CD133 is not present on neurogenic astrocytes in the adult
subventricular zone, but on embryonic neural stem cells, ependymal
cells, and glioblastoma cells. Cancer Res. 67:5727–5736. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
57.
|
Weigmann A, Corbeil D, Hellwig A and
Huttner WB: Prominin, a novel microvilli-specific polytopic
membrane protein of the apical surface of epithelial cells, is
targeted to plasmalemmal protrusions of non-epithelial cells. Proc
Natl Acad Sci USA. 94:12425–12430. 1997. View Article : Google Scholar
|
|
58.
|
Shmelkov SV, Butler JM, Hooper AT, et al:
CD133 expression is not restricted to stem cells, and both
CD133+ and CD133− metastatic colon cancer
cells initiate tumors. J Clin Invest. 118:2111–2120.
2008.PubMed/NCBI
|