Introduction
Interstitial pulmonary fibrosis is thought to arise
as a result of a response to persistent lung injury and
inflammation. An oxidant-antioxidant imbalance in the lower
respiratory tract has been proposed as a cause of pulmonary
fibrosis (1). Cigarette smoking is
causally related to the development of desquamative interstitial
pneumonia, pulmonary Langerhans cell histiocytosis and idiopathic
pulmonary fibrosis (IPF). However, cigarette smoke exposure (CSE)
appears to protect against the development of hypersensitivity
pneumonitis, a lymphocytic alveolitis provoked by the exposure to
organic particles, and sarcoidosis, an inflammatory disorder of
unknown etiology (2). The
mechanisms by which CSE affects in one or another manner the
inflammatory and fibrotic responses in the lung remain to be
elucidated. Smoking is a strong oxidant which has a role in the
development of pulmonary fibrosis (3).
Bleomycin (BLM) causes pulmonary fibrosis by
increasing the free oxygen radical content which causes severe
pulmonary damage. An imbalance between oxidants and antioxidants
has been proposed in the pathogenesis of BLM-induced lung fibrosis.
BLM-induced free oxygen radical production has been shown to
stimulate fibroblasts to secrete collagen, and to proliferate and
differentiate into myofibroblasts, resulting in a histological
appearance that is similar to IPF (4).
The purpose of this study was to evaluate the effect
of smoking on oxidative injury in lung tissue of rats with
BLM-induced pulmonary fibrosis.
Materials and methods
Adult male Sprague-Dawley rats (body weight, 315–375
g) were maintained at 22°C and 20–50% humidity, with a 12-h light
period and supplied with a standard laboratory chow diet and water
ad libitum. The animals were assigned to three groups of
rats (n=10). One group, the control, received intratracheal normal
saline; another group (IT) received intratracheally one dose of BLM
(Nippon Kayaku Co., Ltd., Tokyo, Japan) 0.5 U/100 g body weight in
0.3 ml 0.15 M sterile NaCl. The rats in the third group received
intratracheal BLM and were later placed in a plastic cage (40 cm x
26 cm x 16 cm) two times a day and exposed to tobacco smoke for 4
weeks. BLM solution was prepared immediately before administration
and was administered intraperitoneally directly into the trachea
under light anesthesia with sodium pentobarbital (50 mg/kg body
weight).
After 4 weeks, the animals were sacrificed at the
end of the experiment. The right lungs were removed and were shock
frozen immediately after resection and were stored at −80°C until
use. Each lung was excised, rinsed in ice-cold physiological saline
and homogenized in Tris-HCl buffer, using a tissue homogenizer. The
resultant whole tissue homogenate was used for biochemical
measurements.
The levels of malondialdehyde (MDA), nitric oxide
(NO) and the activity superoxide dismutase (SOD), xanthine oxidase
(XO) in the tissue samples were analyzed using the following
methods.
The MDA level was estimated by the double-heating
method of Wasowicz et al (5). The concentration of MDA was
calculated by the absorbance coefficient of the MDA-TBA complex
(absorbance coefficient e = 1.56x105 l/mol/cm) and
expressed as nmol/g for the lung. Total SOD activity (Cu/Zn and Mn)
was determined using the method of Sun et al (6). Activity was expressed as U/mg for the
lung.
NO was measured using a colorimetric kit method
(cat. no. CM780001, Cayman Chemical Co., USA). The concentration of
NO was expressed as μM/g protein. XO activity was determined by the
method of Prajda and Weber (7).
This activity was expressed as U/g protein.
After sacrifice, each left lung was fixed in a
buffered 10% formalin solution for 24 h and embedded in paraffin.
Longitudinal sections of the lungs were stained with hematoxylin
and eosin (H&E) and were examined for pulmonary fibrosis. Each
successive field was individually assessed for the severity of
interstitial fibrosis using the semi-quantitative grading system
described by Ashcroft et al (8). The entire lung section was reviewed
at a magnification of x100. For each of the 30–35 microscopic
fields needed to review the section, a score ranging from 0
(normal) to 8 (total fibrosis) was assigned.
Statistical analysis
Results are expressed as the means ± SD. A
non-parametric analysis of variance (Kruskal-Wallis method) with
post-hoc Bonferroni’s correction was used to determine any
significant variance among the five groups. The Mann-Whitney U test
was performed for comparison between groups. All analyses were
carried out using the SPSS statistical software package, and a
probability value of <0.05 was considered to be statistically
significant.
Ethical approval
Ethical approval was provided from the Ethics
Committee of Meram Medical Faculty, affiliated at the time of the
study to Selcuk University, Konya, Turkey.
Results
There was more intensive fibrosis in the IT and IT-S
groups than that in the control samples (P<0.001). There was no
significant difference between the IT and IT-S groups with respect
to fibrosis score (P>0.05).
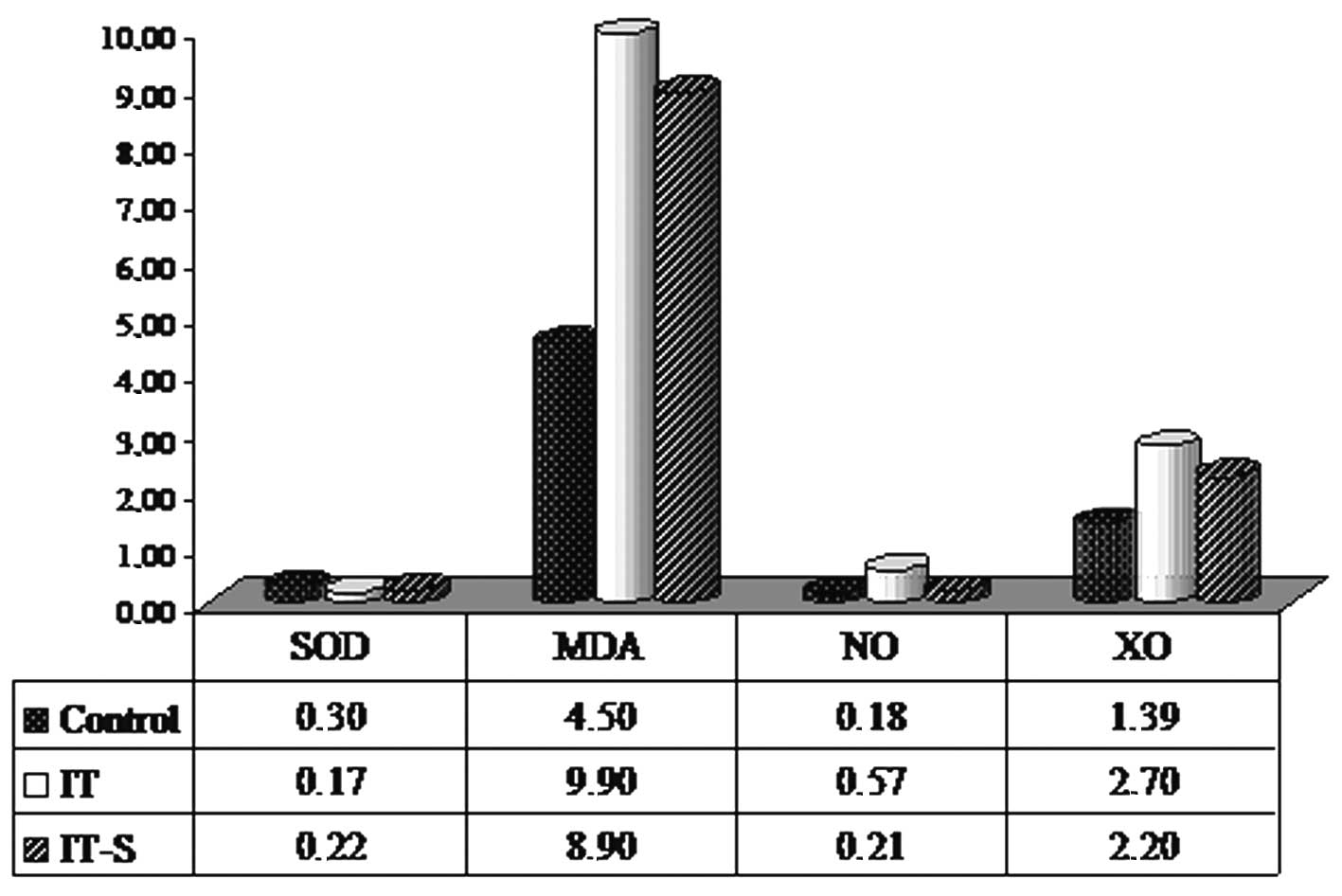
The results of oxidative stress marker levels of the
control, IT and IT-S groups are shown in Table I and Fig. 1. When compared with the control
group, oxidative stress was increased significantly in the
BLM-administered IT group (P<0.05). When the effect of smoking
on oxidative stress in the BLM-treated groups was evaluated, a
significant increase in SOD activity and a significant decrease in
NO level (determinants of decreased oxidative stress) in the IT-S
group compared to the IT group. There was no statistically
significant decrease in MDA level and XO activity in the IT-S group
when compared to the IT group. In Table II a comparison between groups
carried out with the Mann-Whitney U test is shown.
 | Table I.Levels of oxidative stress markers in
lung tissue. |
Table I.
Levels of oxidative stress markers in
lung tissue.
| Group | Lung
|
|---|
| SOD | MDA | NO | XO |
|---|
| C | 0.3±0.07 | 4.5±0.7 | 0.18±0.05 | 1.39±0.5 |
| IT | 0.17±0.04 | 9.9±2.8 | 0.57±0.2 | 2.7±0.7 |
| IT-S | 0.22±0.04 | 8.9±2.9 | 0.21±0.1 | 2.2±0.7 |
 | Table II.P-values for comparison between
groups. |
Table II.
P-values for comparison between
groups.
| SOD | MDA | NO | XO |
|---|
| C vs. IT | <0.001 | <0.001 | <0.002 | <0.002 |
| IT vs. IT-S | <0.05 | NS | <0.002 | NS |
Discussion
The results of the present study indicated increased
oxidative stress in the lung tissue of rats that developed after
BLM-induced pulmonary fibrosis, and CSE reduced the oxidative
injury induced by BLM.
There is limited information on the role of tobacco
smoking in the development and outcome of BLM-induced lung
fibrosis. The mechanisms by which CSE affects in one way or another
the incidence or severity of various interstitial lung diseases are
not entirely clear (3). Several
studies have aimed to reveal whether a smoking history increases
the risk of BLM-induced lung fibrosis. An increased risk in smokers
is strongly suggested by Lower et al (9). These authors showed radiographic
alterations consistent with BLM-induced lung fibrosis in 55% of
smoking patients receiving BLM compared with 0% of non-smokers. An
earlier report by Takada et al (10) found that an intratracheal
administration of BLM induced fibrotic changes in the lungs of
hamsters exposed to cigarette smoke (CS). It was also shown that
exposure to CS increased the number of myofibroblasts in alveolar
septa of guinea pigs and potentialized pulmonary injury induced by
BLM (3).
Various studies have shown a high percentage of ever
smokers among individuals with IPF and that CSE is an independent
risk factor for the development of IPF (11,12).
However, cigarette smoking has only recently been shown to be
associated with improved survival in this disease (13). Osanai et al (14) found that tobacco smoke reduced the
fibrotic response to BLM. In addition, a number of clinical and
experimental studies suggest that CSE reduces the frequency of
radiation-induced pneumonitis (15,16).
Theoretically, the combination of CS inhalation and
BLM should have a synergistic effect on the development of
BLM-induced lung injury. We hypothesized that CS contributes to the
development and outcome of BLM-induced lung fibrosis by increasing
oxidative stress. Our study tested this hypothesis in rats, and we
observed that in contrast to this hypothesis, CSE had no additional
effect on the development of fibrosis.
Reduced oxidative stress after CS exposure in rats
with BLM-induced lung injury can be explained by hyperoxia and
reduced oxidative injury caused by hyperoxia. Firstly, one of the
few indicators of improved prognosis in IPF is active cigarette
smoking at the time of diagnosis. This phenomenon may be related to
the ∼11 mg of carbon monoxide (CO) (or 1–6% CO gas) inhaled with
each cigarette (17). Secondly,
smokers are now known to have increased airway expression of heme
oxygenase-1 (HO-1). HO-1 catalyzes heme degradation to generate CO,
biliverdin and free iron. Extensive data suggest that CO can often
substitute for HO-1 (18). The
toxic properties of CO are well known in the field of pulmonary
medicine. The toxic actions of CO relate to its high affinity for
hemoglobin (240-fold greater than that of O2). CO
replaces O2 rapidly from hemoglobin, causing tissue
hypoxia (19). Only recently has
it become known that, at very low concentrations, CO participates
in many physiological reactions. CO exposure of 100–250 parts per
million (ppm) stimulate physiological effects without apparent
toxicity. The majority of endogenous CO production originates from
active heme metabolism. CSE represents a major source of chronic
low level exposure to CO (20).
Inhaled CO initially targets alveolar macrophages
and respiratory epithelial cells. CO has been shown to decrease
proliferation of fibroblasts (21). CO may limit the generation of ROS,
lower the presence of free metal ions and downregulate
pro-inflammatory cytokines. Exogenous administration of low
concentrations of CO provided protection against oxidative stress
in a model of inflammation (22).
A low concentration of CO was also found to provide protection from
lipopolysaccha-ride-induced injury via directly inhibiting lipid
peroxidation and decreasing ROS. Liu et al (23) investigated the effect of CO
inhalation on oxidative stress and demonstrated that the MDA level
and myeloperoxidase activity increased significantly in rats with
lung injury related to lipopolysaccharide; and CO inhalation
significantly decreased the MDA and myeloperoxidase accumulation
and significantly increased the SOD activity in lungs which
indicates that CO repairs oxidative lung injury.
Hyperoxia generates ROS, e.g., superoxide anion and
hydrogen peroxide, which can injure the lung (24). Rats exposed to hyperoxia in the
presence of a low concentration of CO exhibit less lung injury than
control rats exposed to oxygen alone (25). A similar study by Clayton et
al (26) demonstrated a
statistically significant reduction in pulmonary edema upon
exposure to CO and hyperoxia. Since BLM is assumed to induce its
toxicity partially by the induction of free radicals, the
administration of high inspired oxygen could be hazardous (27). In hamsters treated with BLM and 70%
oxygen for 72 h, the mortality was 90% compared with 15% in those
animals that received BLM only (28). Zhou et al (21) documented that CO may protect
against BLM-induced lung injury in mice. Mice treated with CO and
BLM were found to have less severe lung injury than mice treated
with BLM alone. In humans, clear data showing an increased risk of
BLM-induced lung injury with concomitant oxygen supplementation are
lacking; however, because of the data obtained from animal studies,
hyperoxia is discouraged during BLM treatment (29).
In conclusion, the inhalation of cigarette smoke,
which is similar to the exogenous administration of low
concentrations of CO, can provide protection against oxidative
stress in the lung tissue of rats with bleomycin-induced pulmonary
fibrosis.
References
|
1
|
Kinnula VL, Fattman CL, Tan RJ and Oury
TD: Oxidative stress in pulmonary fibrosis: a possible role for
redox modulatory therapy. Am J Respir Crit Care Med. 172:417–422.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Murin S, Smith BK and Matthay R: Other
smoking affected pulmonary diseases. Clin Chest Med. 21:121–137.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cisneros-Lira J, Gaxiola M, Ramos C,
Selman M and Pardo A: Cigarette smoke exposure potentiates
bleomycin-induced lung fibrosis in guinea pigs. Am J Physiol Lung
Cell Mol Physiol. 285:L949–L956. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vlalov SL, Gabbiani G and Kapanci Y: Rat
alveolar myofibro-blasts acquire alpha-smooth muscle actin
expression during bleomycin-induced pulmonary fibrosis. Am J
Pathol. 143:1754–1765. 1993.
|
|
5
|
Wasowicz W, Nève S and Peretz A: Optimized
steps in fluorometric determination of thiobarbituric acid-reactive
substances in serum: importance of extraction pH and influence of
sample preservation and storage. Clin Chem. 39:2522–2526.
1993.PubMed/NCBI
|
|
6
|
Sun Y, Oberley LW and Ying L: A simple
method for clinical assay of superoxide dismutase. Clin Chem.
34:497–500. 1988.PubMed/NCBI
|
|
7
|
Prajda N and Weber G: Malignant
transformation-linked imbalace: decreased XO activity in hepatomas.
FEBS Lett. 59:245–249. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ashcroft T, Simpson JM and Timbrell V:
Simple method of estimating severity of pulmonary fibrosis on a
numerical scale. J Clin Pathol. 41:467–470. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lower EE, Strohofer S and Baughman RP:
Bleomycin causes alveolar macrophages from cigarette smokers to
release hydrogen peroxide. Am J Med Sci. 295:193–197. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Takada K, Takahashki K, Sato S and Yasui
S: Cigarette smoke modifies bleomycin-induced lung injury to
produce lung emphysema. Tohoku J Exp Med. 153:137–144. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Schwartz DA, Helmers RA, Galvin JR, et al:
Determinants of survival in idiopathic pulmonary fibrosis. Am J
Respir Crit Care Med. 149:450–454. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Baumgartner KB, Samet J, Stidley CA, et
al: Cigarette smoking: a risk factor for idiopathic pulmonary
fibrosis. Am J Respir Crit Care Med. 155:242–248. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
King TE, Tooze JA, Schwarz MI, Brown KR
and Cherniack RM: Predicting survival in idiopathic pulmonary
fibrosis: scoring system and survival model. Am J Respir Crit Care
Med. 164:1171–1181. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Osanai K, Takahashi K, Suwabe A, et al:
The effect of cigarette smoke on bleomycin-induced pulmonary
fibrosis in hamsters. Am Rev Respir Dis. 138:1276–1281. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Johansson S, Bjermer L, Franzen L and
Henriksson R: Effects of ongoing smoking on the development of
radiation-induced pneumonitis in breast cancer and oesophagus
cancer patients. Radiother Oncol. 49:41–47. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nilsson K, Henriksson R, Cai YQ, Hellstrom
S, Hornqvist BS and Bjermer L: Effects of tobacco-smoke on
radiation-induced pneumonitis in rats. Int J Radiat Biol.
62:719–727. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jarvis MJ: Trends in sales weighted tar,
nicotine, and carbon monoxide yields of UK cigarettes. Thorax.
56:960–963. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Morse D: The role of heme oxygenase-1 in
pulmonary fibrosis. Am J Respir Cell Mol Biol. 29:S82–S86.
2003.PubMed/NCBI
|
|
19
|
Piantadosi CA: Carbon monoxide poisoning.
N Engl J Med. 347:1054–1055. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Vremen HJ, Wong RJ and Stevenson DK:
Carbon monoxide in breath, blood, and other tissues. Carbon
Monoxide Toxicity. Penney DG: CRC Press; FL: pp. 19–60. 2000,
View Article : Google Scholar
|
|
21
|
Zhou Z, Song R, Fattman CL, et al: Carbon
monoxide suppresses bleomycin-induced lung fibrosis. Am J Pathol.
166:27–37. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ng CS, Wan S and Yim AP: Pulmonary
ischemia-reperfusion injury: role of apoptosis. J Eur Res.
25:356–363. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu SH, Ma K, Xu B and Xu XR: Carbon
monoxide inhalation protects lung from lipopolysaccharide-induced
injury in rat. Sheng Li Xue Bao. 58:483–489. 2006.PubMed/NCBI
|
|
24
|
Brigham KL and Meyrick B: Endotoxin and
lung injury. Am Rev Respir Dis. 133:913–927. 1986.PubMed/NCBI
|
|
25
|
Otterbein LE, Mantell LL and Choi AM:
Carbon monoxide provides protection against hyperoxic lung injury.
Am J Physiol. 276:L688–L694. 1999.PubMed/NCBI
|
|
26
|
Clayton CE, Carraway MS, Suliman HB, et
al: Inhaled carbon monoxide and hyperoxic lung injury in rats. Am J
Physiol Lung Cell Mol Physiol. 281:L949–L957. 2001.PubMed/NCBI
|
|
27
|
Blom-Muilwijk MC, Vriesendorp R, Veninga
TS, et al: Pulmonary toxicity after treatment with bleomycin alone
or in combination with hyperoxia. Br J Anaesth. 60:91–97. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tryka AF, Skornik WA, Godleski JJ and
Brain JD: Potentiation of bleomycin-induced lung injury by exposure
to 70% oxygen. Am Rev Respir Dis. 126:1074–1076. 1982.PubMed/NCBI
|
|
29
|
Sleijfer S: Bleomycin-induced pneumonitis.
Chest. 120:617–624. 2001. View Article : Google Scholar
|