Introduction
Lung cancer is the leading cause of cancer-related
death in the United States and throughout the world in both men and
women (1,2). Due to diagnosis in late disease
stages and the poor treatment efficacy of metastatic disease,
overall survival is >15% and has not improved substantially in
the last 30 years (3). Tyrosine
kinase inhibitors (TKIs) targeting epidermal growth factor receptor
(EGFR), including gefitinib and erlotinib, have become the standard
first-line therapy for patients with advanced non-small cell lung
cancers (NSCLCs) harboring activating EGFR mutations (4,5).
However, almost all patients eventually develop resistance to EGFR
TKIs.
Laminin 5 (Ln5, also known as laminin 332) is an
extracellular matrix (ECM) protein that plays an important role in
cell migration and tumor invasion (6,7). It
has a cruciform structure with one long arm and three short arms.
The coiled-coil structure of the long arm is formed by three chains
(α3, β3 and γ2) covalently linked via interchain disulfide bonds
(8). The rod-like regions in the
short arms are composed of EGF-like repeats intercalated with
globular domains (9). Given the
multi-domain architecture of Ln5, it seems conceivable that other
receptors in addition to integrins interact with one of its many
potential ligand sites to mediate its diverse cellular functions,
including activation of EGFR signaling.
EGFR is a key mediator of oncogenesis in NSCLCs,
with its activation inducing tumor proliferation and growth,
angiogenesis, invasion and metastasis, and inhibiting apoptosis
(10). Activation of Akt (murine
thymoma viral oncogene homolog) is one of the mechanisms that
mediate the effects of EGFR (11).
The Akt pathway regulates diverse biological functions (12). The tumor-suppressor gene
PTEN negatively regulates the PI3K/Akt signaling pathway
(13).
Interactions between Ln5 and PTEN, phospho-EGFR
(p-EGFR) and phospho-Akt (p-Akt) in patients with NSCLC are not
well understood at the clinical level. Thus, we measured the
expression levels of Ln5, PTEN, p-EGFR and p-AKT and analyzed their
relationships to prognosis. The results may be helpful for
determining the relationships between these factors and potential
prognostic factors and may have important implications for
individualized therapy.
Materials and methods
Patient selection
A total of 98 tissue samples were obtained from the
tumor bank of Guangdong Lung Cancer Institute (Guangzhou, China)
between 2004 and 2006. All specimens were collected after informed
consent was obtained. Data on histological type, clinical stage,
smoking status, gender and patient age were collected from medical
records. Patients were followed through telephone calls or
re-examination of records by the hospital follow-up group. Survival
was determined from the date of surgical resection until the date
of the last time of follow-up (August 1, 2011). The median
follow-up time for overall survival was 53.9 months. A total of
46/98 patients in the study (46.9%) died during this period.
Antibody selection and
immunohistochemistry
We immunohistochemically examined the protein
expression of Ln5, PTEN, p-EGFR and p-Akt in 98 frozen tumor
samples from the lung cancer bank. Frozen sections (6 to 8 μm
thick) were prepared, immediately fixed through incubation in cold
methanol for 10 min and then air-dried. These sections were washed
in PBS. Subsequently, endogenous peroxidase activity was blocked
through incubation with 3% H2O2 for 10 min.
Sections were incubated overnight at 4°C with antibodies against
human Ln5 (dilution 1:1,000; Abcam, Cambridge, UK), PTEN (1:80;
Fuzhou Maixin Biotechnology Development, Co., Fuzhou, China),
phospho-EGFR (Tyr 1086, 1:100; Cell Signaling Technology, Inc.,
Danvers, MA, USA) and phospho-Akt (Ser 473, 1:100; Cell Signaling
Technology, Inc.), then rinsed with PBS and subsequently treated
with components of a ready-to-use Histostain-Plus secondary
antibody kit (Shenzhen Jingmei Biotech, Co, Ltd., Shenzhen, China).
Finally, the chromogenic substrate 3,3-diaminobenzidine
tetrahydrochloride (DAB) was added. The specimens were
counterstained with hematoxylin, mounted and examined with a BX50
light microscope (Olympus). Negative controls were treated with PBS
instead of primary antibody to verify specificity.
Two authors (Q.X. Lin and S.J. An), who were blinded
to clinical details of the specimens, reviewed all of the slides
simultaneously. When the opinions of the two evaluators differed, a
consensus was reached through discussion.
Assessment of immunohistochemistry
The intensity of tissue staining was scored on a
semi-quantitative 0–3 scale (with 0 representing no staining and 3
the strongest staining). Aberrant expression of PTEN, p-EGFR and
p-AKT was judged by cytoplasmic staining. The expression pattern of
Ln5 was assessed as follows: 3, continuous linear immuno staining
in the basement membrane; 2, continuous and discontinuous linear
immunostaining in the same specimen; 1, discontinuous linear
immunostaining; and 0, no immunostaining. Immunohistochemistry
results were then dichotomized as negative (score =0) or positive
(score >0).
Statistical analysis
The relationships between Ln5 and clinical
parameters and PTEN, p-EGFR and p-Akt expression levels were
analyzed by the χ2 test. Kaplan-Meier survival curves
and the log-rank test were used to analyze overall survival. A Cox
regression model was used to analyze the relationships between the
influencing factors and patient prognosis.
Results
Patients and protein expression
All patients enrolled in this study had
histologically confirmed NSCLC (Table
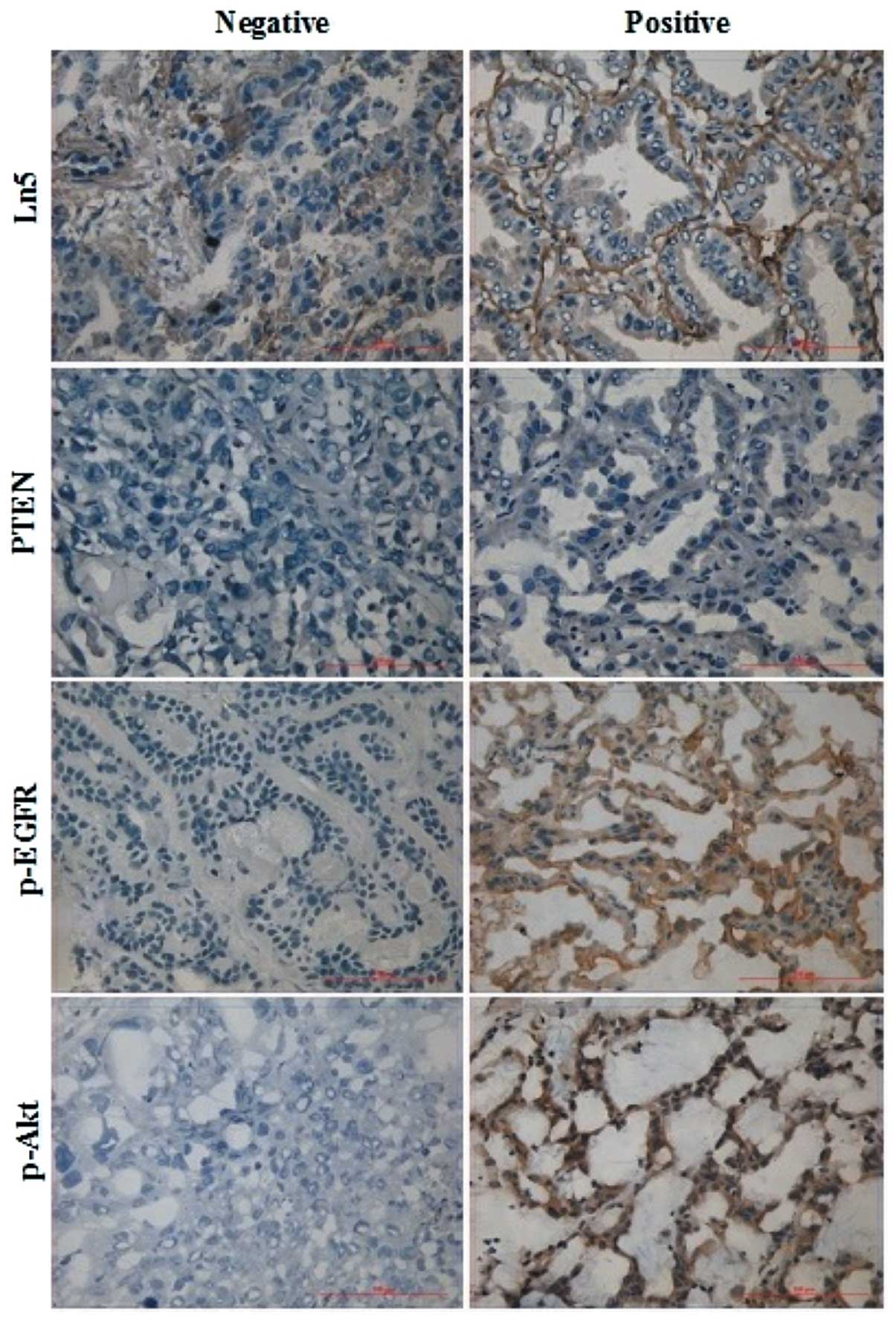
I). Ln5 and p-EGFR expression levels were successfully measured
in 98 patients (Fig. 1). Detection
procedures for PTEN and p-Akt failed for two and three slides,
respectively. These slides were excluded from further analyses.
Ln5, p-EGFR and p-Akt were detected in 61.2 (60/98), 60.2 (59/98)
and 45.3% (43/95) of patients with NSCLC, respectively. Loss of
PTEN expression was found in 67.7% of tumors (65/96).
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Variable | Group | n (%) |
|---|
| Gender | Male | 56 (57.1) |
| Female | 42 (42.9) |
| Age (years) | Mean | 56.7 |
| Range | 26–77 |
| Cigarette
smoking | No | 60 (61.2) |
| Yes | 38 (38.8) |
| Histological
status | AC | 62 (63.3) |
| SCC | 24 (24.5) |
| ASC | 5 (5.1) |
| LCC | 7 (7.1) |
| Stage | I | 47 (48.0) |
| II | 14 (14.3) |
| III | 27 (27.6) |
| IV | 10 (10.2) |
| Follow-up status | Survival | 52 (53.1) |
| Death | 46 (46.9) |
| Total | | 98 |
Relationships between Ln5 expression and
clinical parameters
We analyzed the relationships between Ln5 expression
levels and clinical parameters (Table
II). Ln5 expression was related to patient gender and
histology. Positive Ln5 expression was more common in males than in
females (69.6 vs. 50.0%, respectively; χ2=3.901;
P=0.048) and less common in adenocarcinomas (AC) than in tumors of
other histological types [53.2 vs. 75.0%, respectively;
χ2=4.549 (two-sided test); P=0.033]. There was no
relationship between Ln5 expression and smoking status or tumor
stage (Table II).
 | Table II.Relationships between Ln5 and clinical
parameters (n=98). |
Table II.
Relationships between Ln5 and clinical
parameters (n=98).
| Variable | Negative, n (%) | Positive, n (%) | χ2 | P-value | OR (95% CI) |
|---|
| Gender | | | 3.901 | 0.048 | 0.436
(0.190–1.001) |
| Male | 17 (30.4) | 39 (69.6) | | | |
| Female | 21 (50.0) | 21 (50.0) | | | |
| Smoking status | | | 0.098 | 0.755 | 1.143
(0.495–2.640) |
| No | 24 (40.0) | 36 (60.0) | | | |
| Yes | 14 (36.8) | 24 (63.2) | | | |
| Histology | | | 4.549 | 0.033 | 2.636
(1.067–6.513) |
| AC | 29 (46.8) | 33 (53.2) | | | |
| Others | 9 (25.0) | 27 (75.0) | | | |
| Stage | | | 0.500 | 0.480 | 0.740
(0.321–1.705) |
| I+II | 22 (36.1) | 39 (63.9) | | | |
| III+IV | 16 (43.2) | 21 (56.8) | | | |
Co-alteration of Ln5 and PTEN, p-EGFR and
p-Akt
The associations between PTEN, p-EGFR and p-Akt have
been well studied. Therefore, we focused on their associations with
Ln5 (Table III). Positive Ln5
expression was less common in the p-Akt-positive group than in the
p-Akt-negative group [46.5 vs. 73.1%; χ2=6.985
(two-sided test); P=0.008]. No correlations were found between Ln5
and PTEN or p-EGFR.
 | Table III.Relationships between Ln5 and PTEN,
p-EGFR and p-Akt. |
Table III.
Relationships between Ln5 and PTEN,
p-EGFR and p-Akt.
| Variable | Ln5 negative (%) | Ln5 positive (%) | χ2 | P-value | OR (95% CI) |
|---|
| PTEN negative | 23 (35.4) | 42 (64.6) | 0.847 | 0.357 | 0.665
(0.278–1.589) |
| positive | 14 (45.2) | 17 (54.8) | | | |
| p-EGFR negative | 14 (35.9) | 25 (64.1) | 0.226 | 0.634 | 0.817
(0.354–1.883) |
| positive | 24 (40.7) | 35 (59.3) | | | |
| p-Akt negative | 14 (26.9) | 38 (73.1) | 6.985 | 0.008 | 0.320
(0.136–0.755) |
| positive | 23 (53.5) | 20 (46.5) | | | |
Prognostic significance of Ln5, PTEN,
p-EGFR and p-Akt
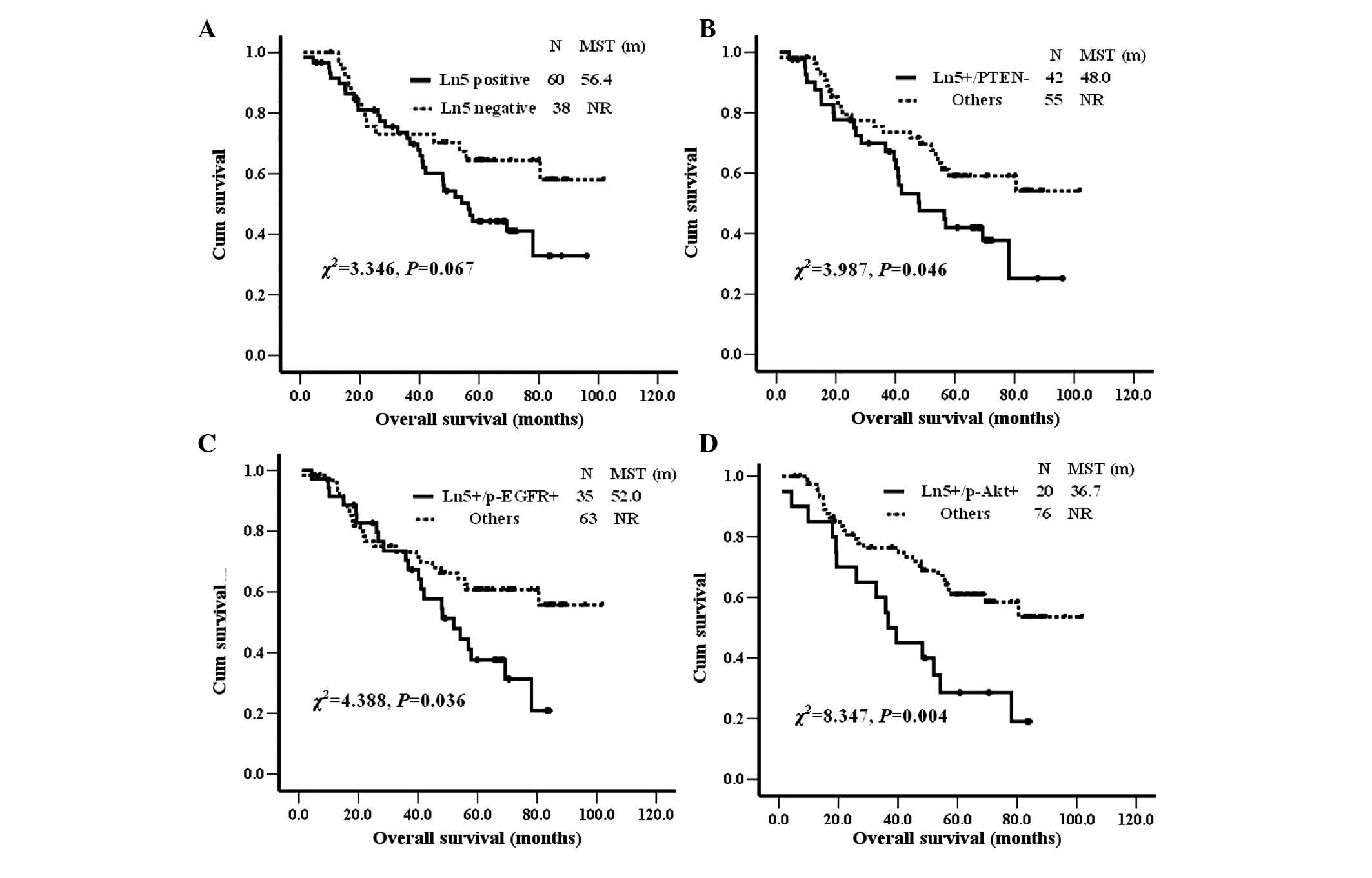
Ln5, PTEN, p-EGFR and p-Akt were subjected to
survival analysis alone and in combination (Fig. 2). Ln5-positive patients had a
marginally shorter survival time than Ln5-negative patients [median
survival time 56.4 months vs. not reached; χ2=3.346;
P=0.067 (Kaplan-Meier analysis, log-rank test)]. There were no
survival differences when PTEN, p-EGFR and p-Akt expression were
analyzed alone. However, when combined with Ln5, all of them showed
statistically significant differences between positive and negative
patients (Fig. 2). Overall
survival in NSCLC patients was significantly shorter in patients
with positive expression of Ln5 plus loss of PTEN expression,
positive expression of Ln5 plus p-EGFR and positive expression of
Ln5 plus p-AKT than in other patients. All of the analyzed clinical
parameters (gender, smoking status, histology and stage) and
molecules (Ln5, PTEN, p-EGFR, p-Akt, Ln5 plus PTEN, Ln5 plus p-EGFR
and Ln5 plus p-Akt) were entered into a multivariate analysis. Cox
regression analysis showed that stage, Ln5 plus Akt and PTEN were
the three most independent prognostic factors in patients with
NSCLC (χ2=27.906; P<0.0005; forward: Wald; P=0.05,
entry; P=0.10, removal) (Table
IV).
 | Table IV.Multivariate analysis of overall
survival. |
Table IV.
Multivariate analysis of overall
survival.
| Variable(s) | Wald | P-value | OR | 95% CI |
|---|
| Stage | 12.557 | 0.000 | 1.769 | 1.290–2.425 |
| PTEN | 6.167 | 0.013 | 0.400 | 0.195–0.825 |
| Ln5 plus p-Akt | 12.840 | 0.000 | 3.384 | 1.737–6.593 |
Discussion
Interactions between tumor cells and laminin or
other components of the extracellular matrix have been shown to
play an important role in tumor invasion and metastasis (14). Previous studies have shown that Ln5
is frequently expressed at the invasive front of several types of
cancers, including colorectal, gastric, pancreatic and breast
adenocarcinomas; uterine, cervical and oral squamous cell
carcinomas; malignant melanoma; and small-sized lung
adenocarcinomas (maximum dimension, ≤2 cm) and that overexpression
of Ln5 is associated with poor patient prognosis (15). PTEN, p-EGFR and p-Akt play
important roles in tumorigenesis. Yet, the relationships among them
in NSCLC patients have not been clarified. Our study revealed for
the first time that patients with co-alteration of Ln5 plus PTEN,
p-EGFR or p-Akt have poorer overall survival.
It has been reported that gefitinib inhibits the
growth of hepatocellular carcinoma cells, Ln5 reduces the ability
of gefitinib to inhibit cell growth and the addition of exogenous
Ln5 has no effect on p-EGFR but restores p-Akt (16). Another in vitro study
performed using A431 cutaneous squamous cell carcinoma cells
reported that Ln5-γ2 siRNA significantly suppressed EGF-stimulated
A431 cell invasion (17). In
addition, the introduction of Ln5 may activate a survival signal
through EGF-independent EGFR activity in certain types of human
lung adenocarcinoma cell lines (18). These results suggest that the
signaling pathways mediating carcinoma cell survival, growth and
invasion resulting from the action of Ln5 and EGF share common
downstream signal transduction molecules, such as p-Akt in some
tumors. Our study of lung cancer tumor tissues indicates that Ln5
expression has no relationship with p-EGFR, but has a strong
relationship with p-Akt.
In the present study, Ln5 expression was negatively
correlated with p-Akt expression. p-Akt-negative patients were more
frequently positive for Ln5 expression than p-Akt-positive
patients. Further analysis of the relationship between p-Akt and
p-EGFR demonstrated that p-Akt was positively correlated with
p-EGFR. p-Akt is a downstream signal transduction molecule in many
pathways. These results suggest that activation of p-Akt primarily
results from the upstream activation of p-EGFR and that activation
of p-Akt can have a negative feedback effect on the expression of
Ln5. The biological significance of Ln5 in invading tumor cells is
controversial and appears to be somewhat tumor-specific (19). Although Ln5 is negatively
correlated with p-Akt, some patients simultaneously express both
Ln5 and p-Akt. Individualized treatment is an attractive challenge
that may allow for safer and more effective treatment of human
disease (20). This phenomenon
suggests the existence of complicated relationships between
different therapy-targeted genes.
Survival analysis showed that Ln5 expression and
loss of PTEN expression were associated with a trend toward worse
prognosis in NSCLC patients. p-EGFR and p-Akt had no prognostic
significance when analyzed separately. However, when combined with
Ln5, they both showed prognostic significance. The multiple Cox
regression analysis showed that stage, Ln5 plus p-Akt and PTEN were
the three most independent prognostic factors in patients with
NSCLC. Stage is a well-known independent prognostic factor. PTEN is
one of the key components in the EGFR signaling pathway, and recent
studies have suggested that loss of its expression is an
independent predictor of poor prognosis in patients with NSCLC
(13,21). Ln5 and p-Akt may play important
roles in the development of NSCLC and may represent new predictors
of poor prognosis in patients with NSCLC. Our results suggest that
Ln5 plays an important role in tumor development, and that altered
expression of Ln5 plus PTEN, p-EGFR or p-Akt defines a distinct
subset of lung cancers. Patients with these cancers have worse
rates of survival and may need to receive earlier treatment that
impacts survival.
In conclusion, our study highlights the complex
relationships between the extracellular matrix protein Ln5 and key
signaling pathway intermediates in tumorigenesis. It also
demonstrates the important prognostic significance of Ln5
expression, either alone or in combination with loss of PTEN
expression, positive p-EGFR expression or positive p-Akt
expression. These changes may serve as unfavorable prognostic
factors for NSCLC and may allow the identification of a subset of
patients with a poorer prognosis who can be targeted with novel
treatments that target Ln5, restore PTEN expression and/or target
activated EGFR, Akt or other downstream signal transduction
molecules.
Abbreviations:
|
Ln5
|
laminin 5
|
|
NSCLC
|
non-small cell lung cancer
|
|
EGFR
|
epidermal growth factor receptor
|
|
ECM
|
extracellular matrix
|
Acknowledgements
We wish to thank Dr Jian-Hua Chen for
her excellent follow-up work. This work was supported by grants
from the National Natural Science Foundation of China (no.
81101549), the Natural Science Foundation of Guangdong Province
(S2011010000792) and the Foundation of Guangdong Science and
Technology Department (2006B60101010 and 2007A032000002).
References
|
1.
|
Paez JG, Janne PA, Lee JC, et al: EGFR
mutations in lung cancer: correlation with clinical response to
gefitinib therapy. Science. 304:1497–1500. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Bremnesa RM, Sirerab R and Camps C:
Circulating tumour-derived DNA and RNA markers in blood: a tool for
early detection, diagnostics, and follow-up? Lung Cancer. 49:1–12.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Al-Saad S, Donnem T, Al-Shibli K, Persson
M, Bremnes RM and Busund LT: Diverse prognostic roles of Akt
isoforms, PTEN and PI3K in tumor epithelial cells and stromal
compartment in non-small cell lung cancer. Anticancer Res.
29:4175–4183. 2009.PubMed/NCBI
|
|
4.
|
Mok TS, Wu YL, Thongprasert S, et al:
Gefitinib or carboplatinpaclitaxel in pulmonary adenocarcinoma. N
Engl J Med. 361:947–957. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Kalikaki A, Koutsopoulos A, Hatzidaki D,
et al: Clinical outcome of patients with non-small cell lung cancer
receiving front-line chemotherapy according to EGFR and K-RAS
mutation status. Lung Cancer. 69:110–115. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Miyazaki K: Laminin-5 (laminin-332):
unique biological activity and role in tumor growth and invasion.
Cancer Sci. 97:91–98. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Fukai Y, Masuda N, Kato H, et al:
Correlation between laminin-5 gamma2 chain and epidermal growth
factor receptor expression in esophageal squamous cell carcinomas.
Oncology. 69:71–80. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Yurchenco PD and Cheng YS: Self-assembly
and calcium-binding sites in laminin A three-arm interaction model.
J Biol Chem. 268:17286–17299. 1993.PubMed/NCBI
|
|
9.
|
Engel J: Domain organizations of modular
extracellular matrix proteins and their evolution. Matrix Biol.
15:295–299. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Lee SY, Kim MJ, Jin G, et al: Somatic
mutations in epidermal growth factor receptor signaling pathway
genes in non-small cell lung cancers. J Thorac Oncol. 5:1734–1740.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Li F, Liu Y, Chen H, Liao D, Shen Y, Xu F
and Wang J: EGFR and COX-2 protein expression in non-small cell
lung cancer and the correlation with clinical features. J Exp Clin
Cancer Res. 30:272011. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Yoshizawa A, Fukuoka J, Shimizu S, et al:
Overexpression of phospho-eIF4E is associated with survival through
AKT pathway in non-small cell lung cancer. Clin Cancer Res.
16:240–248. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Tang JM, He QY, Guo RX and Chang XJ:
Phosphorylated Akt overexpression and loss of PTEN expression in
non-small cell lung cancer confers poor prognosis. Lung Cancer.
51:181–191. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Menard S, Castronovo V, Tagliabue E and
Sobel ME: New insights into the metastasis-associated 67 kD laminin
receptor. J Cell Biochem. 67:155–165. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Moriya Y, Niki T, Yamada T, Matsuno Y,
Kondo H and Hirohashi S: Increased expression of laminin-5 and its
prognostic significance in lung adenocarcinomas of small size. An
immunohistochemical analysis of 102 cases. Cancer. 91:1129–1141.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Giannelli G, Azzariti A, Fransvea E,
Porcelli L, Antonaci S and Paradiso A: Laminin-5 offsets the
efficacy of gefitinib (‘Iressa’) in hepatocellular carcinoma cells.
Br J Cancer. 91:1964–1969. 2004.PubMed/NCBI
|
|
17.
|
Hamasaki H, Koga K, Aoki M, et al:
Expression of laminin 5-γ2 chain in cutaneous squamous cell
carcinoma and its role in tumour invasion. Br J Cancer.
105:824–832. 2011.
|
|
18.
|
Kodama K, Ishii G, Miyamoto S, et al:
Laminin 5 expression protects against anoikis at aerogenous spread
and lepidic growth of human lung adenocarcinoma. Int J Cancer.
116:876–884. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Yuen HW, Ziober AF, Gopal P, et al:
Suppression of laminin-5 expression leads to increased motility,
tumorigenicity, and invasion. Exp Cell Res. 309:198–210. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Uramoto H, Shimokawa H, Hanagiri T, Kuwano
M and Ono M: Expression of selected gene for acquired drug
resistance to EGFR-TKI in lung adenocarcinoma. Lung Cancer.
73:361–365. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Wang L, Yue W, Zhang L, Zhao X, Wang Y and
Xu S: mTOR and PTEN expression in non-small cell lung cancer:
analysis by real-time fluorescence quantitative polymerase chain
reaction and immunohistochemistry. Surg Today. 42:419–425. 2012.
View Article : Google Scholar : PubMed/NCBI
|