Introduction
Radiation pneumonia, an interstitial pulmonary
inflammation, and subsequent radiation lung fibrosis are
significant dose-limiting complications, and may threaten the
quality of life of patients receiving radiation to the thorax. At
the cellular and tissue levels, radiation pneumonia presents as an
edema of the interstitial space, infiltration of inflammatory cells
and thickening of the alveolar septa. Although the molecular
mechanism underlying radiation pneumonia is complex and obscure,
pro-inflammatory cytokines, chemokines and cell adhesion molecules
are likely to be implicated (1,2).
Many researchers have shown that cytokines play important roles in
the pathogenesis of radiation pneumonia (3,4).
Interleukin-6 (IL-6), which was originally identified as a B-cell
differentiation factor (5), is now
known to be a multifunctional cytokine that regulates acute phase
response, immune response and inflammation (6,7).
IL-6 is produced by a variety of cells such as T cells, B cells,
monocytes, macrophages, fibroblasts, endothelial cells and several
tumor cells (8). Clinical as well
as experimental findings have suggested the involvement of IL-6 as
a pro-inflammatory cytokine in radiation pneumonia (9–12).
Indeed, IL-6 production has been found to be elevated in both
humans and animals with radiation pneumonia. Therefore, we
previously investigated whether monoclonal anti-IL-6 receptor
antibody (IL-6RA) treatment could provide a new pharmacological
intervention strategy for ameliorating radiation-induced lung
injury (13). However, early
intervention using IL-6RA was not able to prevent radiation-induced
lung injury. One possible explanation for this finding is that
long-term continuous administration of IL-6RA may be necessary to
reduce lung toxicity. Radiation-induced lung injury is a chronic
phenomenon mediated by a variety of cells such as inflammatory
cells responding to the release or activation of downstream
cytokines, growth factors or chemokines. In this study, we examined
the effect of a higher dose and longer course of IL-6RA treatment
on radiation pneumonia in mice following lethal whole thorax
irradiation.
Materials and methods
Mice and irradiation
Eight-week-old specific pathogen-free female
C57Bl/6J mice were obtained from Charles River Laboratories Japan,
Inc. (Yokohama, Japan) a week before testing. The mice were
maintained and the study protocol was established according to
guidelines of the Institutional Animal Care and Use Committee at
Osaka University Graduate School of Medicine. The mice were
anesthetized by an intraperitoneal (i.p.) injection of
pentobarbital (40 mg/kg) immediately before irradiation. The whole
thorax was irradiated with 4 MV X-rays from a linear accelerator
(EXL-6SP; Varian Medical Systems, Palo Alto, CA, USA) at a dose of
12 Gy delivered at a dose rate of ∼1.8 Gy/min with a 1.0-cm bolus
material on the surface. This dose is estimated to be an
approximately lethal dose (LD) 50/360 (the lethal dose required to
kill 50% of the C57Bl/6J mice within 360 days) (14). The field was 2.5 cm in length in
the cephalocaudal direction. Mice were randomly assigned to one of
four treatment groups (n=6/group): radiation and IL-6RA (IL-6RA 12
Gy), radiation and control (IgG 12 Gy), sham radiation and IL-6RA
(IL-6RA 0 Gy), and sham radiation and control (IgG 0 Gy). Mice were
sacrificed 30 days after irradiation.
IL-6RA (MR16-1) injection
Basic characteristics of the rat anti-mouse IL-6
receptor monoclonal antibody, MR16-1 (Chugai Pharmaceutical, Co.,
Ltd., Tokyo, Japan), have been described in a previously published
study (15). Mice were first
injected i.p. with 2 mg of MR16-1, or control rat IgG antibody (ICN
Biomedicals, Inc., Irvine, CA, USA) immediately following
irradiation. Subsequent weekly injections of IgG control or 0.5 mg
of antibody were administered from 1 to 3 weeks after
irradiation.
Analysis of circulating IL-6 and SAA
Blood samples were collected via cardiac puncture at
the time of euthanasia and plasma was obtained by
microcentrifugation at 4,000 x g for 5 min. Plasma concentrations
of IL-6 (Pierce Biotechnology, Inc., Rockford, IL, USA) and serum
amyloid A (SAA) (Immunology Consultants Laboratory, Inc., Newberg,
OR, USA) were measured using commercial enzyme-linked immunosorbent
assay (ELISA) kits according to the manufacturer’s
instructions.
Histological analysis
For histological examination, lung tissue was fixed
in 10% neutral-buffered formalin, embedded in paraffin wax,
sectioned (5-μm) and stained with haematoxylin and eosin (H&E).
Following H&E staining, we evaluated edema of the interstitial
space, infiltration of inflammatory cells, thickening of the
alveolar septa and vessel thrombosis.
Statistical analysis
Comparison between pairs of groups was performed
using the non-parametric Mann-Whitney U test due to the small
sample number. A P-value of <0.05 was considered to indicate
statistical significance.
Results
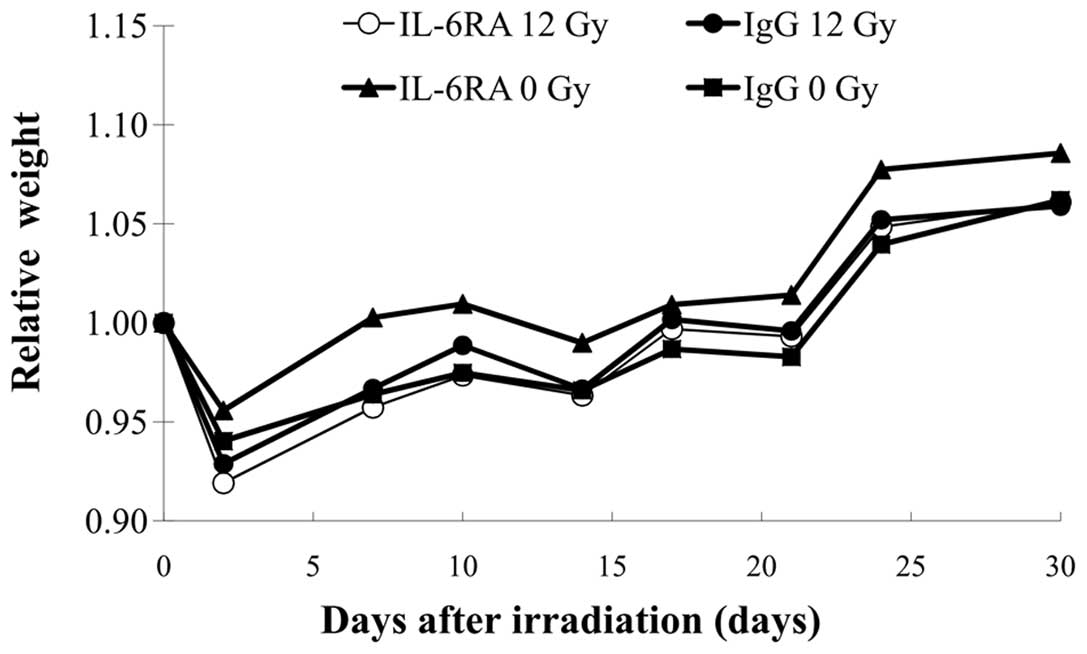
Body weight as an indicator of the systemic toxicity
of radiation pneumonia is presented graphically in Fig. 1; no differences in body weight were
observed among the 4 groups.
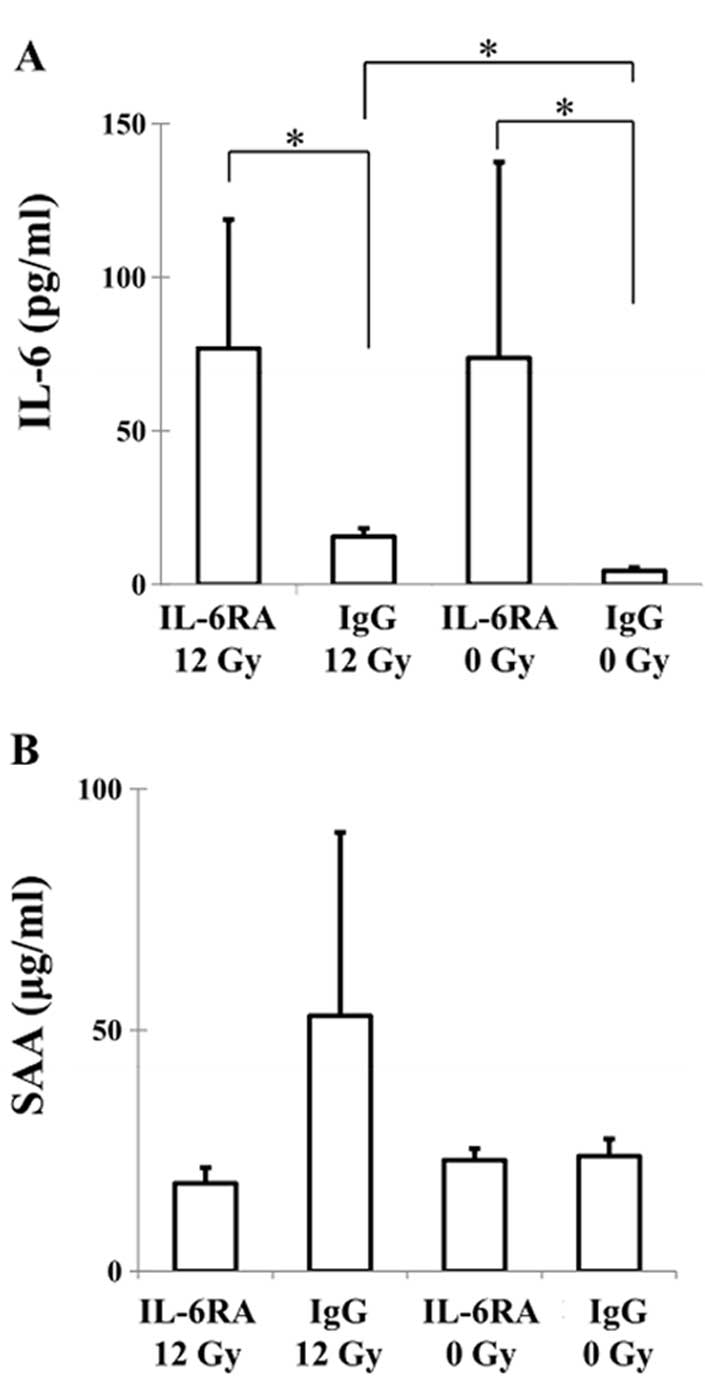
Mouse plasma levels of IL-6 and SAA, one of the
major acute-phase proteins of inflammation and tissue damage in
mammals, were determined using specific ELISAs. The IL-6 levels in
the IgG 0 Gy, IL-6RA 0 Gy, IgG 12 Gy and IL-6RA 12 Gy groups were
4.4±1.2, 73.9±63.8, 15.6±2.7 and 77.0±41.8 pg/ml, respectively
(Fig. 2A). A significant increase
was observed in the IL-6 level in the IL-6RA 12 Gy group compared
to the IgG 12 Gy group. The mice in the IL-6RA 12 Gy group also had
significantly higher levels than the IgG 0 Gy group. A marked
increase in the level of IL-6 was found in the IgG 12 Gy group
compared to the IgG 0 Gy group. The SAA level in the IgG 0 Gy,
IL-6RA 0 Gy, IgG 12 Gy and IL-6RA 12 Gy groups were 23.9±3.6,
23.1±2.4, 52.9±38.1 and 18.2±3.3 μg/ml, respectively (Fig. 2B). The mice in the IgG 12 Gy group
exhibited higher SAA protein levels, but this was not statistically
significant, compared to the other groups (Fig. 2B). The IL-6RA 12 Gy group showed
low SAA protein levels in plasma. No significant difference was
found in the SAA level between the IL-6RA 0 Gy and IgG 0 Gy
groups.
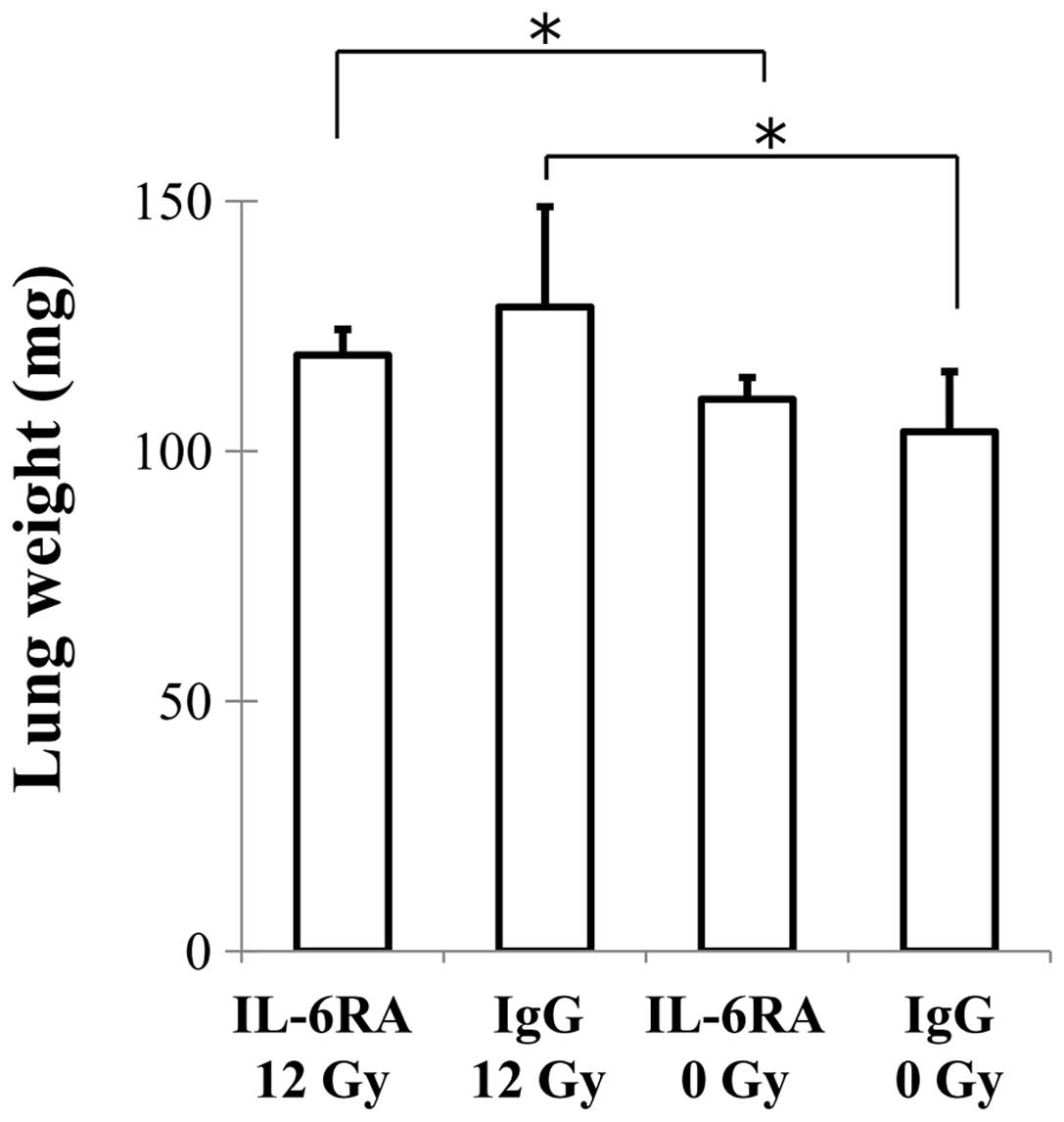
Wet lung weight was determined as a measure of
pulmonary edema and consolidation. There were no differences in the
lung weight of the IgG 12 Gy and IL-6RA 12 Gy groups (Fig. 3).
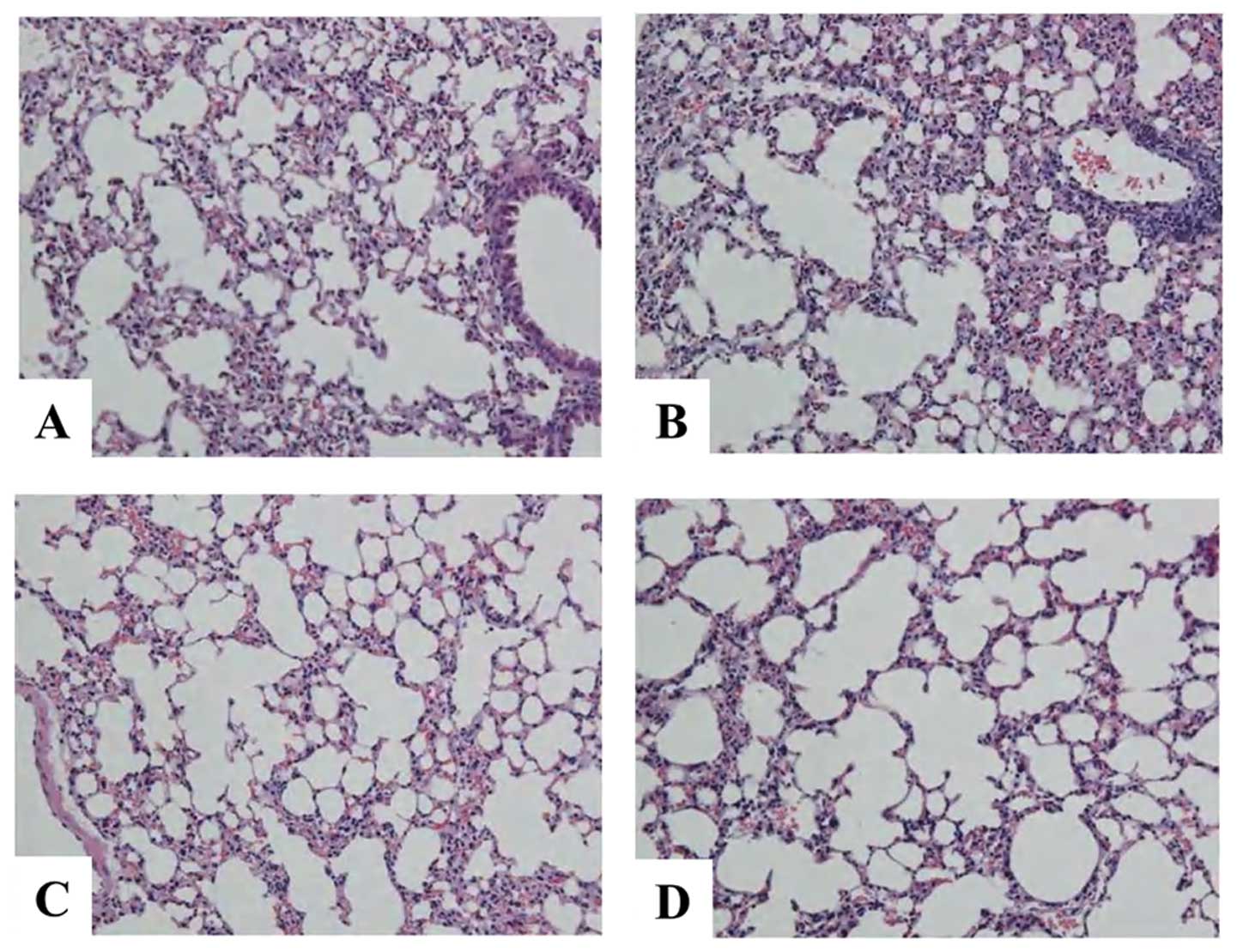
The H&E-stained lungs of the mice in both the
IgG 12 Gy and IL-6RA 12 Gy groups showed an increased acute
inflammatory infiltration in the interstitium (Fig. 4). However, no significant
difference was found between the IgG 12 Gy and IL-6RA 12 Gy groups.
No damage to the lung structure was observed in the IgG 0 Gy or
IL-6RA 0 Gy groups.
Discussion
Radiation pneumonia and subsequent radiation lung
fibrosis are major dose-limiting complications for patients
undergoing thoracic radiotherapy. Recent research findings support
the existence of a mechanism of cellular interaction between lung
parenchymal cells and circulating immune cells, which is mediated
by a variety of pro-inflammatory cytokines, chemokines, adhesion
molecules and pro-fibrotic cytokines (1,2).
Since IL-6 is a pleiotropic cytokine that plays essential roles in
the regulation of the immune response and inflammation, IL-6
receptor monoclonal antibody treatment has been identified as a
promising treatment for rheumatoid arthritis, juvenile idiopathic
arthritis, Castleman’s disease and Crohn’s disease (16–19).
IL-6 has also been implicated in the pathogenesis of radiation
pneumonia (9–12) and is synthesized by type II
pneumocytes, alveolar macrophages, T lymphocytes and lung
fibroblasts (3). We therefore
hypothesized that blockage of the IL-6 signaling pathways could
offer an attractive therapeutic target for the amelioration of
radiation-induced lung injury. However, early IL-6RA treatment was
not able to mitigate radiation-induced lung injury (13).
Rübe et al (12) showed that radiation-induced release
of IL-6 in the bronchiolar epithelium of C57Bl/6J mice could be
detected a few hours and several weeks after irradiation. Anscher
et al (20) reported that
long-term administration of the small-molecule inhibitor of TGF-β
was more effective in reducing radiation-induced lung toxicity than
short-term administration. Rabbani et al (21) demonstrated that prolonged
administration of the novel catalytic anti-oxidant, AEOL 10150,
after irradiation protects against radiation-induced lung injury.
However, treatment with AEOL 10150 before and for a short time
after irradiation had no significant benefits. Therefore, we
hypothesized that long-term continuous administration of IL-6RA
might be necessary to reduce lung toxicity. In this study, we used
a higher dose and longer course (2 mg of MR16-1 initially, followed
by 3 doses of 0.5 mg MR16-1, weekly for 3 weeks) of IL-6RA
treatment than we used in our previous study (2 doses of 0.2 mg
MR16-1, weekly) (13). In
addition, we used the C57Bl/6 strain of mice, which more easily
develops radiation-induced lung injury than the Balb/c mice used in
the previous study. Usage of a different mice strain or irradiation
dose may have resulted in changes in the results from our previous
study.
In our previous study, we were not able to
administer IL-6RA more than twice, since we were concerned that
repeated treatment with a rat antibody would result in the
production of mouse anti-rat antibodies. Recently,
Tomiyama-Hanayama et al (22) examined the effect of IL-6RA
concentration, by using the treatment regimen that we used in this
study, on renal injury in apolipoprotein E-deficient mice and
confirmed the safety of an intensive dose.
We found a significant increase in the IL-6 levels
in the radiation and IL-6RA treatment group compared to the
radiation only group. Nishimoto et al (23) reported that serum IL-6 markedly
increased after IL-6RA administration in both rheumatoid arthritis
and Castleman’s disease through inhibition of IL-6R-mediated
consumption of IL-6. Despite the increase in serum IL-6 levels,
IL-6RA treatment has been shown to dramatically ameliorate
inflammatory manifestations and to normalize the levels of acute
phase proteins such as C-reactive protein in rheumatoid arthritis
and Castleman’s disease. Since one possible explanation for the
increase in serum IL-6 following IL-6RA treatment is that IL-6RA
may inhibit the clearance of IL-6 from serum, the measurement of
serum IL-6 levels only may be a limitation in evaluating radiation
pneumonia. Consistent with this report, our data revealed that
IL-6RA treatment maintained the same SAA protein level as in the
IgG 0 Gy group. Acute phase protein SAA is known as a sensitive
systemic marker of inflammation and tissue damage (24). Furthermore, IL-6, acting
synergistically with tumor necrosis factor or IL-1, plays an
important role in the induction of the SAA gene and IL-6RA inhibits
this synergistic effect of IL-6 on SAA production (25). Since SAA did not increase in the
IL-6RA-treated mice receiving irradiation in this study, IL-6
action may be inhibited. We previously observed that IL-6RA
treatment suppressed the radiation-induced increase in IL-6 as
compared with the IgG control group 50 days after irradiation
(13). Such a discrepancy may be
due to differences in the protocol of antibody administration and
time of assessment.
Our findings suggest that elevation of IL-6 may not
be involved to a great extent in the mechanism behind the
development of radiation pneumonia, but instead reflects the
inflammatory state of the lung due to the development of radiation
pneumonia. Measurement of plasma IL-6, as an acute phase
inflammatory cytokine, may therefore indicate the severity of
inflammatory state of the radiation-induced lung injury, although
Rübe et al (26) reported
that IL-6 levels do not provide a predictive risk assessment for
radiation pneumonia in patients irradiated for non-small cell lung
cancer. The utility of IL-6 measurement should be validated in
future studies, since IL-6 also increases in patients with
pulmonary diseases such as infectious pneumonia, interstitial
pneumonia and chronic obstructive pulmonary disease (27).
Limitations of our study included the lack of
evaluation of data over long periods of time and the relatively
small number of mice used. We evaluated radiation-induced lung
injury in only acute interstitial inflammation (30 days) as IL-6
has been implicated in the pathogenesis of radiation pneumonia.
Saito-Fujita T et al (28)
demonstrated that IL-6-knockout mice exhibited attenuated
radiation-induced lung fibrosis. Additional research is required to
determine the optimal timing, antibody dose and duration for
therapy using this approach for the prevention of lung injury after
radiation therapy.
In conclusion, the findings demonstrated that
intervention using IL-6RA does not ameliorate radiation pneumonia.
Therefore, more detailed studies are required to identify the
possible strategies for the inhibition of cytokine signaling as a
method for mitigating lung injury caused by radiation therapy.
Acknowledgements
The authors are indebted to Kumie
Hirai, Kazumasa Minami and Masaru Isono for the excellent technical
support.
References
|
1.
|
Tsoutsou PG and Koukourakis MI: Radiation
pneumonitis and fibrosis: mechanisms underlying its pathogenesis
and implications for future research. Int J Radiat Oncol Biol Phys.
66:1281–1293. 2006. View Article : Google Scholar
|
|
2.
|
Brush J, Lipnick SL, Phillips T, Sitko J,
McDonald JT and McBride WH: Molecular mechanisms of late normal
tissue injury. Semin Radiat Oncol. 17:121–130. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Rubin P, Johnston CJ, Williams JP,
McDonald S and Finkelstein JN: A perpetual cascade of cytokines
postirradiation leads to pulmonary fibrosis. Int J Radiat Oncol
Biol Phys. 33:99–109. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Chen Y, Williams J, Ding I, et al:
Radiation pneumonitis and early circulatory cytokine markers. Semin
Radiat Oncol. 12:S26–S33. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Hirano T, Yasukawa K, Harada H, et al:
Complementary DNA for a novel human interleukin (BSF-2) that
induces B lymphocytes to produce immunoglobulin. Nature. 324:73–76.
1986. View
Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Nishimoto N and Kishimoto T: Inhibition of
IL-6 for the treatment of inflammatory diseases. Curr Opin
Pharmacol. 4:386–391. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Nishimoto N and Kishimoto T:
Interleukin-6: from bench to bedside. Nat Clin Pract Rheumatol.
2:619–626. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Kishimoto T, Akira S, Narazaki M and Taga
T: Interleukin-6 family of cytokines and gp130. Blood.
86:1243–1254. 1995.PubMed/NCBI
|
|
9.
|
Chen Y, Rubin P, Williams J, Hernady E,
Smudzin T and Okunieff P: Circulating IL-6 as a predictor of
radiation pneumonitis. Int J Radiat Oncol Biol Phys. 49:641–648.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Chen Y, Hyrien O, Williams J, Okunieff P,
Smudzin T and Rubin P: Interleukin (IL)-1A and IL-6: applications
to the predictive diagnostic testing of radiation pneumonitis. Int
J Radiat Oncol Biol Phys. 62:260–266. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Rübe CE, Wilfert F, Palm J, et al:
Irradiation induces a biphasic expression of pro-inflammatory
cytokines in the lung. Strahlenther Onkol. 180:442–448.
2004.PubMed/NCBI
|
|
12.
|
Rübe CE, Uthe D, Wilfert F, et al: The
bronchiolar epithelium as a prominent source of pro-inflammatory
cytokines after lung irradiation. Int J Radiat Oncol Biol Phys.
61:1482–1492. 2005.PubMed/NCBI
|
|
13.
|
Ogata T, Yamazaki H, Teshima T, et al:
Early administration of IL-6RA does not prevent radiation-induced
lung injury in mice. Radiat Oncol. 5:262010. View Article : Google Scholar
|
|
14.
|
Chiang CS, Liu WC, Jung SM, et al:
Compartmental responses after thoracic irradiation of mice: strain
differences. Int J Radiat Oncol Biol Phys. 62:862–871. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Okazaki M, Yamada Y, Nishimoto N,
Yoshizaki K and Mihara M: Characterization of anti-mouse
interleukin-6 receptor antibody. Immunol Lett. 84:231–240. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Nishimoto N, Yoshizaki K, Miyasaka N, et
al: Treatment of rheumatoid arthritis with humanized
anti-interleukin-6 receptor antibody: a multicenter, double-blind,
placebo-controlled trial. Arthritis Rheum. 50:1761–1769. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Yokota S, Miyamae T, Imagawa T, et al:
Therapeutic efficacy of humanized recombinant anti-interleukin-6
receptor antibody in children with systemic-onset juvenile
idiopathic arthritis. Arthritis Rheum. 52:818–825. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Nishimoto N, Kanakura Y, Aozasa K, et al:
Humanized anti-interleukin-6 receptor antibody treatment of
multicentric Castleman disease. Blood. 106:2627–2632. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Ito H, Takazoe M, Fukuda Y, et al: A pilot
randomized trial of a human anti-interleukin-6 receptor monoclonal
antibody in active Crohn’s disease. Gastroenterology. 126:989–996.
2004.PubMed/NCBI
|
|
20.
|
Anscher MS, Thrasher B, Zgonjanin L, et
al: Small molecular inhibitor of transforming growth factor-beta
protects against development of radiation-induced lung injury. Int
J Radiat Oncol Biol Phys. 71:829–837. 2008. View Article : Google Scholar
|
|
21.
|
Rabbani ZN, Batinic-Haberle I, Anscher MS,
et al: Long-term administration of a small molecular weight
catalytic metalloporphyrin antioxidant, AEOL 10150, protects lungs
from radiation-induced injury. Int J Radiat Oncol Biol Phys.
67:573–580. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Tomiyama-Hanayama M, Rakugi H, Kohara M,
et al: Effect of interleukin-6 receptor blockage on renal injury in
apolipoprotein E-deficient mice. Am J Physiol Renal Physiol.
297:F679–F684. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Nishimoto N, Terao K, Mima T, Nakahara H,
Takagi N and Kakehi T: Mechanisms and pathologic significances in
increase in serum interleukin-6 (IL-6) and soluble IL-6 receptor
after administration of an anti-IL-6 receptor antibody,
tocilizumab, in patients with rheumatoid arthritis and Castleman
disease. Blood. 112:3959–3964. 2008. View Article : Google Scholar
|
|
24.
|
Gabay C and Kushner I: Acute-phase
proteins and other systemic responses to inflammation. N Engl J
Med. 340:448–454. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Hagihara K, Nishikawa T, Isobe T, Song J,
Sugamata Y and Yoshizaki K: IL-6 plays a critical role in the
synergistic induction of human serum amyloid A (SAA) gene when
stimulated with proinflammatory cytokines as analyzed with an SAA
isoform real-time quantitative RT-PCR assay system. Biochem Biophys
Res Commun. 314:363–369. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Rübe CE, Palm J, Erren M, et al: Cytokine
plasma levels: reliable predictors for radiation pneumonitis? PLoS
One. 3:e28982008.PubMed/NCBI
|
|
27.
|
Barthelemy-Brichant N, Bosquée L, Cataldo
D, et al: Increased IL-6 and TGF-β1 concentrations in
bronchoalveolar lavage fluid associated with thoracic radiotherapy.
Int J Radiat Oncol Biol Phys. 58:758–767. 2004.
|
|
28.
|
Saito-Fujita T, Iwakawa M, Nakamura E, et
al: Attenuated lung fibrosis in interleukin 6 knock-out mice after
C-ion irradiation to lung. J Radiat Res. 52:270–277. 2011.
View Article : Google Scholar : PubMed/NCBI
|