Introduction
The excessive secretion of sebum on skin is a
significant factor in various skin diseases (including acne and
seborrheic dermatitis) (1,2). Medication remains the main method of
treatment for diseases in which excessive sebum is secreted.
Cis-retinoic acid has been shown to inhibit the proliferation of
sebaceous gland cells and reduce lipid secretion (3–6), and
has been suggested to be the most effective anti-acne drug
currently in use. However, studies have shown that cis-retinoic
danshen/tanshin acid may induce several side effects, including
cerebral damage (7,8), abnormal function of the skin barrier
(9), intestinal mucosa damage
(10), teratogenicity (11) and psychological problems (12), therefore there are limitations to
its clinical use. Thus, identifying more effective and safer drugs
to treat sebum secretion is clearly of practical significance.
Tanshinone is the main substance among the
fat-soluble extracts obtained from the Chinese herbal medicine
danshen/tanshin, which comes from a member of the Labiatae family
commonly used for the treatment of cardio-cerebrovascular diseases.
There have been numerous Chinese clinical studies concerning its
use in the treatment of acne, seborrheic dermatitis and diseases of
excessive sebum secretion (13–15).
To date, more than ten monomers have been identified, among which
Tanshinone IIA (Tan IIA) has been the most extensively studied. A
wide range of pharmacological uses have been reported for Tan IIA,
for example, neuroprotective effects (16), prevention of cardiac hypertrophy
(17), antitumor (18), anti-atherosclerosis (19) and anti-inflammatory (20) effects and protection of vascular
endothelial cells (21). In
addition, a number of Chinese studies have reported that Tan IIA is
effective against the secretion of sebum, either by inhibiting the
proliferation of sebaceous glands and the synthesis of lipids, or
by indirectly downregulating the expression of mRNA of androgen
receptors in sebaceous gland cells (22). Our previous studies have
demonstrated that the topical application of Tan IIA sodium
sulfonate is able to significantly inhibit the proliferation of
Syrian hamster sebaceous glands (23). However, further study is required
to identify the underlying mechanisms.
In recent years, the sterol regulatory element
binding protein (SREBP) pathway has been a main focus in studies of
the mechanism of lipid secretion. SREBPs are one of the most
significant regulatory factors for lipid synthesis in animals and
regulate the synthesis of cholesterol, fatty acids and
triglycerides through regulating the gene transcription of lipid
synthesis-related enzymes (24–26).
Rosignoli et al (27) found
that androgenic hormones regulate the gene transcription of lipid
synthesis-related enzymes through activating the SREBP pathway in
sebaceous gland tissues, as a result of increased synthesis and
secretion of lipid. Apart from sebaceous glands, keratinocytes
(KCs) are another significant source of lipids on the skin surface.
Harrison et al (28) found
that SREBP-1c was expressed in KCs and played a role in lipid
synthesis. In this study, we observed that Tan IIA inhibits
dihydrotestosterone (DHT)-induced lipid synthesis and secretion in
HaCaT cells, which may occur via blocking of the activity of the
PI3K/Akt pathway. This is the first study on the mechanism of the
inhibitory effect of Tan IIA on lipid secretion in HaCaT cells.
Materials and methods
Drugs
DHT powder was purchased from Sigma Co. Ltd. (St.
Louis, MO, USA) and Tan IIA powder was purchased from Nanjing
Zelang Medical Technological Co. Ltd. (Jiangsu, China). These were
diluted with DMSO prior to use, prepared in serum-free medium at
the required concentrations and subpackaged by filtration
sterilization. Anti-SREBP-1 (polyclonal) was purchased from Beijing
Boao Bio-tech Co. Ltd., (Beijing, China); rabbit anti-phospho-Akt
(Ser473; monoclonal) was purchased from Cell Signaling Technology,
Inc. (Danvers, MA, USA); mouse/rabbit anti-actin, PI3K inhibitor
LY294002 and BCA Protein Assay kit were purchased from Beyotime
Institute of Biotechnology (Haimen, Jiangsu, China); TRIzol was
purchased from Invitrogen (Carlsbad, CA, USA); real-time PCR Assay
kits were purchased from Nanjing KGI Bioteknologi Development Co,
Ltd. (Nanjing, China); Nile red powder was purchased from Shanghai
XinRan Bio-tech Co. Ltd. (Shanghai, China), diluted with methanol
and stored at a concentration of 10 μg/ml at 4°C, away from
light.
Cell culture
Cells of the HaCaT KC cell line were cultured in a
cell incubator at 37°C in 5% CO2, in DMEM medium
containing 10% fetal bovine serum, 1% penicillin and streptomycin.
After the cells became polygonal, arranged as a single layer, they
were vaccinated at a density of 1x109 cells/l with 0.25%
trypsin solution. The cultured cells were used for experimentation
when they adhered to the culture plate and the confluence reached
70–80%.
Experimental grouping and treatment of
cells
The cells were cultured in serum-free medium and,
after growing synchronously for 24 h, they were divided randomly
into five groups: i) control; ii) DHT, administered DHT at a
concentration of 100 nmol/l; iii) Tan IIA (1.25 μmol/l) + DHT; iv)
Tan IIA (2.5 μmol/l) + DHT; v) Tan IIA (5 μmol/l) + DHT. The cells
were treated with Tan IIA for 24 h before adding 100 nmol/l DHT and
were then cultured for a further 24 h prior to use.
To observe the effect of Tan IIA and LY294002 on
DHT-induced lipid synthesis and lipid synthetase-related genes in
HaCaT cells, the cells were cultured in serum-free medium, and
after growing synchronously for 24 h they were divided randomly
into four groups: i) control; ii) DHT, administered DHT at a
concentration of 100 nmol/l; iii) an LY294002 + DHT group,
pre-treated with LY294002 at 50 μM for 40 min, then with DHT added
at concentration of 100 nmol/L, and then cultured for a further 24
h prior to use; iv) a Tan IIA 2.5 μmol/l + DHT group in which the
Tan IIA was pre-treated for 24 h prior to being added to 100 nmol/l
DHT, and then cultured for a further 24 h prior to use.
Real-time PCR detection of the expression
of SREBP-1c mRNA and the expression of lipogenic enzyme [fatty acid
synthase (FAS), acyl-CoA synthetase (ACS), stearoyl-CoA synthetase
(SCD) and HMG-CoA reductase (HMGCR)] mRNA in HaCaT cells
The cells were grouped and TRIzol was added to break
down the cells, followed by extraction of total RNA, measurement of
concentration and then measurement of purity. After ensuring that
the quality met the requirements of the experiment, cDNA was
obtained by reverse transcription. cDNA was diluted 10 times and
amplified in a 20-μl reaction system. Primers were synthesized by
Nanjing Kaiji Bio-tech Co, Ltd. (Table
I). The amplification conditions were as follows:
pre-denaturation at 95°C for 5 min, entering reaction cycles,
denaturation at 95°C for 15 min, annealing for 30 sec at 60°C,
extending for 30 sec at 72°C and maintained at 72°C for 10 min
following 40 cycles.
 | Table I.Primers and amplified products. |
Table I.
Primers and amplified products.
| Genes | Primers | Products (bp) |
|---|
| SREBP-1c | Forward: 5′
GGAGCCATGGATTGCACTTT 3′ | 77 |
| Reverse: 5′
TCAAATAGGCCAGGGAAGTCA 3′ |
| FAS | Forward: 5′
CAGGCACACACGATGGAC 3′ | 92 |
| Reverse: 5′
CGGAGTGAATCTGGGTTGAT 3′ |
| ACS | Forward: 5′
CCCAGTTTATCCCAATGCTG 3′ | 74 |
| Reverse: 5′
GGGCGCCATAGAACTGATT 3′ |
| SCD | Forward: 5′
CCGGGAGAATATCCTGGTTT 3′ | 97 |
| Reverse: 5′
GCGGTACTCACTGGCAGAGT 3′ |
| HMGCR | Forward: 5′
TGGCTCTTTCAGAGAGGTCTCA 3′ | 158 |
| Reverse: 5′
TGCCTTCAGAGGTGAGCTGTA 3′ |
| Actin | Forward: 5′
GCAGAAGGAGATCACAGCCCT 3′ | 136 |
| Reverse: 5′
GCTGATCCACATCTGCTGGAA 3′ |
Western blot tests for the protein
expression of SREBP-1 and p-Akt
The cells were treated according to group. The
culture solution was discarded, the cells were washed three times
with PBS solution pre-cooled to 4°C, 300 μl cell degradation
solution containing protease inhibitor was added, then the mixture
was placed on ice for 15 min and, following cell detachment,
centrifugal separation was conducted at 4°C at 12,000 rpm. The
upper layer of the solution was tested for protein using the BCA
method. A 30-μg sample of protein was taken from each group for
SDS-PAGE, protein was transferred onto PVDF films and covered with
a 5% BSA blocking buffer at 37°C for 1 h. The primary antibody was
added according to the cell group (rabbit antibody SREBP-1, diluted
at 1:500; rabbit antibody phospho-Akt, diluted at 1:1,000; actin
antibody diluted at 1:1,000) and the cells were incubated at 4°C
throughout the night, washed prior to incubation with the secondary
antibody, which was diluted at 1:1,000, and marked by horseradish
peroxidase at 37°C for 40 min. ECL detection reagent was added for
5 min. Finally, a fixation procedure was conducted.
Detecting the effects of Tan IIA on the
DHT-induced synthesis of lipids in HaCaT cells using flow
cytometry
HaCaT cells at the exponential phase of growth were
inoculated in 6-well plates, 3x104 cells/well and
cultured for 24 h. The HaCaT cells were treated according to group
and 0.25% trypsin solution containing 0.02% EDTA was added. The
degradation process was terminated with 10% fetal bovine serum
medium. The cells were washed twice with PBS, a single-cell
suspension was prepared (in PBS), 100 ng/ml Nile red fluorescent
dye was added, samples were incubated at room temperature for 15
min, filtered with 300 mesh nylon membrane and flow cytometry was
then used to test 10,000 cells from each sample. The average
fluorescence intensity of every cell was determined, with an
excitation wavelength at 485 nm and emission wavelength at 565
nm.
Statistical analysis
SPSS 13.0 software (SPSS, Inc., Chicago, IL, USA)
was used for data analysis, and the form of mean ± standard
deviation was used to indicate measurement data. A t-test was used
for inter-group comparison, and P<0.05 was considered to
indicate a statistically significant result.
Results
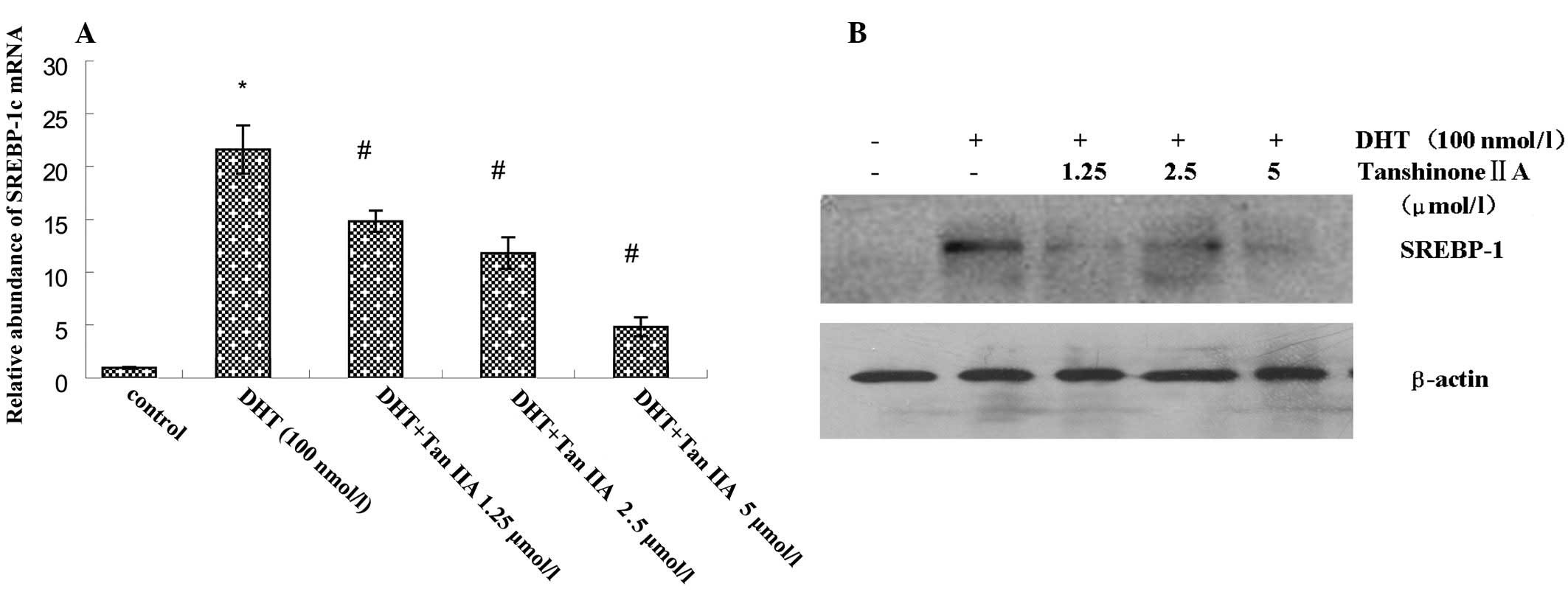
Effect of Tan IIA on the DHT-activated
SREBP-1 pathway in HaCaT cells
Compared with the low expression of SREBP-1c mRNA
and SREBP-1 protein in the untreated control group, significantly
higher levels of expression of SREBP-1c mRNA and SREBP-1 protein
were observed in DHT-treated HaCaT cells. Different concentrations
of Tan IIA were added prior to DHT treatment, resulting in a
decrease in SREBP-1c mRNA and SREBP-1 protein expression in a
concentration-dependent manner (Fig.
1). Overall, in quantitative analysis, compared with the
unexposed control epidermis, after 24 h exposure to DHT, the
SREBP-1 protein accumulation was 21.71%. This was decreased by
pre-application of different concentrations of Tan IIA (1.25, 2.5
and 5 μmol/l) to 6.34, 16.82 (P<0.05) and 10.17% SREBP-1 protein
expression, respectively.
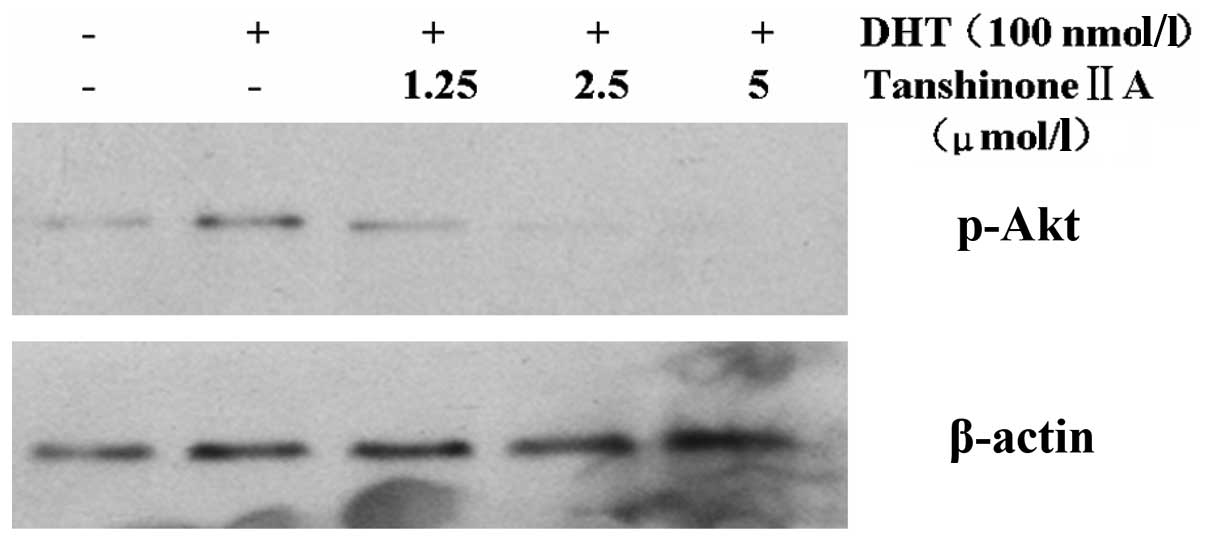
Effect of Tan IIA on the DHT-activated
p-Akt in HaCaT cells
Compared with a low protein expression for p-Akt in
the untreated control group, a significantly higher expression of
p-Akt protein was observed in DHT-treated HaCaT cells. A different
concentration of Tan IIA was added prior to DHT treatment, however,
this resulted in a decrease in p-Akt protein expression in a
concentration-dependent manner (Fig.
2). Overall, in quantitative analysis, compared with the
unexposed control epidermis, after 24 h exposure to DHT the p-Akt
protein accumulation was 18.32%. This was decreased by
pre-application of different concentrations of Tan IIA (1.25, 2.5
and 5 μmol/l) to 11.22, 6.18 (P<0.05) and 2.43% p-Akt protein
expression, respectively.
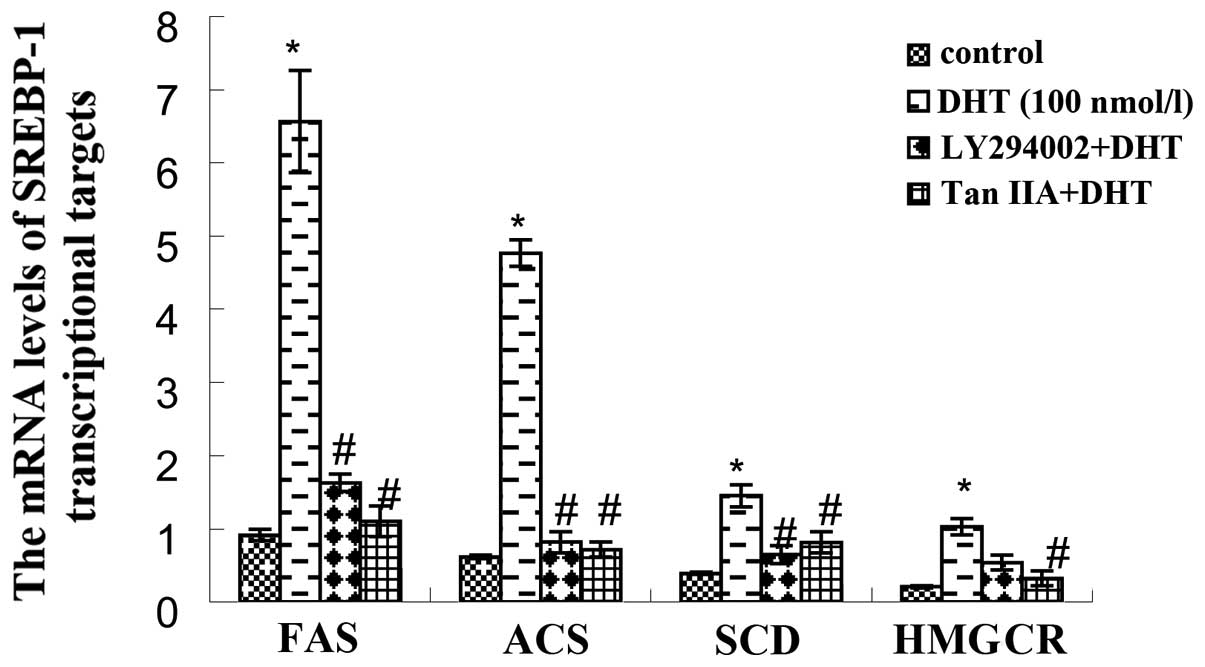
Effect of Tan IIA and LY294002 on the
expression of lipid synthetase-related genes in HaCaT cells
According to the results of real-time PCR tests
(Fig. 3), DHT (100 nmol/l)
increased the transcription of FAS, ACS, SCD and HMGCR (P<0.01)
when compared with the untreated control group. Following
pre-treatment with LY294002 for 40 min, the induction effect of DHT
on the expression of FAS, ACS, SCD and HMGCR was significantly
inhibited (P<0.01). Tan IIA played a role similar to LY294002
and significantly inhibited the SREBP-1-regulated gene
transcription of lipid synthetase (P<0.01), which was consistent
with the inhibitory effects of Tanshinone on SREBP-1c.
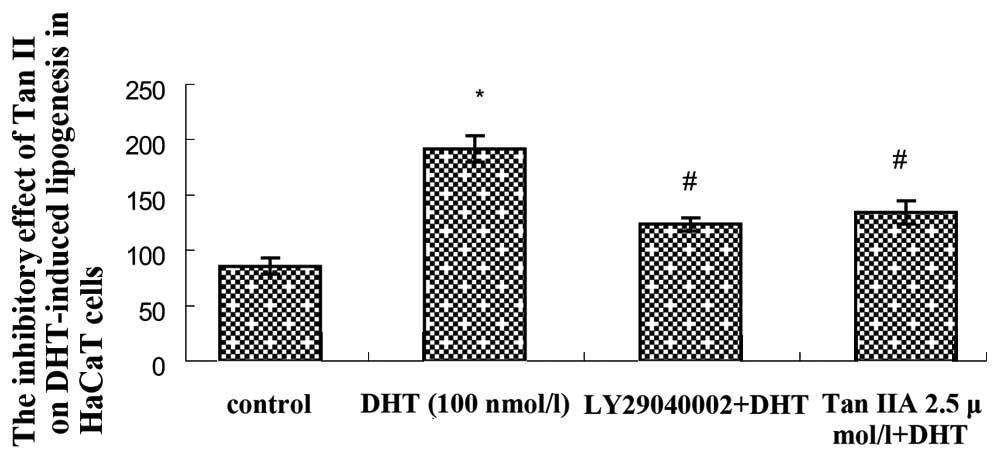
Effect of Tan IIA on DHT-induced lipid
synthesis in HaCaT cells
The results of flow cytometry showed that the
average fluorescence intensity of the DHT group was significantly
increased (P<0.05) compared with the untreated control group.
The average fluorescence intensity of the LY294002+DHT group and
the Tan IIA+DHT group were decreased compared with the blank
control group (P<0.05; Fig. 4).
The results above indicate that Tan IIA has an inhibitory effect on
the lipid synthesis of HaCaT cells induced by DHT, an effect
similar to that of LY294002.
Discussion
In recent years, much attention has been focused on
intra-cellular signal transduction mediating the activation of the
SREBP pathway, which plays a role in lipogenesis. The PI3K/Akt
signaling and mitogen-activated protein kinase (MAPK) pathways play
critical roles in mediating the activation of the SREBP pathway in
different cell types (29–32). Previous studies have indicated that
the activation of Akt is involved in the transport of the SREBP
cleavage-activating protein (SCAP)/SREBP complex from the
endoplasmic reticulum to the Golgi (33). This is a major regulatory step in
SREBP activity. In the present study, we demonstrated for the first
time that DHT-induced SREBP-1 expression is regulated by the
PI3K/Akt pathway. Our data indicate that DHT increases the amount
of cleaved (mature) SREBP protein and that this increase is
inhibited in the presence of LY294002. This suggests that, in
addition to possible transcriptional and translational control, Akt
activation may also affect SREBP processing in HaCaT cells. Akt
activation has also been shown to increase the expression of
lipogenic genes (34). The
conclusion is further supported by our observation that the
inhibition of Akt activation by LY294002 blocks the increase in
mRNA expression of lipogenic genes and lipogenesis induced by
SREBP-1.
Chinese clinical studies have shown that Tanshinone
has significant effects on reducing sebum secretion and is used to
treat acne and seborrheic dermatitis (13–15).
Tan IIA is the most effective pharmacological ingredient of
Tanshinone and has become a popular topic of research, with
extensive studies in numerous fields of pharmacological effects,
including anti-oxidant, anti-inflammatory and antibiotic
properties, liver-protection and the ability to prevent tumors and
improve circulation. However, no studies concerning the specific
pathways and mechanisms of the anti-lipid effects of Tan IIA have
been performed. In the present study, we found that Tan IIA
significantly inhibits the expression of SREBP-1 induced by DHT,
downregulates the transcription of enzyme genes associated with
lipid synthesis, including FAS, ACS, SCD and HMGCR, and
significantly reduces lipid production and secretion in HaCaT
cells. The results of this study demonstrate that Tan IIA is able
to counter lipid secretion in KCs, a property associated with its
regulation of the expression of SREBP-1. In addition, we found that
Tan IIA is able to produce an inhibitory effect on the SREBP-1
pathway through the PI3K/Akt signaling pathway; its inhibitory
effect on the DHT-induced expression of SREBP-1 and lipid synthesis
in HaCaT cells is identical to the effect of the PI3K inhibitor
LY294002. Therefore, we consider that in the suppression of skin
lipid secretion, Tan IIA may produce effects similar to a PI3K
inhibitor.
In conclusion, this study indicates that Tan IIA is
capable of inhibiting lipid secretion caused by an excess of
androgenic hormones and that it may be used for the treatment of
skin diseases including acne and seborrheic dermatitis in which an
excessive amount of lipid is secreted.
Acknowledgements
This study was supported by grants
from the China National Natural Science Foundation (81000700,
81171518). We appreciate the assistance of NT Pharma (China)
Investment Co., Ltd. during the editing process.
References
|
1.
|
Zouboulis CC: Acne and sebaceous gland
function. Clin Dermatol. 22:360–366. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Plewig G: How acne vulgaris develops.
Hautarzt. 61:99–100. 106(In German).
|
|
3.
|
McDonald SK, Goh MS and Chong AH:
Successful treatment of cyclosporine-induced sebaceous hyperplasia
with oral isotretinoin in two renal transplant recipients.
Australas J Dermatol. 52:227–230. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Orfanos CE and Zouboulis CC: Oral
retinoids in the treatment of seborrhoea and acne. Dermatology.
196:140–147. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Doran TI and Shapiro SS: Retinoid effects
on sebocyte proliferation. Methods Enzymol. 190:334–338. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
De Marchi MA, Maranhão RC, Brandizzi LI
and Souza DR: Effects of isotretinoin on the metabolism of
triglyceride-rich lipoproteins and on the lipid profile in patients
with acne. Arch Dermatol Res. 297:403–408. 2006.PubMed/NCBI
|
|
7.
|
Wong A, Williams M and Gibb W:
Isotretinoin-induced encephalopathy. J Dermatolog Treat.
21:361–362. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Yaman M, Albayram S, Altintas A, et al: A
cerebellar demyelinating lesion following treatment of acne with
isotretinoin. Clin Exp Dermatol. 33:118–121. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Tinoco MP, Tamler C, Maciel G, et al:
Pyoderma gangrenosum following isotretinoin therapy for acne
nodulocystic. Int J Dermatol. 47:953–956. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Papageorgiou NP, Altman A and Shoenfeld Y:
Inflammatory bowel disease: adverse effect of isotretinoin. Isr Med
Assoc J. 11:505–506. 2009.PubMed/NCBI
|
|
11.
|
Malvasi A, Tinelli A, Buia A and De Luca
GF: Possible long-term teratogenic effect of isotretinoin in
pregnancy. Eur Rev Med Pharmacol Sci. 13:393–396. 2009.PubMed/NCBI
|
|
12.
|
Azoulay L, Blais L, Koren G, et al:
Isotretinoin and the risk of depression in patients with acne
vulgaris: a case-crossover study. J Clin Psychiatry. 69:526–532.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Gao YG, Wang LZ and Tang JX: Sex
hormone-like activity of Tanshinone (author's transl). Zhongguo Yi
Xue Ke Xue Yuan Xue Bao. 2:189–191. 1980.(In Chinese).
|
|
14.
|
Wang DB and Liu AS: Tanshinone in the
treatment of acne (a primary report of 20 cases) (author’s transl).
Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2:187–188. 1980.(In
Chinese).
|
|
15.
|
Wang DB: Tanshinone in the treatment of
acne. J Tradit Chin Med. 3:227–228. 1983.PubMed/NCBI
|
|
16.
|
Hei M, Luo Y, Zhang X and Liu F:
Tanshinone IIa alleviates the biochemical changes associated with
hypoxic ischemic brain damage in a rat model. Phytother Res.
25:1865–1869. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Tan X, Li J, Wang X, et al: Tanshinone IIA
protects against cardiac hypertrophy via inhibiting
calcineurin/NFATc3 pathway. Int J Biol Sci. 7:383–389. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Cheng CY and Su CC: Tanshinone IIA may
inhibit the growth of small cell lung cancer H146 cells by
up-regulating the Bax/Bcl-2 ratio and decreasing mitochondrial
membrane potential. Mol Med Report. 3:645–650. 2010.PubMed/NCBI
|
|
19.
|
Bian Z, Li LM, Tang R, et al:
Identification of mouse liver mitochondria-associated miRNAs and
their potential biological functions. Cell Res. 20:1076–1078. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Dong X, Dong J, Zhang R, et al:
Anti-inflammatory effects of tanshinone IIA on radiation-induced
microglia BV-2 cells inflammatory response. Cancer Biother
Radiopharm. 24:681–687. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Lin R, Wang WR, Liu JT, et al: Protective
effect of tanshinone IIA on human umbilical vein endothelial cell
injured by hydrogen peroxide and its mechanism. J Ethnopharmacol.
108:217–222. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Qiang J, Xingping Y, Jihai S, et al:
Effects of Cryptotanshinone and Tanshinone A on proliferation,
lipid synthesis and expression of androgen receptor mRNA in human
sebocytes in vitro. Chin J Dermatol. 38:98–101. 2005.(In
Chinese).
|
|
23.
|
Huang Q, Zhou B, Guo X, et al:
Sulfotanshinone sodium suppresses sebaceous hyperplasia in Syrian
hamsters. Chin J Dermatol. 44:643–645. 2011.(In Chinese).
|
|
24.
|
Horton JD and Shimomura I: Sterol
regulatory element-binding proteins: activators of cholesterol and
fatty acid biosynthesis. Curr Opin Lipidol. 10:143–150. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Shimano H: Sterol regulatory
element-binding proteins (SREBPs): transcriptional regulators of
lipid synthetic genes. Prog Lipid Res. 40:439–452. 2001. View Article : Google Scholar
|
|
26.
|
Eberlé D, Hegarty B, Bossard P, et al:
SREBP transcription factors: master regulators of lipid
homeostasis. Biochimie. 86:839–848. 2004.PubMed/NCBI
|
|
27.
|
Rosignoli C, Nicolas JC, Jomard A and
Michel S: Involvement of the SREBP pathway in the mode of action of
androgens in sebaceous glands in vivo. Exp Dermatol. 12:480–489.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Harrison WJ, Bull JJ, Seltmann H, et al:
Expression of lipogenic factors galectin-12, resistin, SREBP-1, and
SCD in human sebaceous glands and cultured sebocytes. J Invest
Dermatol. 127:1309–1317. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Smith TM, Gilliland K, Clawson GA and
Thiboutot D: IGF-1 induces SREBP-1 expression and lipogenesis in
SEB-1 sebocytes via activation of the phosphoinositide 3-kinase/Akt
pathway. J Invest Dermatol. 128:1286–1293. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Chang Y, Wang J, Lu X, et al: KGF induces
lipogenic genes through a PI3K and JNK/SREBP-1 pathway in H292
cells. J Lipid Res. 46:2624–2635. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Hao J, Liu S, Zhao S, et al: PI3K/Akt
pathway mediates high glucose-induced lipogenesis and extracellular
matrix accumulation in HKC cells through regulation of SREBP-1 and
TGF-β1. Histochem Cell Biol. 135:173–181. 2011.PubMed/NCBI
|
|
32.
|
Yang YA, Han WF, Morin PJ, et al:
Activation of fatty acid synthesis during neoplastic
transformation: role of mitogen-activated protein kinase and
phosphatidylinositol 3-kinase. Exp Cell Res. 279:80–90. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Du X, Kristiana I, Wong J and Brown AJ:
Involvement of Akt in ER-to-Golgi transport of SCAP/SREBP: a link
between a key cell proliferative pathway and membrane synthesis.
Mol Biol Cell. 17:2735–2745. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Porstmann T, Griffiths B, Chung YL, et al:
PKB/Akt induces transcription of enzymes involved in cholesterol
and fatty acid biosynthesis via activation of SREBP. Oncogene.
24:6465–6481. 2005.PubMed/NCBI
|