Introduction
A combination of docetaxel (D) and cisplatin (P) is
one of the standard regimens for the initial treatment of advanced
non-small cell lung cancer (NSCLC). The landmark Eastern
Cooperative Oncology Group (ECOG) 1594 (1) and Tax 326 (2) studies used three weekly D doses of 75
mg/m2. Whereas in a Japanese phase II trial (3), three weekly doses of 60
mg/m2 were used. In Korea, the recommended dose of D for
NSCLC is 75 mg/m2 in three weekly doses. Because of
toxicities associated with 75 mg/m2 of D, there have
been concerns related to using this dosage in the Korean
population.
The primary objective of this study was to evaluate
the efficacy of a lower D dose of 60 mg/m2 with 60
mg/m2 P as a first-line treatment for NSCLC compared to
the 75 mg/m2 D dose with 60 mg/m2 P. We also
performed single-nucleotide polymorphism (SNP) analysis of the
cytochrome (CYP)3A5, CYP3A4 and the ABCB1 genes in the patients to
evaluate any toxicity profile differences or responses according to
genotypes.
D is eliminated mainly by hepatic CYP450, CYP3A4 and
CYP3A5 (4). The presence of a SNP
in the 5′-regulatory region of the CYP3A4 gene (−392A>G),
referred to as CYP3A4*1B, has been associated in
vitro with enhanced CYP3A4 expression (5), and a CYP3A5*3 polymorphism
(A6986G) has been shown to lead to an inactive truncated protein
(6).
Moreover, intestinal P-glycoprotein (P-gp, multidrug
resistance 1, ABCB1) plays a main role in fecal elimination of D by
modulating reabsorption of the drug after hepatobiliary secretion
(7). Reduced clearance of D has
been associated with an increased risk of hematologic toxicity
(8). The silent ABCB1 3435C>T
polymorphism has been associated with a lower expression of P-gp
(9). Another polymorphism of the
ABCB1 gene, G2677T/A, has also been reported as a predictor of
response to D chemotherapy (10).
Although controversy exists, 2677GG and 3435CC genotypes have been
associated with a better chemotherapy response and increased
D-related toxicity, probably due to lower P-gp expression levels
(10).
Patients and methods
Study design
In this phase III trial, chemotherapy-naive patients
with stage IIIB or IV NSCLC were randomized into one of two arms,
respectively. The control arm received 75 mg/m2 of D and
60 mg/m2 of P in three weekly doses (75/60 group). The
experimental arm followed the same schedule and received the same
amount of P but only 60 mg/m2 of D (60/60 group). The
randomization was stratified in accordance with ECOG performance
scale (PS) 0–1 vs. 2, weight loss in the previous 6 months <5
vs. ≥5%, and stage IIIB vs. IV or relapsed.
The primary endpoint of the study was to evaluate
the non-inferiority of the experimental arm in terms of the
response rate as measured by the Response Evaluation Criteria in
Solid Tumors (RECIST). The non-inferiority margin was set at −15%.
Ninety-five percent confidence interval for the difference between
two proportions was calculated according to the method described by
Newcombe (11) without a
correction for continuity. Secondary endpoints were
progression-free survival and safety.
A neutrophil count ≥1,500/μl and a platelet count
≥100,000/μl were required to receive the next dose. A 20% dosage
reduction was allowed for grade 4 hematologic toxicities or for
grade 3 or 4 non-hematologic toxicities. Treatment could be delayed
up to two weeks. Toxicity was evaluated with the National Cancer
Institute Common Toxicity Criteria version 3.0.
This study was approved by the Institutional Review
Board of Chonnam National University Hwasun Hospital (#HCRE
07-018-3) (Jeonnam, Korea) and written informed consent was
obtained from all patients.
Patient selection
Enrolled patients met the following inclusion
criteria: stage IIIB/IV or relapsed NSCLC, ≥18 years of age, ECOG
performance status 0–2, presence of uni-dimensionally measurable
lesion(s) by RECIST version 1.0 (12), no prior chemotherapy or
radiotherapy for NSCLC, no other previous investigational therapy,
adequate bone marrow function, adequate liver and renal function,
no prior malignancy and written informed consent.
Exclusion criteria included: carcinoid tumors,
small-cell carcinoma of the lung, a history of another malignancy
within the last five years (except cured basal cell carcinoma of
the skin and cured carcinoma in situ of the uterine cervix),
any other morbidity or situation with contraindications for
chemotherapy (e.g. active infection, myocardial infarction in the
preceding six months, symptomatic heart disease including unstable
angina, congestive heart failure or uncontrolled arrhythmias,
immunosuppressive treatment), pregnant or lactating women, and
women and men of childbearing potential who did not wish to use
adequate contraception.
Definitions of the study populations were as
follows. The intent to treat (ITT) population included 132 patients
who were randomized and treated with at least one cycle of
chemotherapy. All randomized patients received at least one cycle
of chemotherapy. A safety evaluation was performed in this
population. The response evaluable (RE) population included 119
patients whose responses were evaluated.
Polymorphism analysis
Genomic DNA samples were obtained from 87 patients.
DNA was isolated from 0.5 ml EDTA-treated whole blood using a
QIAamp DNA Blood Midi kit (Qiagen, Valencia, CA, USA) according to
the manufacturer's instructions. Primers and enzymes used for the
CYP3A and ABCB1 polymorphism analyses are listed in Table I. The observed frequency of each
genotype was compared with the expected frequency using a
χ2 test (Hardy-Weinberg equilibrium).
 | Table I.Primers and enzymes for CYP3A and
ABCB1 polymorphism analysis. |
Table I.
Primers and enzymes for CYP3A and
ABCB1 polymorphism analysis.
| SNP | Primers (5′→3′) | Enzyme | Size (bp) |
|---|
| CYP3A4 | F,
TGAGGACAGCCATAGAGACAAGG | Direct
sequencing | 98 |
| (−392A>G) | R,
CAAGGGTTCTGGGTTCTTATCA | | |
| CYP3A5 | F,
ACCACCCAGCTTAACGAATG | Direct
sequencing | 98 |
| (A6986G) | R,
ATGTGGTCCAAACAGGGAAG | | |
| ABCB1 exon 21 | F,
TGCAGGCTATAGTTCCAGG | RsaI | 220 |
| G2677A | R,
GTTTGACTCACCTTCCCAG | | |
| ABCB1 exon 21 | F,
TGCAGGCTATAGTTCCAGG | BanI | 224 |
| G2677T | R,
TTTAGTTTGACTCACCTTCCCG | | |
| ABCB1 exon 26 | F,
TGTTTTCAGCTGCTTGATGG | Sau3AI | 197 |
| C3435T | R,
AAGGCATGTATGTTGGCCTC | | |
Genotyping of CYP3A4 (−392A>G, RefSNP,
rs2740574) and CYP3A5 (A6986G, RefSNP, rs776746)
Genotyping of CYP3A4 and CYP3A5 was
determined by polymerase chain reaction (PCR) followed by direct
sequencing. PCR was performed using a Takara PCR Thermal Cycler
Dice TP600 (Takara Shuzo, Tokyo, Japan). Amplified DNA was purified
using a QIAquick DNA purification system (Qiagen). Genotyping for
the CYP3A4*1A/*1A,
CYP3A4*1A/*1B and
CYP3A4*1B/*1B genotypes (AA, AG and GG,
respectively, at nucleotide −392A>G in CYP3A4) and the
CYP3A5*1/*1,
CYP3A5*1/*3 and
CYP3A5*3/*3 genotypes (AA, AG, and GG,
respectively, at nucleotide 6986A>G in CYP3A5) were carried out
by direct-sequencing using an ABI-PRISM® 3100 genetic
analyzer (Applied Biosystems).
Genotyping of ABCB1 exon 21 G2677T/A
(RefSNP, rs2032582) and ABCB1 exon 26 C3435T (RefSNP, rs1045642)
polymorphisms
Genotyping of the ABCB1 exon 21 G2677T/A and exon 26
C3435T polymorphisms was performed using polymerase chain
reaction-restrict fragment length polymorphism (PCR-RFLP). In
brief, the PCR assay was performed in a 10-μl reaction system. The
PCR products for ABCB1 exon 21 and exon 26 were
digested by the restriction enzymes, RsaI (Takara Shuzo),
BanI and Sau3AI (New England BioLabs, Inc., Ipswich,
MA, USA) in a total volume of 20 μl for 3 h, 20 μl for 3 h, and 20
μl for 4 h at 37°C. The digested products were separated on an 8%
acrylamide gel. Restriction fragments were visualized after
ethidium bromide staining of the acrylamide gel with the use of an
ultraviolet transilluminator. Sequencing with an
ABI-PRISM® 3100 genetic analyzer (Applied Biosystems)
was used to confirm the PCR-RFLP results.
Results
Clinical efficacy and toxicity
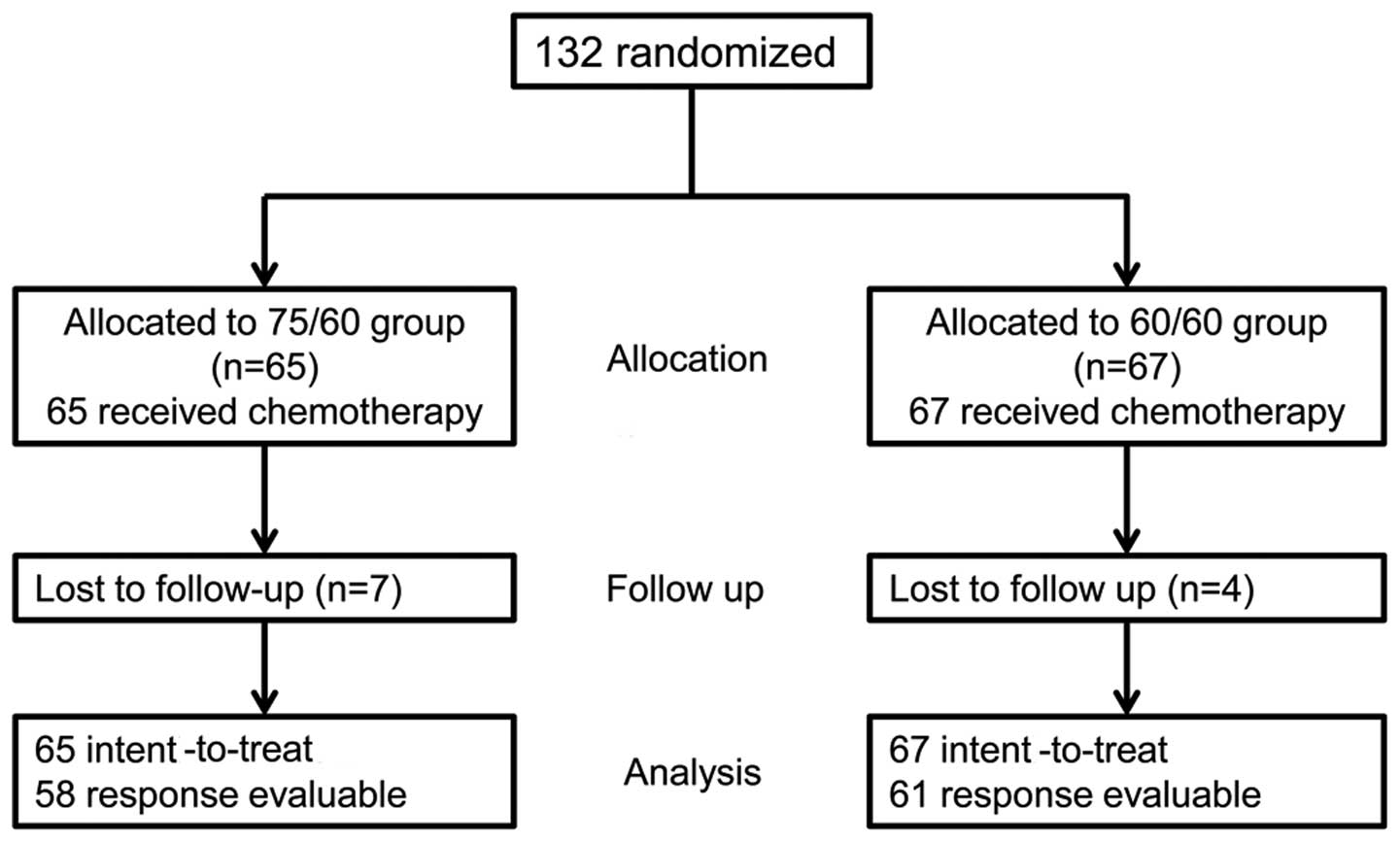
From September 2007 to September 2009, 132 patients
were enrolled in the study and randomized to the 75/60 (n=65) or
60/60 group (n=67) (Fig. 1). As
shown in Table II, both groups
were well-matched in terms of age, gender, histology, performance
status, stage and weight loss. After the first-line treatment, the
number of subsequent treatment lines and proportion of patients
treated with epidermal growth factor tyrosine kinase inhibitor were
not different between the two groups.
 | Table II.Characteristics of the intent to treat
(ITT) population (n=132). |
Table II.
Characteristics of the intent to treat
(ITT) population (n=132).
| Characteristics | 75/60 group
(n=65) | 60/60
group(n=67) | P-value |
|---|
| Age (mean ± standard
dev) | 59.9±10.1 | 59.3±10.8 | >0.05 |
| Male/female | 46/19 | 50/17 | >0.05 |
| Histology
(ADC/SQC/LCC/NSCLC-NOS) | 40/22/1/2 | 42/17/4/4 | >0.05 |
| ECOG PS 0-1 or 2 | 56/9 | 57/10 | >0.05 |
| Stage IIIB/IV or
relapsed | 6/59 | 7/60 | >0.05 |
| Weight loss (<5 or
≥5%) | 57/8 | 58/9 | >0.05 |
| Subsequent treatment
after first-line treatment failure | | | |
| Treatment
linesa (mean ±
standard dev) | 2.8±1.5 | 3.1±1.7 | >0.05 |
| Use of EGFR-TKI
(%) | 41 (63.1) | 46 (68.7) | >0.05 |
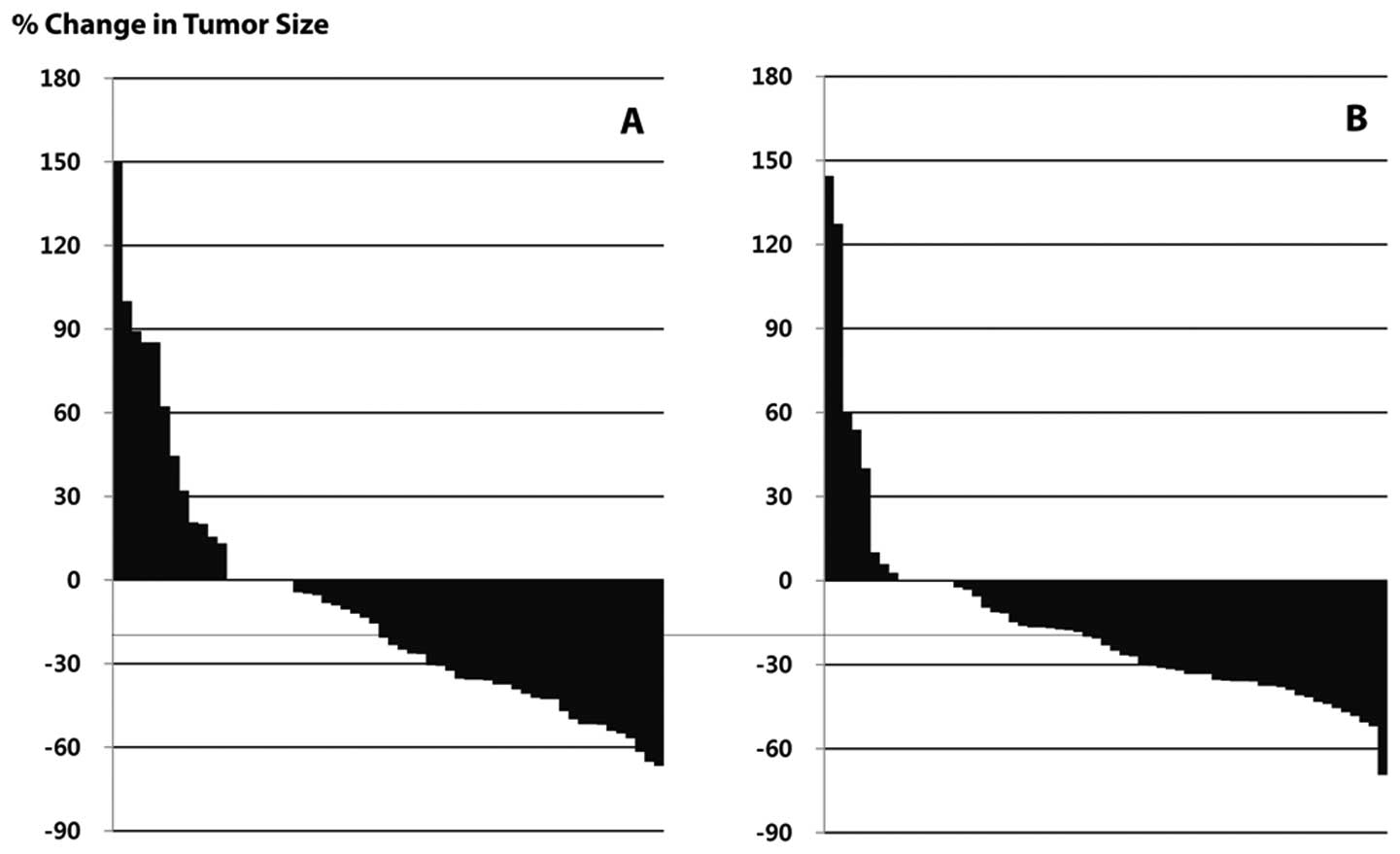
In the ITT population, response rates (RR) were
38.5% in the 75/60 group and 40.3% in the 60/60 group (Fig. 2 and Table III). The 95% confidence interval
for the difference in response rate was −14.8 to 18.5%. Since the
range was higher than the predefined non-inferiority limit, we
concluded that the response rate of the 60/60 group was not
inferior to the 75/60 group. There were no significant differences
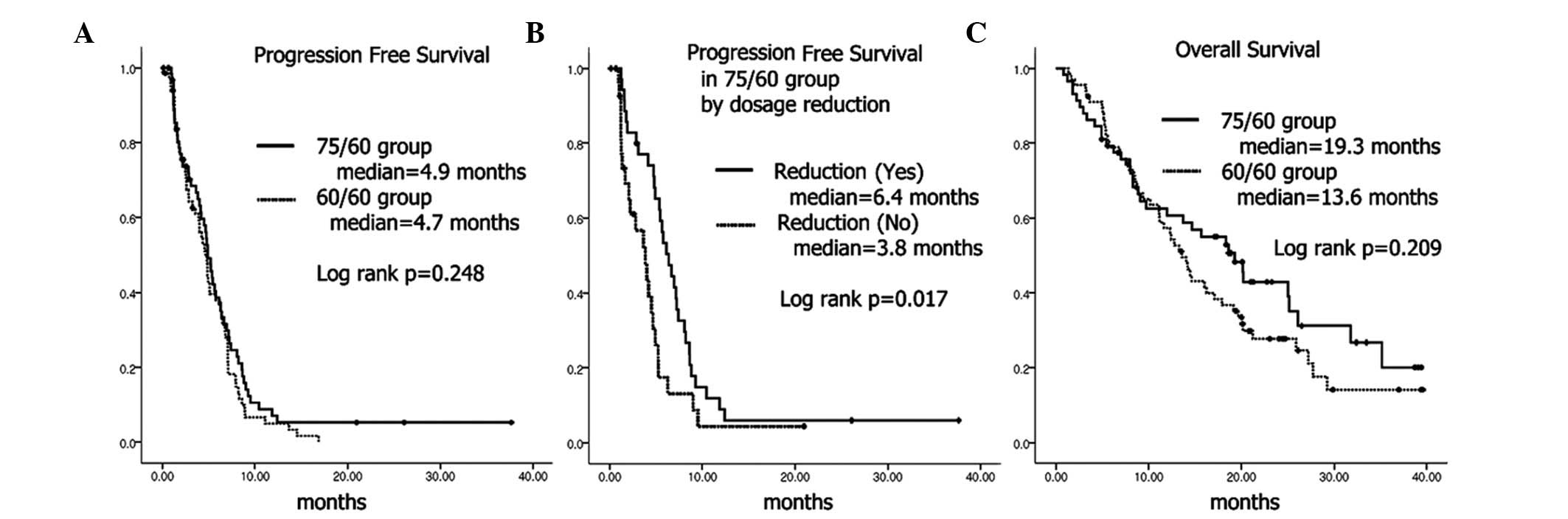
in the number of cycles (3.42 vs. 3.57), or progression-free
survival (median, 4.9 vs. 4.7 months) between the two groups
(Fig. 3A). However, the dose
reduction rate (53.8 vs. 22.4%, P<0.001), and incidence of grade
3–4 neutropenia (81.3 vs. 60.3%, P=0.009) were significantly higher
in the 75/60 group compared to the 60/60 group. No significant
difference in overall survival was noted between the two groups
(Fig. 3C).
 | Table III.Comparison of the results after
treatment in the intent to treat (ITT) population. |
Table III.
Comparison of the results after
treatment in the intent to treat (ITT) population.
| 75/60 group
(n=65) | 60/60 group
(n=67) | P-value |
|---|
| PR/SD/PD/NE | 25/23/10/7 | 27/27/7/6 | |
| Response rate
(ITT) | 38.5% | 40.3% | >0.05 |
| Response rate
(RE) | 43.1% | 44.3% | >0.05 |
| Relative dose
intensity (mean ± standard dev) | 93.8±6.2 | 97.9±4.1 | <0.001 |
| Dose reduction | | | |
| Yes/no (%) | 35/30 (53.8) | 15/52 (22.4) | <0.001 |
| Cycles (mean ±
standard dev) | 3.42±1.69 | 3.57±1.71 | >0.05 |
| Grade 3 or 4
toxicities (%) | | | |
| Leukopenia | 33/64 (51.6) | 22/63 (34.9) | 0.058 |
| Neutropenia | 52/64 (81.3) | 38/63(60.3) | 0.009 |
| Anemia | 0/52 | 2/47 (4.3) | >0.05 |
| Anorexia | 4/49 (8.2) | 4/52 (7.7) | >0.05 |
| Diarrhea | 4/18 (22.2) | 1/22 (4.5) | >0.05 |
|
Nausea/vomiting | 3/20 (15.0) | 4/28 (14.3) | >0.05 |
Within the 75/60 group, dosage reductions were made
for 35 patients, while no dosage modification was performed in 30
patients. Age of patients, number of delivered cycles, response
rate and progression-free survival were significantly higher in
patients with dosage reductions compared to those whose doses were
not reduced (Table IV and Fig. 3B).
 | Table IV.Comparison of the characteristics
between the patients whose doses were reduced vs. those whose doses
were not reduced in the 75/60 group. |
Table IV.
Comparison of the characteristics
between the patients whose doses were reduced vs. those whose doses
were not reduced in the 75/60 group.
| Dose reduced
(n=35) | Not reduced
(n=30) | P-value |
|---|
| Age (mean ±
standard dev) | 62.3±10.2 | 57.2±9.3 | <0.05 |
| ECOG PS 0–1/2 | 31/4 | 25/5 | >0.05 |
| PR/SD/PD/NE | 21/12/2/0 | 4/11/8/7 | |
| Response
rate | 60.0% | 13.3% | <0.001 |
| Relative dose
intensity (mean ± standard dev) | 89.1±4.0 | 99.3±2.5 | <0.001 |
| Cycles (mean ±
standard dev) | 4.06±1.42 | 2.67±1.67 | <0.05 |
Genotyping analysis
Genotyping for the CYP3A4, CYP3A5 and ABCB1 genes
were performed in 87 patients. None of the observed frequencies
were significantly different from expected values (Table V). Among 86 patients who were
tested for a CYP3A4 polymorphism, all had the AA genotype, while
other alleles of CYP3A5 and the ABCB1 gene showed certain
proportions in 87 patients.
 | Table V.Hardy-Weinberg equilibrium for SNPs
of the CYP3A5, CYP3A4 and ABCB1 genes. |
Table V.
Hardy-Weinberg equilibrium for SNPs
of the CYP3A5, CYP3A4 and ABCB1 genes.
CYP3A5 A6986G
| CYP3A4 −392A>G
| ABCB1 C3435T
| ABCB1 G2677T/A
|
|---|
| Obs | Exp | | Obs | Exp | | Obs | Exp | | Obs | Exp |
|---|
| AA | 5 | 5.8 | AA | 86 | 86 | CC | 44 | 40.7 | GG | 22 | 18.9 |
| AG | 35 | 33.4 | AG | 0 | 0 | CT | 31 | 37.6 | GT | 21 | 27.9 |
| GG | 47 | 47.8 | GG | 0 | 0 | TT | 12 | 8.7 | TT | 12 | 10.3 |
| | | | | | | | | GA | 16 | 15.4 |
| | | | | | | | | TA | 15 | 11.4 |
| | | | | | | | | AA | 1 | 3.1 |
| χ2 | 0.210 | | | | | χ2 | 2.691 | | χ2 | 5.137 | |
| df | 1 | | | | | df | 1 | | df | 3 | |
| P | 0.647 | | | | | P | 0.100 | | P | 0.162 | |
| A | 0.26 | | A | 1 | | C | 0.68 | | G | 0.47 | |
| G | 0.74 | | G | 0 | | T | 0.32 | | T | 0.34 | |
| | | | | | | | | A | 0.19 | |
When related to hematologic toxicities, the CYP3A5
A6986G polymorphism showed a significant correlation. Patients with
G alleles (non-expressing variant) showed significantly higher
incidence of grade 3–4 neutropenia (Table VI). No significant correlation was
found between ABCB1 and CYP3A4 genotypes, and hematologic
toxicities. Haplotype analysis with CYP3A5 and ABCB1 SNPs showed no
significant association with hematologic toxicities. There was also
no significant correlation between genotypes and chemotherapy
response.
 | Table VI.Genotypes of the CYP3A5, CYP3A4 and
ABCB1 genes and hematologic toxicities. |
Table VI.
Genotypes of the CYP3A5, CYP3A4 and
ABCB1 genes and hematologic toxicities.
| Single-nucleotide
polymorphism | Genotype | Patients with grade
3–4 neutropenia | Patients without
grade 3–4 neutropenia |
P-value(χ2 test) |
|---|
| CYP3A5
(A6986G) | *1/*1
(AA) | 2 | 3 | 0.047 |
| *1/*3
(AG) | 22 | 12 | |
| *3/*3
(GG) | 38 | 8 | |
| CYP3A4
(-A392G) | *1A/*1A
(AA) | 62 | 22 | |
| *1A/*1B
(AG) | 0 | 0 | |
| *1B/*1B
(GG) | 0 | 0 | |
| ABCB1 (C3435T) | C/C | 29 | 13 | 0.718 |
| C/T | 24 | 7 | |
| T/T | 9 | 3 | |
| ABCB1
(G2677T/A) | G/G | 16 | 6 | 0.424 |
| G/T(A) | 24 | 12 | |
| T(A)/T(A) | 22 | 5 | |
Discussion
Lung cancer is a heterogeneous disease. In the past
it was only necessary to know whether it was small-cell lung cancer
or NSCLC to choose a chemotherapy regimen. Recently, we have begun
using different chemotherapy regimens for squamous cell carcinoma
or non-squamous cell carcinoma, even though both types are in the
NSCLC category. In addition, different small-molecule inhibitors
are being used based on the molecular characteristics of tumors
even though they may have the same histology (13).
This personalized treatment for lung cancer requires
knowledge not only of the characteristics of tumor cells but also
of the individual characteristics of patients harboring the tumors.
Since the pharmacokinetic or pharmacodynamic profile may vary
according to individual or racial characteristics, an attempt has
been made to explain most differences by SNPs.
In this study, we showed the non-inferiority of a 60
mg/m2 dose of D compared to a 75 mg/m2 dose
of D in a Korean population, which is similar to previously
published Japanese data (3).
Therefore, we propose that D 60 mg/m2 in three weekly
doses, which is lower than the standard dose of D 75
mg/m2, is optimal for East Asian populations in terms of
its proven efficacy and toxicity profile.
To answer the question as to where genetic
differences may arise, we used genomic DNA acquired from the
subjects in this trial to study polymorphisms of CYP3A4, CYP3A5 and
ABCB1 which could affect the activity of proteins dealing with D.
We found that the frequencies of those three genotypes were not
different from previous reports (14) which studied Asians, Caucasians and
Africans. In our study, the proportion of G allele in CYP3A5 was
0.74, which has been reported as 0.69–0.78 in Asians, 0.81–0.94 in
Caucasians and 0.14–0.53 in Africans. This ethnic difference
suggests that the optimal dose of D for Asians and Caucasians can
differ from that of Africans. In terms of different incidence of
neutropenia according to the CYP3A5 genotype, we suggest that the
optimal dose of D can be tailored based on this genotype.
According to our data, the incidence of toxicity and
response rates did not differ according to the SNPs of CYP3A4 and
ABCB1. In this study, every patient had the A allele in the CYP3A4
locus, suggesting low activity of CYP3A4. The frequency of the A
allele was reported as 0.96–1.0 in Caucasians and Asians, while in
a study of Africans, it was 0.3. The frequency of C allele in the
ABCB1 C3435T locus was 0.68 compared to Asians and Caucasians
(0.36–0.66) and Africans (0.70–0.86). In the ABCB1 G2677T/A locus,
the frequency of the G allele was 0.47 in comparison to Asians and
Caucasians (0.29–0.61) and Africans (0.89–0.97).
One can raise the question as to whether there was a
possibility that the higher dose reduction rate in the 75/60 group
decreased its efficacy. To address this issue, we compared the
response rate within the 75/60 group. In 35 patients, doses of D
were reduced during the course of treatment and in 23 patients
doses were not reduced. Although the dose intensity was lower in
the reduced group, the response rate was rather higher in patients
whose dose was reduced compared to patients without dose reduction.
In the 75/60 group, progression-free survival was significantly
superior to the patients whose dosage was reduced compared to those
without dosage reduction (Fig.
3B). This trend was consistent even in the 60/60 group.
Progression-free survival tended to be longer in the dosage reduced
group, although not significant. Thus, the answer to the question
as to whether dose reduction reduced efficacy was negative because
even in the 75/60 group, patients with dose reduction showed higher
response rates and longer progression-free survival.
In conclusion, docetaxel 60 mg/m2 with
cisplatin was not inferior to docetaxel 75 mg/m2 with
cisplatin in response rate, and the lower docetaxel dose provides
Korean patients with a better safety profile. Pharmacogenomic and
racial differences should be considered in the next clinical trial
design.
Acknowledgements
This study was supported by funding
from Sanofi-Aventis Korea.
References
|
1.
|
Schiller JH, Harrington D, Belani CP, et
al: Comparison of four chemotherapy regimens for advanced non-small
cell lung cancer. N Engl J Med. 346:92–98. 2002. View Article : Google Scholar
|
|
2.
|
Fossella F, Pereira JR, von Pawel J, et
al: Randomized, multinational, phase III study of docetaxel plus
platinum combinations versus vinorelbine plus cisplatin for
advanced non-small cell lung cancer: the TAX 326 study group. J
Clin Oncol. 21:3016–3024. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Okamoto H, Watanabe K, Segawa Y, et al:
Phase II study of docetaxel and cisplatin in patients with
previously untreated metastatic non-small cell lung cancer. Int J
Clin Oncol. 5:316–322. 2000. View Article : Google Scholar
|
|
4.
|
Shou M, Martinet M, Korzekwa KR, Krausz
KW, Gonzalez FJ and Gelboin HV: Role of human cytochrome P450 3A4
and 3A5 in the metabolism of taxotere and its derivatives: enzyme
specificity, interindividual distribution and metabolic
contribution in human liver. Pharmacogenetics. 8:391–401. 1998.
View Article : Google Scholar
|
|
5.
|
Amirimani B, Ning B, Deitz AC, Weber BL,
Kadlubar FF and Rebbeck TR: Increased transcriptional activity of
the CYP3A4*1B promoter variant. Environ Mol Mutagen.
42:299–305. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Kuehl P, Zhang J, Lin Y, et al: Sequence
diversity in CYP3A promoters and characterization of the genetic
basis of polymorphic CYP3A5 expression. Nat Genet. 27:383–391.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
van Zuylen L, Verweij J, Nooter K, Brouwer
E, Stoter G and Sparreboom A: Role of intestinal P-glycoprotein in
the plasma and fecal disposition of docetaxel in humans. Clin
Cancer Res. 6:2598–2603. 2000.PubMed/NCBI
|
|
8.
|
Bruno R, Hille D, Riva A, et al:
Population pharmacokinetics/pharmacodynamics of docetaxel in phase
II studies in patients with cancer. J Clin Oncol. 16:187–196.
1998.PubMed/NCBI
|
|
9.
|
Hoffmeyer S, Burk O, von Richter O, et al:
Functional polymorphisms of the human multidrug-resistance gene:
multiple sequence variations and correlation of one allele with
P-glycoprotein expression and activity in vivo. Proc Natl Acad Sci
USA. 97:3473–3478. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Pan JH, Han JX, Wu JM, Huang HN, Yu QZ and
Sheng LJ: MDR1 single nucleotide polymorphism G2677T/A and
haplotype are correlated with response to docetaxel-cisplatin
chemotherapy in patients with non-small cell lung cancer.
Respiration. 78:49–55. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Newcombe RG: Interval estimation for the
difference between independent proportions: comparison of eleven
methods. Stat Med. 17:873–890. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Therasse P, Arbuck SG, Eisenhauer EA, et
al: New guidelines to evaluate the response to treatment in solid
tumors. European Organization for Research and Treatment of Cancer,
National Cancer Institute of the United States, National Cancer
Institute of Canada. J Natl Cancer Inst. 92:205–216. 2000.
View Article : Google Scholar
|
|
13.
|
Gandara DR, Mack PC, Li T, Lara PN Jr and
Herbst RS: Evolving treatment algorithms for advanced non-small
cell lung cancer: 2009 looking toward 2012. Clin Lung Cancer.
10:392–394. 2009. View Article : Google Scholar
|
|
14.
|
Turolo S, Tirelli AS, Ferraresso M, et al:
Frequencies and roles of CYP3A5, CYP3A4 and ABCB1 single nucleotide
polymorphisms in Italian teenagers after kidney transplantation.
Pharmacol Rep. 62:1159–1169. 2010. View Article : Google Scholar : PubMed/NCBI
|