Introduction
Osteosarcoma (OS), as a malignant primary bone
tumor, typically occurs in children, adolescents and young adults
(1), with an age-standardized
incidence of approximately 5 per million per year (2). Despite the improvement of
multidisciplinary treatment, including aggressive surgical
resection and intensive multiagent chemotherapy and radiotherapy
(3), 30% of patients with
localized disease and 80% with metastatic disease at diagnosis will
relapse (4,5), and 40% of patients succumb to lung
metastases (6). Identifying
prognostic factors in OS aids selection of those patients for more
aggressive management at early time points (7–9).
CD133, also known as prominin-1, is a member of the
pentaspan transmembrane glycoproteins (10,11).
Although it was initially expressed in hematopoietic stem cells,
CD133 presentation was also found in various solid tumors, such as
hepatocarcinoma (12), melanoma
(13) and synovial sarcoma
(14), but not in OS to date.
However, Tirino et al (15)
identified CD133+ cells within OS cell lines SAOS2, U2OS
and MG-63 in 2008, and other investigators (16,17)
have confirmed their presence in various OS cell lines.
Furthermore, we investigate the expression of CD133 in OS tissues
in this study.
In addition, CD133 has been found to be a prognostic
factor for certain cancer types. Several studies have reported that
the presence of CD133 in various tumors was correlated with poor
prognosis. Song et al (18)
found that high expression of CD133 was correlated with increased
tumor grade, advanced disease stage, elevated serum α-fetoprotein
levels and poor survival of patients with hepatocellular carcinoma.
Similarly, Horst et al (19) found that CD133 expression was an
independent prognostic marker for low survival in colorectal
cancer. Furthermore, Zhang et al (20) reported that CD133 expression was a
predictor of poor response to chemotherapy and of reduced
disease-free survival time for patients with ovarian cancer.
However, Qin et al (21)
revealed that CD133 was not associated with cisplatin-based
chemotherapy resistance or shorter overall survival of patients
with advanced serous ovarian cancer. Moreover, Fan et al
(22) demonstrated that
CD133-negative expression correlated with poor prognosis, whereas
CD133-positive expression predicted favorable outcome in
cholangiocarcinoma patients.
To date, the association between CD133 expression
and prognosis of OS remains unknown. In this study, we analyzed the
association of CD133 expression in OS with clinical factors and
overall survival, and further investigated its potential role in
metastasis in vitro.
Materials and methods
Patient data collection
Paraffin-embedded OS sections from 70 patients who
were diagnosed with primary OS and had undergone initial surgery at
the Sixth People’s Hospital, Shanghai Jiao Tong University,
Shanghai, China, between January 2002 and January 2010 were
obtained from the Department of Pathology for immunohistochemical
staining. Follow-up information was updated through December 31,
2011, by reviewing medical records and telephone contact. The use
of tissue blocks and the chart reviews were approved by the Ethics
Committee of the Sixth People’s Hospital, Shanghai JiaoTong
University. The relevant clinical data included gender, age, tumor
location, tumor size, Ennecking stage, local recurrence status,
lung metastasis status and overall survival. Overall survival was
calculated as the time from the date of diagnosis to the date of
death or the date of last follow-up if the patient was still
surviving.
Immunohistochemistry
Paraffin sections (4-μm thickness) were
deparaffinized and treated with 3% hydrogen peroxide for 10 min to
quench the endogenous peroxidase activity. Antigenic retrieval was
performed by submerging in citric acid (pH 6.0) and microwaving.
The slides were then allowed to cool at room temperature, followed
by incubation in normal goat serum for 1 h to block nonspecific
binding, then incubated overnight at 4°C using CD133 antibody
(1:100, Abcam, Hong Kong), and examined using HRP Envision Systems
(Dako, Shanghai, China). Rabbit IgG was used as the primary
antibody for the negative control. Finally, the sections were
visualized after counterstaining with hematoxylin. Each section was
evaluated by three independent pathologists without knowledge of
the clinical case features. The whole sections were screened for
CD133 expression under ×100 magnification.
Flow cytometry
To measure the proportions of CD133+
cells in the human MG63 OS cell line, cells were detached using
0.02% EDTA in phosphate-buffered saline (PBS), counted and washed
in PBS. At least 105 cells were incubated with
CD133/2(293C3)-APC (1:100; Miltenyi Biotec, Auburn, CA, USA) at 4°C
for 30 min in the dark. After washing steps, the labeled cells were
analyzed by flow cytometry (Beckman Coulter, Brea, CA, USA).
Magnetic-activated cell sorting
(MACS)
CD133+ cells in MG63 were magnetically
labeled using a CD133 microbead kit (Miltenyi Biotec) as described
in the manufacturer’s instructions. Briefly, 108 cells
were dissociated and resuspended in 300 μl PBS supplemented
with 0.5% bovine serum albumin (BSA) and 2 mM EDTA (pH 7.2). CD133
microbeads were used for positive selection by MACS cell separation
using two MACS MS columns consecutively. After separation by MACS,
aliquots of the positive and negative sorted populations were
evaluated for purity by flow cytometry. Purities ranged from 90 to
95% for positive and from 89 to 99% for negative populations.
During the experiment, 4–6 passages of the sorted cells were
used.
Immunofluorescence staining
CD133+ and CD133− cells
cultured in 6-well plates were fixed in 4% paraformaldehyde for 30
min at 4°C, washed in PBS, treated with PBS supplemented with 1%
BSA for 1 h at room temperature and then stained with CD133
antibody (1:100) at 4°C overnight. Goat anti-rabbit IgG-FITC (CW
Biotec, Beijing, China) was used as a secondary antibody, the
nuclei were stained with DAPI. Cells were then washed and observed
under a fluorescence microscope (Olympus BX41, Tokyo, Japan).
Western blotting
Protein (50 μg) prepared from MG-63,
CD133+ and CD133− cells were loaded per lane
and electrophoresed in SDS-PAGE, and then transferred onto
polyvinylidene difluoride Immobilon-P membranes (Bio-Rad, Hercules,
CA, USA) using a transblot apparatus (Bio-Rad). The membranes were
blocked in 10 mmol/l Tri-HCl (pH 8.0), 150 mmol/l NaCl and 0.05%
Tween-20 (TBST) with 5% (w/v) non-fat milk at room temperature,
followed by overnight incubation at 4°C with primary antibodies
diluted in TBST [1:1000 for CD133; 1:1000 for β-actin (CW Biotec)].
After washing with TBST, the membranes were incubated for 1 h with
an HRP-conjugated secondary antibody diluted 1:5000 in TBST, and
the labeled proteins were detected using the enhanced
chemiluminescence reagents and exposed to the film.
Scratch wound-healing assay
Migratory ability was determined using a scratch
wound-healing assay. CD133+ and CD133− cells
were seeded and grown to confluence, and then scratches were made
on the cell layer with a pipette tip running across the dishes.
Plates were washed twice with fresh medium to remove non-adherent
cells. Locations (n=3–4) were visualized and photographed at 0 and
24 h under a phase-contrast inverted microscope (Olympus BX41,
Japan). The distance between the two edges of the scratch was
measured (23).
Matrigel invasion assay
Cell invasion was performed using 24-well Transwells
(8-mm pore size; Corning, NY, USA) coated with Matrigel (1 mg/ml;
BD, NJ, USA) in triplicate. CD133+ and CD133−
cells (105 per well) were seeded in the upper chambers
in culture media containing 0.2% fetal bovine serum (FBS), and the
lower chambers were filled with 500 μl 10% FBS medium to
induce cell migration. Following incubation for 24 h, cells inside
the chamber were wiped off with a cotton swab, invading cells were
stained with Giemsa (Lexiang Biotec, Shanghai, China) and examined
using microscopy (Olympus BX41). Cells in at least six random
microscopic fields (×200 magnification) were counted to determine
relative invasive potential.
Reverse transcription-polymerase chain
reaction (RT-PCR)
Total cellular RNA of CD133+ and
CD133− cells was extracted using TRIzol reagent (Ambion,
Austin, TX, USA) and treated with RNase-free DNase (DNase I,
Ambion) to remove potential genomic DNA contaminants. Total RNA (1
μg) was reverse-transcribed with the RETROscript™
Two-Step RT-PCR system (Ambion). Reactions were performed according
to the manufacturer’s instructions using SYBR green PCR supermix
(Sangon Biotec, Shanghai, China) in a single-color RT-PCR detection
system (Stratagene, Santa Clara, CA, USA). The gene expression
levels (mRNA) of octamer-binding transcription factor 4 (Oct-4),
NANOG, and metastasis-related receptor C-X-C chemokine receptor
type 4 (CXCR4) were normalized to that of the GAPDH transcript.
Sequences for mRNAs from the nucleotide data bank (National Center
for Biotechnology Information, USA) were used to design primer
pairs for RT-PCR reactions (Table
I).
 | Table IList of primer sets used in this
study. |
Table I
List of primer sets used in this
study.
| Gene (GenBank
accession no.) | Sequence (5′ to
3′) | Tm (°C) | Location |
|---|
| Oct 4
(NM_002701) |
| Forward |
CTTGAATCCCGAATGGAAAGGG | 61 | 42–63 |
| Reverse |
GTGTATATCCCAGGGTGATCCTC | | 205–183 |
| NANOG
(NM_024865) |
| Forward |
TTTGTGGGCCTGAAGAAAACT | 61 | 83–103 |
| Reverse |
AGGGCTGTCCTGAATAAGCAG | | 198–178 |
| CXCR4
(NM_003467) |
| Forward |
TGACGGACAAGTACAGGCTG | 61 | 215–234 |
| Reverse |
AGGGAAGCGTGATGACAAAGA | | 277–257 |
| GAPDH
(NM_002046) |
| Forward |
AAGGTGAAGGTCGGAGTCAAC | 61 | 7–27 |
| Reverse |
GGGGTCATTGATGGCAACAATA | | 108–87 |
Statistics
Correlations between CD133 and clinicopathological
features were examined by the Chi-square test. Survival rate was
calculated using the Kaplan-Meier method. Univariate and
multivariate survival analyses were performed to test the
association of clinicopathological features with OS, incorporating
log-rank testing and Cox proportional hazard regression models.
Comparison of the two experimental groups was performed using the
independent-samples T-test. Statistical analyses were conducted
using SPSS 16.0. Data were expressed as the mean ± SEM. A value of
P<0.05 was considered to indicate statistical significance.
Results
Patient clinical characteristics
As shown in Table
II, there were equal numbers of male and female patients, and
29 (41.4%) patients were older than 18 years of age. Tumors were
located in axial (1/70), upper limb (5/70) and lower limb (64/70)
locations. In total, 20 (28.6%) and 54 (77.1%) patients had local
recurrence and lung metastases, respectively. Regarding Ennecking
staging, 58 patients were at stage II and 12 at stage III. Over the
course of the study, 63 patients succumbed to tumor-related causes.
The median overall survival of patients was 20.0 months [95%
confidence interval (CI), 16.6–23.4 months].
 | Table IICorrelation between CD133 expression
and clinico-pathological factors in the osteosarcoma patients. |
Table II
Correlation between CD133 expression
and clinico-pathological factors in the osteosarcoma patients.
| Variable | n | CD133
| P-value |
|---|
| Positive | Negative |
|---|
| Gender | | | | |
| Male | 35 | 26 | 9 | 0.131 |
| Female | 35 | 20 | 15 | |
| Age | | | | |
| ≤18 years | 41 | 24 | 17 | 0.233 |
| >18 years | 29 | 21 | 8 | |
| Tumor location | | | | |
| Axial | 1 | 1 | 0 | 0.773 |
| Upper limb | 5 | 4 | 1 | |
| Lower limb | 64 | 40 | 24 | |
| Tumor size | | | | |
| <10 cm | 38 | 22 | 16 | 0.224 |
| ≥10 cm | 32 | 23 | 9 | |
| Ennecking
stage | | | | |
| II | 58 | 35 | 23 | 0.130 |
| III | 12 | 10 | 2 | |
| Local
recurrence | | | | |
| Yes | 20 | 13 | 7 | 0.937 |
| No | 50 | 32 | 18 | |
| Lung
metastasis | | | | |
| Yes | 54 | 40 | 14 | 0.002 |
| No | 16 | 5 | 11 | |
Correlation between CD133 expression and
clinicopathological characteristics
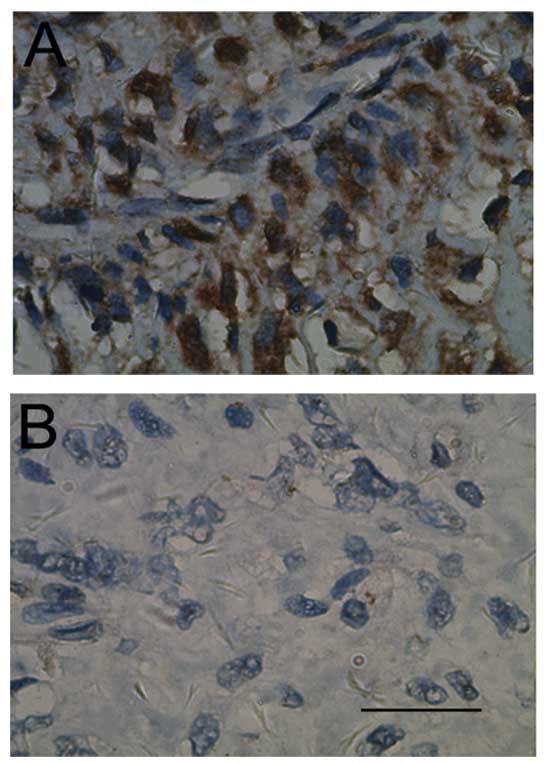
CD133 stained the cytoplasm and membrane of tumor
cells and representative images of immunostaining of OS tissues are
shown in Fig. 1. Cases were
defined as CD133-positive if CD133 staining was detected in >10%
of the entire tumor area (21,24).
As shown in Table
II, CD133 expression was found in 46/70 (65.7%) of OS samples.
Of the 54 patients who developed lung metastasis, 40 cases were in
the CD133-positive group, whereas only 14 cases were in the
negative group (P=0.002), indicating that CD133 expression was
positively correlated with lung metastasis as analyzed by the
Chi-square test. However, no significant association was observed
between CD133 expression and any of the other clinicopathological
characteristics listed in Table
II.
Correlation between CD133 expression and
prognosis of OS patients
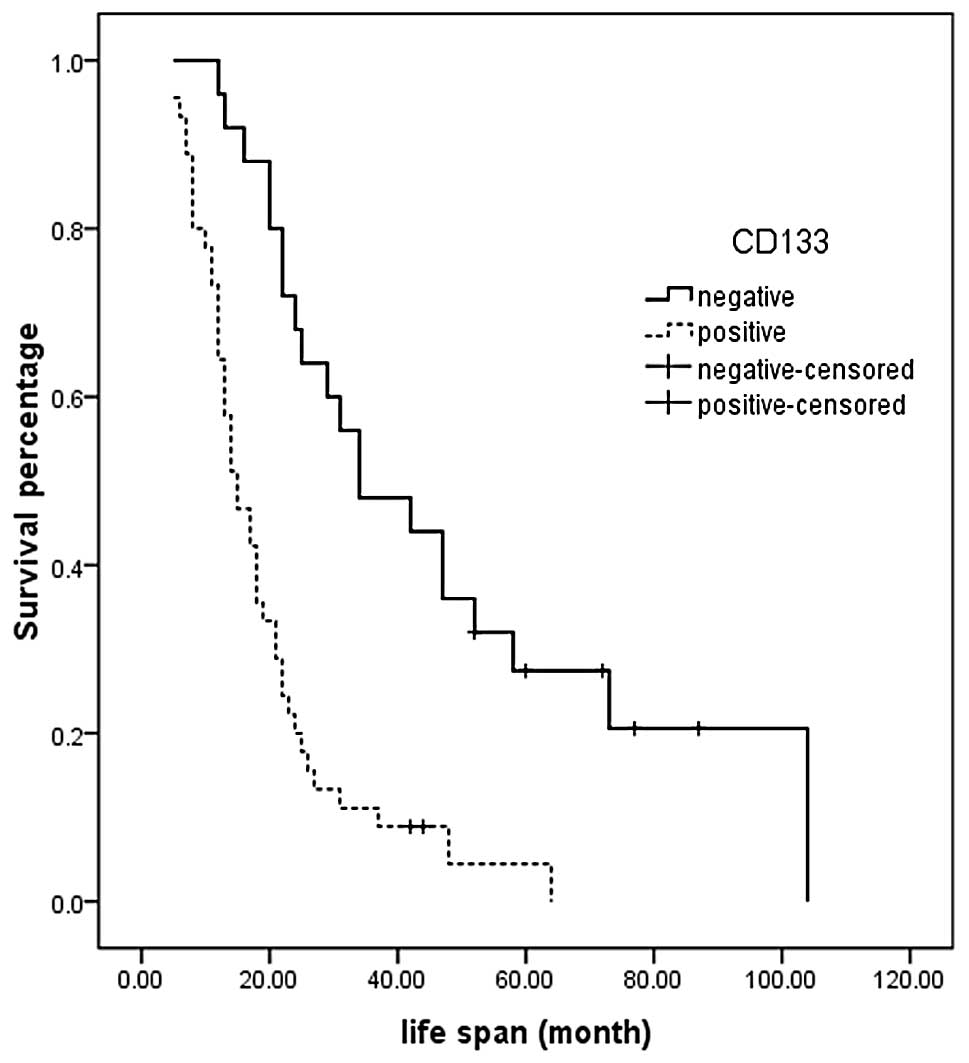
At the cut-off date, the median overall survival was
significantly shorter in the CD133-positive group compared with the
CD133-negative group (34.0 months; 95% CI, 16.0–51.9 vs. 15.0
months; 95% CI, 11.3–18.7; P=0.000) (Fig. 2). Univariate survival analysis
showed that the significant prognostic factors were tumor size,
local recurrence, lung metastasis and expression of CD133.
Multivariate analysis showed that the CD133 expression and tumor
size were independent prognostic factors of patients with OS
(Table III).
 | Table IIIMultivariate Cox regression analysis
of potential prognostic factors for osteosarcoma patients. |
Table III
Multivariate Cox regression analysis
of potential prognostic factors for osteosarcoma patients.
| B | SE | Wald | df | Sig. | Exp (B) | 95% CI for Exp (B)
|
|---|
| Lower | Upper |
|---|
| CD133
expression | 1.135 | 0.310 | 13.391 | 1 | 0.000 | 3.112 | 1.694 | 5.716 |
| Local
recurrence | 0.438 | 0.287 | 2.320 | 1 | 0.128 | 1.549 | 0.882 | 2.720 |
| Lung
metastasis | 0.336 | 0.338 | 0.984 | 1 | 0.321 | 1.399 | 0.721 | 2.716 |
| Tumor size | 0.551 | 0.266 | 4.287 | 1 | 0.038 | 1.734 | 1.030 | 2.921 |
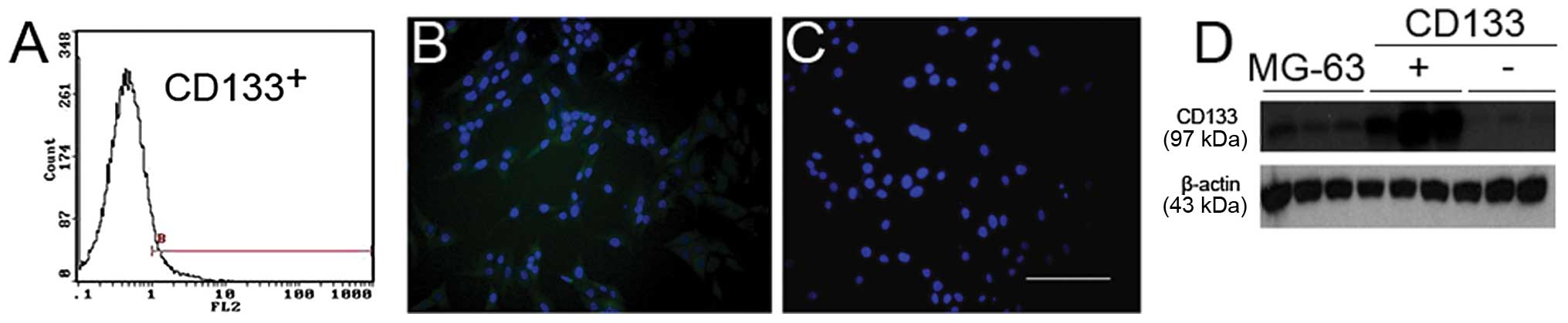
Identification of CD133+ and
CD133− cells
The patient data showed that expression of CD133 was
related with lung metastasis in OS. To investigate the potential
mechanisms, we sorted the OS cell line MG-63 for CD133+
and CD133− populations using MACS, and further confirmed
our results using immunofluorescence staining and western blotting
(Fig. 3).
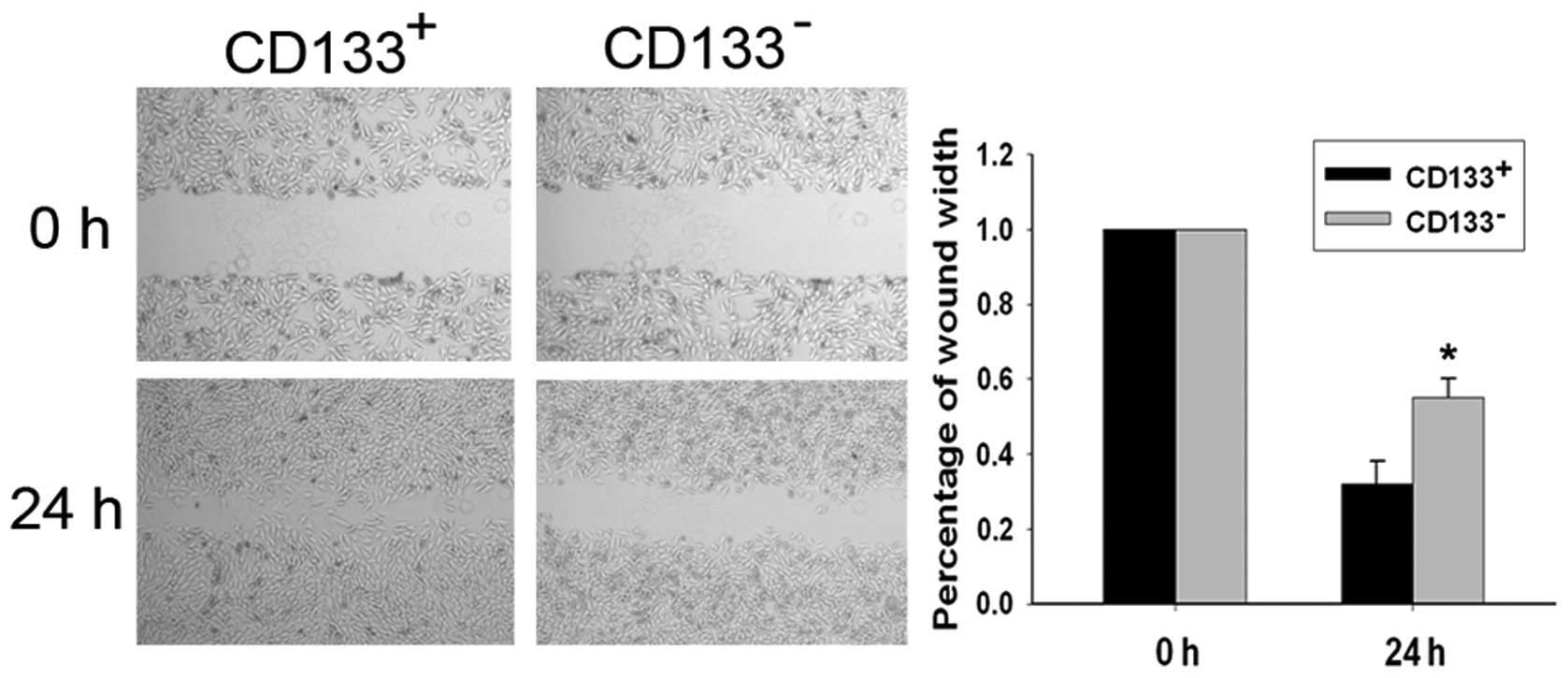
Migratory ability of CD133+
and CD133− cells
Migratory ability of the CD133+ and
CD133− cell populations was examined using a
wound-healing assay (Fig. 4).
Following incubation of physically wounded cells for 24 h,
CD133+ cells were found to have traveled a significantly
longer distance than CD133− cells (P<0.05). The
percentages of wound width were 32.00±6.11 and 54.67±5.21%,
respectively.
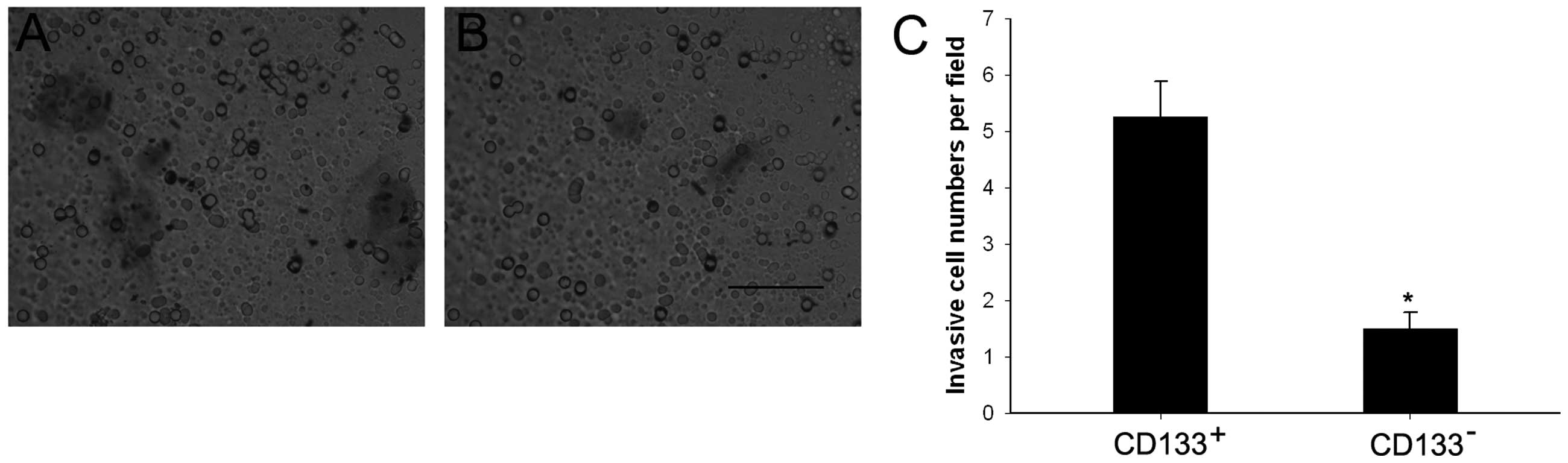
Invasiveness of CD133+ and
CD133− cells
To analyze invasiveness of the CD133+ and
CD133− cell populations, we performed Transwell invasion
assays using cell culture inserts covered with extracellular matrix
components. Cells (5.25±0.63 per field) traveled through the
membranes in the CD133+ group, compared to only
1.50±0.29 cells per field in the CD133− group
(P<0.05) (Fig. 5).
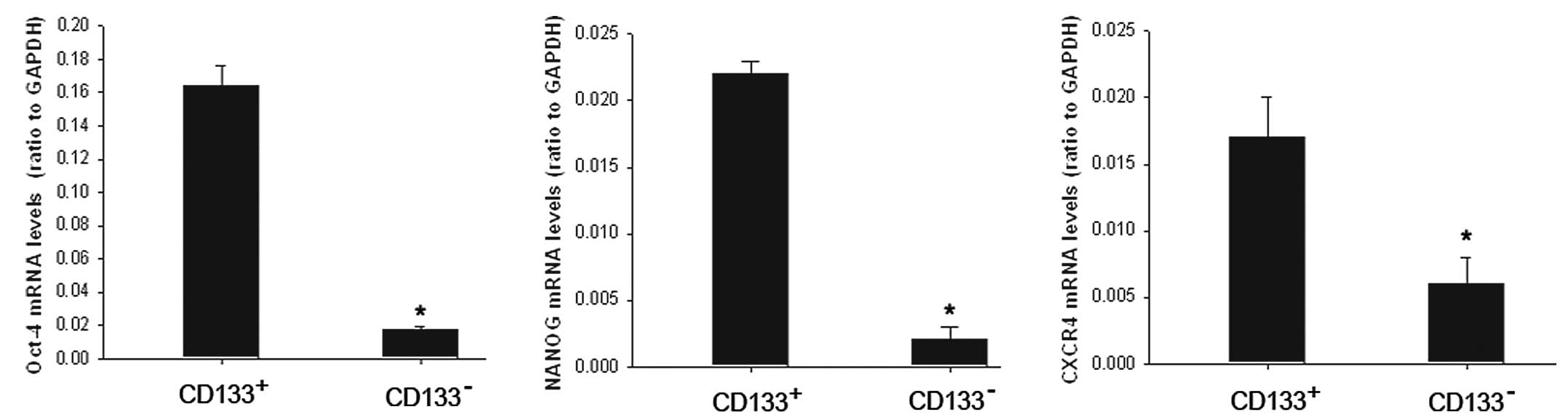
mRNA expression of Oct-4, NANOG and
CXCR4
As CD133 has been considered to be a cancer stem
cell marker in several tumor types, we examined mRNA expression of
the stemness gene Oct-4, NANOG and metastasis-associated receptor
CXCR4 using RT-PCR in the CD133+ population and its
counterpart CD133− cells. The results showed that mRNA
expression was significantly higher in the CD133+
population (Fig. 6).
Discussion
OS is a highly aggressive tumor, comprising
approximately 20% of all bone tumors and 5% of pediatric tumors
(25). However, our current
understanding of OS etiology is limited. Previous cancer studies
have shown that many tumors contain a small population of cells
termed cancer stem cells (CSCs), which are responsible for tumor
progression, metastasis, recurrence and resistance to chemotherapy
and radiation treatments (26,27).
While the experimental evidence for the existence of CSCs was first
proposed for hematological malignancies, more recently CSCs have
been observed in solid tumors, including breast, brain, pancreatic
and bone cancers (28). The
existence of CSCs in primary OS and cell lines derived from human
OS was previously demonstrated (15,29).
CD133 has been considered as a CSCs marker in a
number of tumor types (28,30,31),
such as colorectal, brain, prostate, pancreatic and gastric
cancers, and also in OS. Subsequent studies have confirmed that
CD133+ cells from OS cell lines showed stem-like
features including high proliferation rate, cells detected in the
G2/M phase of the cell cycle and Ki-67 positivity (15). In our patient data, we found that
CD133 was a worse prognostic factor for OS and lung metastasis.
In vitro, we found that CD133+ cells efficiently
invaded and migrated, according to high expression of Oct-4, NANOG
and CXCR4. These findings support the proposed link between CD133
and CSCs.
Oct-4, also known as Oct-3, Oct-3/4 and POU5F1, is
one of the earliest transcription factors expressed in the embryo,
and has been identified as fundamental to the maintenance of
pluripotency and self-renewal in embryonic stem cells and
primordial germ cells (32,33).
NANOG is also a homeodomain transcription factor thought to be a
key factor in sustaining the pluripotency of embryonic stem cells
(34). A recent study of Oct-4 and
NANOG in OS demonstrated that the two markers were highly expressed
in CSCs, which suggests that these transcription factors play a
role in sarcoma stem cell biology (35). CXCR4, a chemokine receptor in the
GPCR gene family, has been proven to play an essential role in the
metastasis of CSCs (36). OS stem
cells were found to express more CXCR4 than normal tumor cells
(37).
In conclusion, our findings revealed for the first
time that high expression of CD133 in OS tissues indicates a high
lung metastasis risk and short survival time in OS patients.
CD133+ cells were more active in invasion and migration
than CD133− cells, in accordance with higher expression
of Oct-4, NANOG and CXCR4. These findings support the proposed link
between CD133 and CSCs. However, further animal experiments using
in vivo cell xenografts are warranted to confirm this.
Prognostic judgment in order to improve the effect of treatment on
OS and more suitable treatment strategies including the application
of CD133 target gene therapy could be accomplished according to the
expression status of CD133.
Acknowledgements
The study was supported by grants from
the National Natural Science Foundation of China (no. 81001191 and
81172105) and Science and Technology Commission of Shanghai, China
(no. 10PJ1408300 and 09140902200).
References
|
1.
|
Arndt CA and Crist WM: Common
musculoskeletal tumors of childhood and adolescence. N Engl J Med.
341:342–352. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Ottaviani G and Jaffe N: The epidemiology
of osteosarcoma. Cancer Treat Res. 152:3–13. 2009. View Article : Google Scholar
|
|
3.
|
Ozaki T, Flege S, Kevric M, et al:
Osteosarcoma of the pelvis: experience of the Cooperative
Osteosarcoma Study Group. J Clin Oncol. 21:334–341. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Saeter G, Hoie J, Stenwig AE, Johansson
AK, Hannisdal E and Solheim OP: Systemic relapse of patients with
osteogenic sarcoma. Prognostic factors for long term survival.
Cancer. 75:1084–1093. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Tabone MD, Kalifa C, Rodary C, Raquin M,
Valteau-Couanet D and Lemerle J: Osteosarcoma recurrences in
pediatric patients previously treated with intensive chemotherapy.
J Clin Oncol. 12:2614–2620. 1994.PubMed/NCBI
|
|
6.
|
Ham SJ, Schraffordt Koops H, van der Graaf
WT, van Horn JR, Postma L and Hoekstra HJ: Historical, current and
future aspects of osteosarcoma treatment. Eur J Surg Oncol.
24:584–600. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Lin F, Zheng SE, Shen Z, et al:
Relationships between levels of CXCR4 and VEGF and blood-borne
metastasis and survival in patients with osteosarcoma. Med Oncol.
28:649–653. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Lee JA, Kim MS, Kim DH, et al: Relative
tumor burden predicts metastasis-free survival in pediatric
osteosarcoma. Pediatr Blood Cancer. 50:195–200. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Yang J, Yang D, Cogdell D, et al: APEX1
gene amplification and its protein overexpression in osteosarcoma:
correlation with recurrence, metastasis, and survival. Technol
Cancer Res Treat. 9:161–169. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Yin AH, Miraglia S, Zanjani ED, et al:
AC133, a novel marker for human hematopoietic stem and progenitor
cells. Blood. 90:5002–5012. 1997.PubMed/NCBI
|
|
11.
|
Weigmann A, Corbeil D, Hellwig A and
Huttner WB: Prominin, a novel microvilli-specific polytopic
membrane protein of the apical surface of epithelial cells, is
targeted to plasmalemmal protrusions of non-epithelial cells. Proc
Natl Acad Sci USA. 94:12425–12430. 1997. View Article : Google Scholar
|
|
12.
|
Yin S, Li J, Hu C, et al: CD133 positive
hepatocellular carcinoma cells possess high capacity for
tumorigenicity. Int J Cancer. 120:1444–1450. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Monzani E, Facchetti F, Galmozzi E, et al:
Melanoma contains CD133 and ABCG2 positive cells with enhanced
tumourigenic potential. Eur J Cancer. 43:935–946. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Terry J and Nielsen T: Expression of CD133
in synovial sarcoma. Appl Immunohistochem Mol Morphol. 18:159–165.
2009. View Article : Google Scholar
|
|
15.
|
Tirino V, Desiderio V, d’Aquino R, et al:
Detection and characterization of CD133+ cancer stem
cells in human solid tumours. PLoS One. 3:e34692008. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Li J, Liu W, Zhao K, et al: Diallyl
trisulfide reverses drug resistance and lowers the ratio of
CD133+ cells in conjunction with methotrexate in a human
osteosarcoma drug-resistant cell subline. Mol Med Report.
2:245–252. 2009.PubMed/NCBI
|
|
17.
|
Veselska R, Hermanova M, Loja T, et al:
Nestin expression in osteosarcomas and derivation of nestin/CD133
positive osteosarcoma cell lines. BMC Cancer. 8:3002008. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Song W, Li H, Tao K, et al: Expression and
clinical significance of the stem cell marker CD133 in
hepatocellular carcinoma. Int J Clin Pract. 62:1212–1218. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Horst D, Kriegl L, Engel J, Kirchner T and
Jung A: CD133 expression is an independent prognostic marker for
low survival in colorectal cancer. Br J Cancer. 99:1285–1289. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Zhang J, Guo X, Chang DY, Rosen DG,
Mercado-Uribe I and Liu J: CD133 expression associated with poor
prognosis in ovarian cancer. Mod Pathol. 25:456–464. 2011.
View Article : Google Scholar
|
|
21.
|
Qin Q, Sun Y, Fei M, et al: Expression of
putative stem marker nestin and CD133 in advanced serous ovarian
cancer. Neoplasma. 1–2. 2012.PubMed/NCBI
|
|
22.
|
Fan L, He F, Liu H, et al: CD133: a
potential indicator for differentiation and prognosis of human
cholangiocarcinoma. BMC Cancer. 11:3202011. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Li S, Li Z, Guo F, et al: miR-223
regulates migration and invasion by targeting Artemin in human
esophageal carcinoma. J Biomed Sci. 18:242011. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Kojima M, Ishii G, Atsumi N, Fujii S,
Saito N and Ochiai A: Immunohistochemical detection of CD133
expression in colorectal cancer: a clinicopathological study.
Cancer Sci. 99:1578–1583. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Tang N, Song WX, Luo J, Haydon RC and He
TC: Osteosarcoma development and stem cell differentiation. Clin
Orthop Relat Res. 466:2114–2130. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Wion D and Berger F: Cancer stem cells. N
Engl J Med. 355:2703author reply 2703. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Pardal R, Clarke MF and Morrison SJ:
Applying the principles of stem-cell biology to cancer. Nat Rev
Cancer. 3:895–902. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Liu B, Ma W, Jha RK and Gurung K: Cancer
stem cells in osteosarcoma: recent progress and perspective. Acta
Oncol. 50:1142–1150. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Gibbs CP, Kukekov VG, Reith JD, et al:
Stem-like cells in bone sarcomas: implications for tumorigenesis.
Neoplasia. 7:967–976. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Singh SK, Hawkins C, Clarke ID, et al:
Identification of human brain tumour initiating cells. Nature.
432:396–401. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Ricci-Vitiani L, Lombardi DG, Pilozzi E,
et al: Identification and expansion of human
colon-cancer-initiating cells. Nature. 445:111–115. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Niwa H, Miyazaki J and Smith AG:
Quantitative expression of Oct-3/4 defines differentiation,
dedifferentiation or self-renewal of ES cells. Nat Genet.
24:372–376. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Looijenga LH, Stoop H, de Leeuw HP, et al:
POU5F1 (OCT3/4) identifies cells with pluripotent potential in
human germ cell tumors. Cancer Res. 63:2244–2250. 2003.PubMed/NCBI
|
|
34.
|
Mitsui K, Tokuzawa Y, Itoh H, et al: The
homeoprotein Nanog is required for maintenance of pluripotency in
mouse epiblast and ES cells. Cell. 113:631–642. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Wang L, Park P and Lin CY:
Characterization of stem cell attributes in human osteosarcoma cell
lines. Cancer Biol Ther. 8:543–552. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Hermann PC, Huber SL, Herrler T, et al:
Distinct populations of cancer stem cells determine tumor growth
and metastatic activity in human pancreatic cancer. Cell Stem Cell.
1:313–323. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Adhikari AS, Agarwal N, Wood BM, et al:
CD117 and Stro-1 identify osteosarcoma tumor-initiating cells
associated with metastasis and drug resistance. Cancer Res.
70:4602–4612. 2010. View Article : Google Scholar : PubMed/NCBI
|