Introduction
Hepatocellular carcinoma (HCC) is a problem
worldwide, particularly in Asian countries (1–3).
Unlike most solid cancers, the incidence and mortality rates for
HCC are projected to increase substantially in many countries over
the next 20 years, mostly as a result of infections with hepatitis
C and hepatitis B viruses (4). It
has become possible to identify a group of patients with chronic
liver disease who are at a high risk of developing HCC. In
addition, improvements in diagnostic imaging have allowed early
diagnosis of HCC. However, the majority of HCC patients are first
seen when the disease has reached an advanced stage at which
curative treatment is no longer possible (4).
Since HCC is considered to be chemoresistant in
general, results of systemic chemotherapy have previously been
disappointing (5). Sorafenib
(Nexavar, Bayer Healthcare Pharmaceuticals), a multi-kinase
inhibitor that blocks tumor growth and cell proliferation, was the
first systemic chemotherapeutic agent found to improve the survival
time of patients with advanced HCC in the SHARP and Asian Pacific
trials (6,7). Sorafenib has opened a novel era for
the treatment of advanced HCC. However, it is associated with a low
tumor response rate, minimal survival advantage and high rates of
adverse events (6,7).
Transcatheter arterial chemoembolization (TACE) is a
procedure whereby an embolizing agent is injected into the hepatic
artery to deprive the tumor of its major nutrient source via
embolization of the nutrient artery, resulting in ischemic necrosis
of the tumor with minimization of systemic side effects. It has
become the most popular palliative treatment for patients with
unresectable HCC (8–10). Patients with well preserved liver
function and multi-nodular HCC without vascular invasion appear to
be the best candidates for TACE (8–10).
However, TACE is no longer considered to be contra-indicated in
advanced HCC with portal vein tumor thrombus (PVTT) (11,12),
and even in advanced HCC patients with extrahepatic metastasis, in
cases in which extrahepatic spread is minimal and local control of
liver tumors is considered more important, TACE is useful and may
obtain survival benefits and has often been used in these cases in
Japan (5,13). However, the long-term outcomes are
less favorable in general for advanced HCCs treated with TACE,
since the devascularization effect induced by TACE is transient,
resulting in tumor progression (14).
Recently, concurrent or sequential treatment methods
of advanced HCC with TACE and sorafenib with a manageable safety
profile and a possibility of promising efficacy have been reported
(15,16). However, regarding comparison of
survival outcomes of advanced HCC patients treated with TACE and
those treated with sorafenib, there have been no reliable data to
the best of our knowledge to date.
The present study aimed to compare overall survival
between stage IVA or IVB HCC patients who received TACE and those
who were treated with sorafenib.
Patients and methods
Patients
This retrospective comparative study included 55
patients with stage IVA or IVB HCC in whom TACE was performed as an
initial treatment (the TACE group) between April 2004 and November
2011 at the department of Gastroenterology and Hepatology, Osaka
Red Cross Hospital, Japan and 56 patients with stage IVA or IVB HCC
in whom sorafenib was administered (the sorafenib group) between
June 2009 and October 2011 at our department. Since the aim of the
present study was to compare clinical outcomes between stage IV HCC
patients treated with TACE and those treated with sorafenib, 6
patients in whom TACE was performed as an initial treatment and
thereafter sorafenib treatment was started were excluded in the
present study. None of the TACE group patients received systemic
chemotherapy and locoregional therapy other than TACE during the
follow-up period. None of the sorafenib group patients received
previous systemic chemotherapy. After patients were provided with
sufficient information regarding TACE and sorafenib treatment, they
themselves decided whether they were treated with TACE or
sorafenib. Written informed consent was obtained from all patients
prior to each treatment and this study protocol complied with all
provisions of the Declaration of Helsinki.
Diagnosis of HCC
HCC was diagnosed using abdominal ultrasound and
dynamic computed tomography (CT) scans (hyperattenuation during the
arterial phase in all or some part of the tumor and hypoattenuation
in the portal-venous phase), mainly based on the recommendations of
the American Association for the Study of Liver Diseases (17). The presence of vascular invasion of
the tumor was confirmed with the demonstration of a low-attenuation
intraluminal mass expanding the portal vein, the bile duct, or the
hepatic vein and/or filling defects in these vascular sites at
dynamic CT. Arterial and portal phase dynamic CT images were
obtained at approximately 30 and 120 sec, respectively, after
injecting contrast material. Abdominal CT, chest CT, bone
scintigraphy, brain CT and/or brain magnetic resonance imaging were
performed prior to treatment in all stage IVB HCC patients.
Diagnosis of stage IVB HCC was determined using these imaging
modalities. Histopathological examination for metastasis was not
performed. All eligible patients in the present study had
bidimensionally measurable, inoperable HCC, no prior systemic
treatments for HCC, an Eastern Cooperative Oncology Group (ECOG)
performance status of 0 or 1, and a Child-Pugh classification of
either A or B.
TACE procedure
TACE for HCC was performed in conformity with
Japanese guidelines for this therapy, comprising catheterization
via the femoral artery with super-selective cannulation to the
hepatic artery feeding the target HCC (18). Farmorubicin (epirubicin
hydrochloride; Pfizer, New York, NY, USA) was infused at 20–60 mg,
mitomycin (mitomycin C; Kyowa Hakko, Tokyo, Japan) was infused at
4–14 mg, and Lipiodol (iodine addition products of ethyl esters of
fatty acids obtained from poppy seed oil; Mitsui, Japan) was also
injected at 2–15 ml according to the tumor size and tumor number.
This was followed by embolization with gelatin (Spongel;
Yamanouchi, Japan), which was injected slowly to prevent reflux
into untreated segments. The sites of injection of the embolizing
agents were segmental or subsegmental in all patients.
In the TACE group, after the initial TACE, another
session of TACE was performed every 4–12 weeks until one of the
following end points were reached: i) technical impossibility in
performing TACE; ii) complete devascularization of the target HCC;
iii) development of contraindications to TACE such as liver
failure.
Sorafenib dose and treatment
Initiated sorafenib dose was determined considering
factors such as patient’s body weight, performance status, and
liver function. In all patients with Child-Pugh B, the initiated
sorafenib dose was 200 mg twice a day (b.i.d.). Sorafenib treatment
continued until one of the following criteria was met: disease
progression, unacceptable drug-related toxicities or patient’s wish
for discontinuation.
Evaluation of treatment efficacy
Tumor response was assessed at 8–12 weeks according
to the modified Response Evaluation Criteria in Solid Tumors
(RECIST) criteria using dynamic CT scans. The change in viable
perfused tumor volume of the targeted lesions as measured on the
arterial phase imaging before and after treatment was evaluated
(19).
Follow-up
In the TACE group, follow-up consisted of monthly
blood tests and monitoring of tumor markers. Dynamic CT scans were
obtained every 8–12 weeks during the follow-up period. No patients
were lost to follow-up in the TACE group. In the sorafenib group,
follow-up consisted of weekly blood tests for the purpose of
detecting adverse events and monitoring of tumor markers. Dynamic
CT scans were obtained every 8–12 weeks during the follow-up
period. No patients were lost to follow-up in the sorafenib
group.
Statistical analysis
The primary end point was overall survival. It was
calculated from the date of first diagnosis with stage IVA or stage
IVB HCC using imaging modalities until death from any cause or the
last follow-up. Differences between the two groups were analyzed
using the unpaired t-test for continuous variables, and the
categorical variables were analyzed using the Fisher’s exact test.
The overall survival curves were generated using the Kaplan-Meier
method and compared using the log-rank test. All statistical tests
were two-sided. All data were analyzed using SPSS software, version
9.0 (SPSS Inc., Chicago, IL, USA) for Microsoft Windows. Data are
expressed as means ± standard deviation (SD). Values of P<0.05
were considered to indicate statistical significance.
Results
Baseline characteristics
Baseline characteristics between the TACE group and
the sorafenib group are shown in Table
I. There were 55 patients in the TACE group and 56 in the
sorafenib group. In the TACE group, there were 46 stage IVA HCC
patients and 9 stage IVB HCC patients, respectively. In the
sorafenib group, there were 26 stage IVA HCC patients and 30 stage
IVB HCC patients, respectively. Fifty-one patients (91.1%) in the
sorafenib group had received previous locoregional therapies such
as percutaneous thermal ablation, percutaneous ethanol injection
therapy or transcatheter arterial infusion chemotherapy without
embolization, and all patients in the sorafenib group received at
least one dose of sorafenib. In terms of HCC stage, there was a
significant difference between the two groups (P<0.001).
However, in terms of gender, age, etiology of liver disease,
maximum tumor size, Child-Pugh classification, and laboratory data
including tumor markers and body mass index, there were no
significant differences between these two groups.
 | Table IBaseline characteristics between the
TACE group and the sorafenib group. |
Table I
Baseline characteristics between the
TACE group and the sorafenib group.
| TACE group
(n=55) | Sorafenib group
(n=56) | P-value |
|---|
| Gender (M/F) | 42/13 | 46/10 | 0.490a |
| Age (years) | 67.9±10.0 | 69.1±12.0 | 0.563b |
| Etiology of liver
disease | | | |
| B/C/B,C/non-B,
non-C | 7/29/4/15 | 9/29/1/17 | 0.575a |
| Child-Pugh
classification | | | |
| Child-Pugh
A/Child-Pugh B | 35/20 | 42/14 | 0.221a |
| HCC stage | | | |
| Stage IVA/stage
IVB | 46/9 | 26/30 | <0.001a |
| Maximum tumor size
(cm) | 7.6±3.1 | 6.3±4.4 | 0.087b |
| Total-bilirubin
(mg/dl) | 1.03±0.69 | 0.91±0.50 | 0.311b |
| Serum albumin
(g/dl) | 3.51±0.56 | 3.66±0.49 | 0.120b |
| Platelets
(×104/mm3) | 16.1±7.7 | 15.3±8.2 | 0.595b |
| ALT (IU/l) | 66.5±86.8 | 45.7±39.7 | 0.106b |
| Prothrombin time
(%) | 85.8±17.0 | 82.7±12.4 | 0.268b |
| AFP (ng/ml) |
25,223.4±96,684.3 |
17,945.7±92,746.3 | 0.674b |
| PIVKAII
(mAU/ml) |
50,535.4±83,206.0 |
28,613.6±117,254.6 | 0.259b |
| Body mass index
(kg/m2) | 22.4±3.3 | 22.2±4.1 | 0.756b |
Overall survival
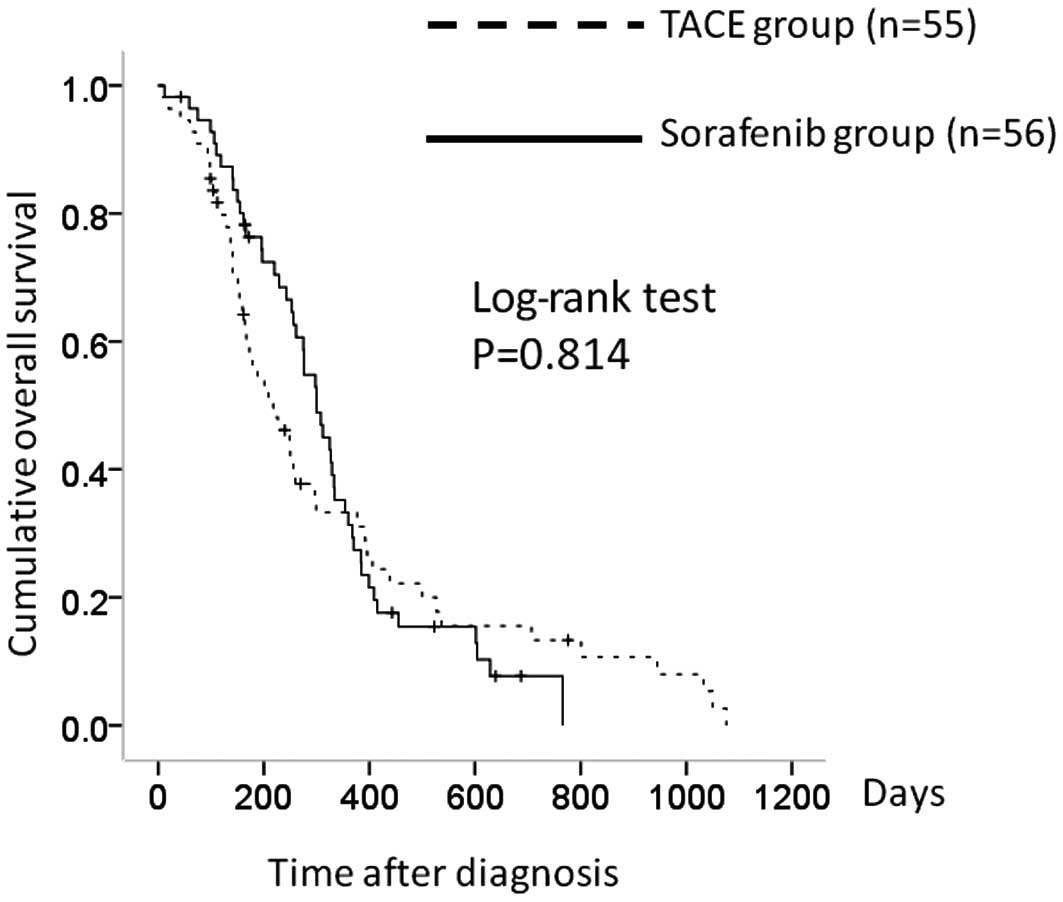
Median overall survival times were 6.6 months in the
TACE group and 9.2 months in the sorafenib group. The 1- and 2-year
overall survival rates were 34.4 and 14.2%, respectively, in the
TACE group and 34.0 and 6.7%, respectively, in the sorafenib group.
In terms of overall survival, there was no significant difference
between the two groups (P= 0.814) (Fig. 1).
Subgroup analyses
Comparison between the TACE and
sorafenib group patients with stage IVA HCC
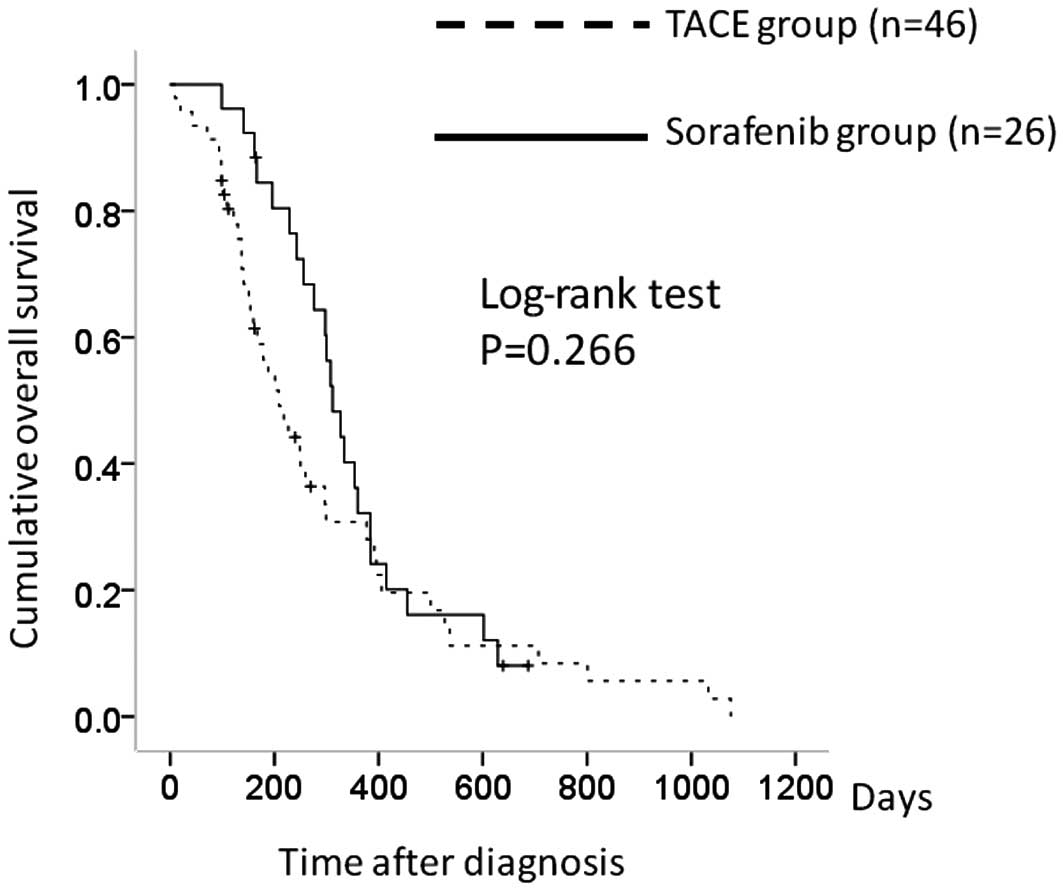
There were 46 patients in the TACE group with stage
IVA HCC and 26 in the sorafenib group. The 1-year overall survival
rates were 30.6% in the TACE group and 32.8% in the sorafenib
group. In terms of overall survival, there was no significant
difference between the two groups (P=0.266) (Fig. 2).
Comparison between the TACE and
sorafenib group patients with stage IVB HCC
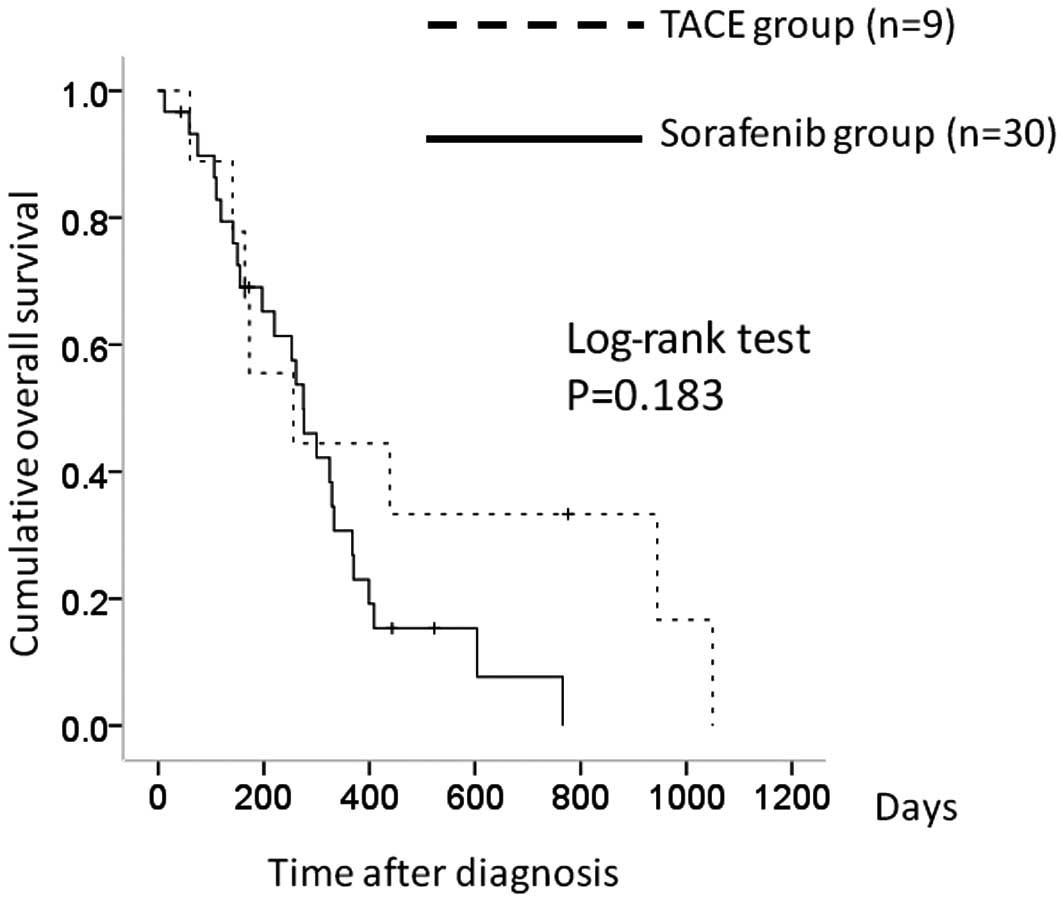
There were 9 patients in the TACE group with stage
IVB HCC and 30 in the sorafenib group. The 1-year overall survival
rates were 43.5% in the TACE group and 30.2% in the sorafenib
group. In terms of overall survival, there was no significant
difference between the two groups (P=0.183) (Fig. 3).
Comparison between the TACE and
sorafenib group patients with Child-Pugh A
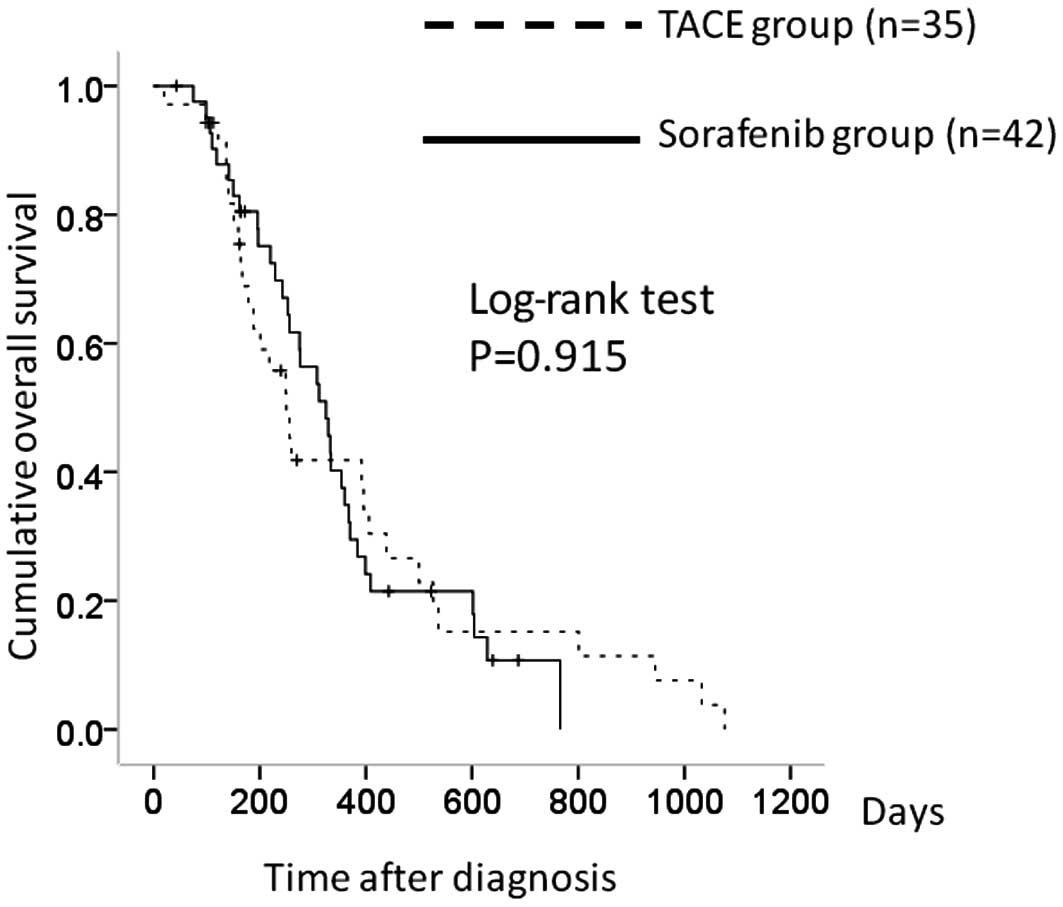
There were 35 patients in the TACE group with
Child-Pugh A and 42 in the sorafenib group. The 1-year overall
survival rates were 41.1% in the TACE group and 30.4% in the
sorafenib group. In terms of overall survival, there was no
significant difference between the two groups (P=0.915) (Fig. 4).
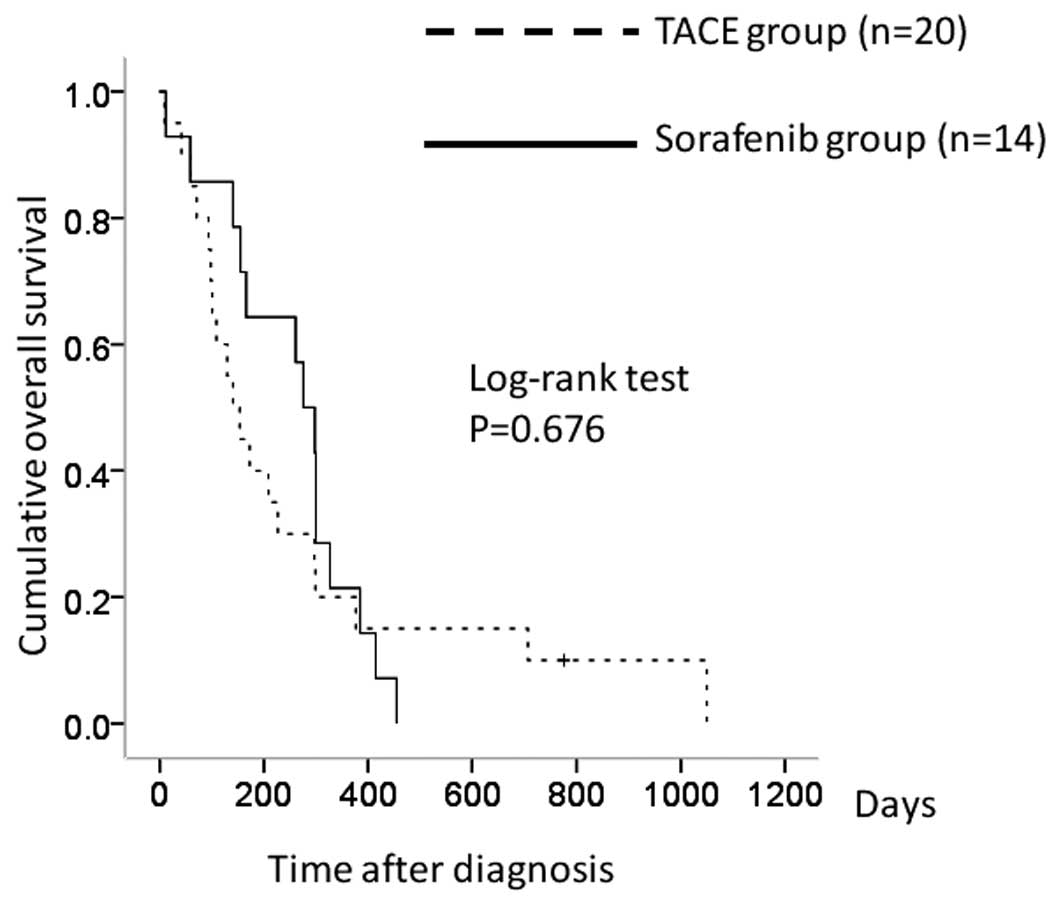
Comparison between the TACE and
sorafenib group patients with Child-Pugh B
There were 20 patients in the TACE group with
Child-Pugh B and 14 in the sorafenib group. The 1-year overall
survival rates were 20.0% in the TACE group and 21.4% in the
sorafenib group. In terms of overall survival, there was no
significant difference between the two groups (P=0.676) (Fig. 5).
Outcomes in the TACE group
During the follow-up period, a mean of 3.0 (range,
1–9) sessions of TACE were performed in the TACE group. Eighteen
patients (32.7%) received 1 session and 37 (67.3%) received more
than 1 session of TACE. Partial response (PR) was obtained in 10
patients (18.2%). Stable disease (SD) was observed in 31 patients
(56.4%). Progressive disease (PD) was observed in 14 patients
(25.5%). The objective response and disease control rates in the
TACE group were 18.2 and 74.5%, respectively.
Adverse events related to TACE
The majority of patients suffered self-limited
post-embolization syndrome consisting of low-grade fever, appetite
loss, abdominal pain, nausea or mild vomiting, which were
effectively controlled and improved within a few days. During the
follow-up period, 23 clinical adverse events with grade 3 or higher
were observed in the TACE group as determined with National Cancer
Institute Common Terminology Criteria for Adverse Events, version
3.0 (20). The details were as
follows: appetite loss in 7 patients (12.7%), hepatotoxicity in 6
patients (10.9%), general fatigue in 7 patients (12.7%) and high
grade fever in 3 patients (5.5%), respectively. All improved during
hospitalization and no patients died of TACE-related adverse
events.
Outcomes in the sorafenib group
In the sorafenib group, the median interval between
first diagnosis date of stage IVA or IVB HCC and initiation date of
sorafenib treatment was 40 days (range, 1–203 days). Median
duration of sorafenib therapy was 73 days (range, 4–377 days) for
all patients treated with sorafenib. In 42 patients with Child-Pugh
A, median duration of sorafenib therapy was 82 days (range, 4–377
days). In 14 patients with Child-Pugh B, median duration of
sorafenib therapy was 35 days (range, 10–287 days). In 16 patients
(28.6%), sorafenib 400 mg b.i.d. was started. In 40 patients
(71.4%), sorafenib 200 mg b.i.d. was started. In 16 of 16 patients
(100%) with initiated sorafenib 400 mg b.i.d., dose reductions were
required. In 29 of 40 patients (72.5%) with initiated sorafenib 200
mg b.i.d., dose reductions were required. Complete response was
obtained in 1 patient (1.8%). PR was obtained in 5 patients (8.9%).
SD was observed in 22 patients (39.3%). PD was observed in 26
patients (46.4%). In 2 patients (3.6%), treatment efficacy was not
determined, since evaluation using dynamic CT was not performed.
The objective response and disease control rates in the sorafenib
group were 11.1 and 51.9%, respectively.
Adverse events associated with
sorafenib treatment
During the follow-up period, 38 clinical adverse
events with grade 3 or higher were observed in the sorafenib group
as determined with National Cancer Institute Common Terminology
Criteria for Adverse Events, version 3.0 (20). The details were as follows: rash in
2 patients (3.6%), hand-foot syndrome in 4 patients (7.1%),
diarrhea in 8 patients (14.3%), appetite loss in 4 patients (7.1%),
hepatotoxicity in 10 patients (17.9%), general fatigue in 5
patients (8.9%), high-grade fever in 3 patients (5.4%), and lung
toxicity in 2 patients (3.6%).
Causes of discontinuation of
sorafenib
In the sorafenib group, 45 patients (80.4%)
discontinued sorafenib treatment. Causes of discontinuation were as
follows: tumor progression in 21 patients, serious adverse events
in 23 patients and patient’s wish in 1 patient.
Causes of death
During the follow-up period, 48 patients (87.3%)
died in the TACE group. Mortality in the TACE group was due to
tumor progression in 34 patients (61.8%), liver failure in 13
patients (23.6%) and pneumonia in 1 patient (1.8%). During the
follow-up period, 48 patients (85.7%) died in the sorafenib group.
Mortality in the sorafenib group was due to tumor progression in 35
patients (62.5%), liver failure in 8 patients (14.3%) and pneumonia
in 5 patients (8.9%).
Discussion
To our best knowledge, there have been no reliable
data to date with regard to comparison between conventional TACE
and sorafenib treatment for advanced HCC with vascular invasion
and/or extrahepatic metastasis. Therefore, in the present study, we
aimed to compare overall survival between stage IV HCC patients
treated with conventional TACE and those treated with
sorafenib.
Sorafenib was the first systemic chemotherapeutic
agent to demonstrate a significant improvement in overall survival
in patients with advanced HCC (6,7).
However, Niu et al reported in their prospective comparative
study that TACE was an effective treatment method for advanced HCC
with PVTT compared to conservative treatment (21). Luo et al also reported in
their prospective study that TACE was safe and feasible in selected
HCC patients with PVTT and that it had survival benefit over
conservative treatment (22).
Chung et al also reported in their large retrospective study
that TACE for advanced HCC patients with main portal vein invasion
can be performed safely and may improve overall survival (23). Thus, several studies with favorable
outcome in patients with stage IV HCC who received TACE have been
reported. In the present study, in terms of overall survival, there
were no significant differences between the TACE and the sorafenib
groups. Our study results suggest that TACE could be a first-line
treatment for stage IV HCC.
In terms of the objective response rate, there was
no significant difference between the two groups (TACE group,
18.2%; sorafenib group, 11.1%; P=0.418). This result also suggests
that TACE can be considered as a therapeutic option for the
treatment of stage IV HCC.
In terms of the disease control rate, there was a
significant difference between the two groups (TACE group, 74.5%;
sorafenib group, 51.9%; P=0.017). Although the reason for this is
unclear, TACE may be more effective at suppressing disease
progression in stage IV HCC than sorafenib therapy.
Serum vascular endothelial growth factor (VEGF)
levels increase with advancing HCC stages (24). Treatment of HCC with TACE is known
to induce VEGF expression (25,26).
In particular, in patients with incomplete response to TACE, TACE
can induce the up-regulation of VEGF (24). Serum VEGF level was an independent
predictor of survival in patients with advanced HCC (27,28).
In the present study, there were 45 patients (81.8%) who did not
obtain CR or PR in the TACE group. In these patients, in order to
suppress VEGF and malignant angiogenesis, concurrent or sequential
therapy with molecular targeted drugs such as sorafenib may be
effective to optimize outcome (29).
Currently, in Japan, advanced HCCs are treated by
hepatologists or radiologists. The former may be less familiar with
the side effects of anticancer drugs, and the latter may not be
prepared to manage problems related to underlying liver cirrhosis.
Collaboration between hepatologists and radiologists is therefore
essential to optimize outcome in the treatment of advanced HCC
patients.
There are several limitations in the present study.
First, this was a retrospective study. Second, in the sorafenib
group, in 40 patients (71.4%), sorafenib 200 mg b.i.d. was started,
leading to underestimated outcomes of patients treated with
sorafenib, although in the SHARP and Asian Pacific trials,
sorafenib 400 mg b.i.d. was started in all eligible patients.
(6,7). Third, in the TACE group, previous
therapies for HCC were not performed, whereas in the sorafenib
group, previous locoregional therapies were performed, leading to
bias. Therefore, a large prospective study will be required in the
future. However, our study results demonstrated that in terms of
overall survival, including subgroup analyses, there were no
significant differences between the TACE group and the sorafenib
group. In conclusion, TACE for stage IV HCC can be a first-line
treatment as well as sorafenib therapy.
Acknowledgements
The authors would like to thank Haruko
Takada for the data collection.
References
|
1.
|
Curley SA, Izzo F, Ellis LM, Nicolas
Vauthey J and Vallone P: Radiofrequency ablation of hepatocellular
carcinoma in 110 patients with cirrhosis. Ann Surg. 232:1694–1702.
2000. View Article : Google Scholar
|
|
2.
|
Allgaier HP, Deibert P, Zuber I,
Olschewski M and Blum HE: Percutaneous radiofrequency interstitial
thermal ablation of small hepatocellular carcinoma. Lancet.
353:1676–1677. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Okuda K: Hepatocellular carcinoma: recent
progress. Hepatology. 15:948–963. 1992. View Article : Google Scholar
|
|
4.
|
Lencioni: Loco-regional treatment of
hepatocellular carcinoma. Hepatology. 52:762–773. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Nishikawa H, Osaki Y, Kita R and Kimura T:
Hepatic arterial infusion chemotherapy for advanced hepatocellular
carcinoma in Japan. Cancer. 4:165–183. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Llovet JM, Ricci S, Mazzaferro V, Hilgard
P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A,
Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR,
Seitz JF, Borbath I, Häussinger D, Giannaris T, Shan M, Moscovici
M, Voliotis D and Bruix J; SHARP Investigators Study Group:
Sorafenib in advanced hepatocellular carcinoma. N Engl J Med.
359:378–390. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S,
Kim JS, Luo R, Feng J, Ye S, Yang TS, et al: Efficacy and safety of
sorafenib in patients in the Asia-Pacific region with advanced
hepato-cellular carcinoma: a phase III randomised, double-blind,
placebo-controlled trial. Lancet Oncol. 10:25–34. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Llovet JM and Bruix J: Systematic review
of randomized trials for unresectable hepatocellular carcinoma:
chemoembolization improves survival. Hepatology. 37:429–442. 2003.
View Article : Google Scholar
|
|
9.
|
Llovet JM, Real MI, Montaña X, Planas R,
Coll S, Aponte J, Ayuso C, Sala M, Muchart J, Solà R, Rodés J and
Bruix J; Barcelona Liver Cancer Group: Arterial embolisation or
chemoembolisation versus symptomatic treatment in patients with
unresectable hepatocellular carcinoma: a randomised controlled
trial. Lancet. 359:1734–1739. 2002. View Article : Google Scholar
|
|
10.
|
Lencioni R, Chen XP, Dagher L and Venook
AP: Treatment of intermediate/advanced hepatocellular carcinoma in
the clinic: how can outcomes be improved? Oncologist. 15:42–52.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Schwarz RE, Abou-Alfa GK, Geschwind JF,
Krishnan S, Salem R and Venook AP; American
Hepato-Pancreato-Biliary Association; Society of Surgical Oncology;
Society for Surgery of the Alimentary Tract: Nonoperative therapies
for combined modality treatment of hepatocellular cancer: expert
consensus statement. HPB (Oxford). 12:313–320. 2010.PubMed/NCBI
|
|
12.
|
Lee HS, Kim JS, Choi IJ, Chung JW, Park JH
and Kim CY: The safety and efficacy of transcatheter arterial
chemoembolization in the treatment of patients with hepatocellular
carcinoma and main portal vein obstruction. A prospective
controlled study. Cancer. 79:2087–2094. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Ishikawa T: Future perspectives on the
treatment of hepatocellular carcinoma with cisplatin. World J
Hepatol. 1:8–16. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Peng ZW, Guo RP, Zhang YJ, Lin XJ, Chen MS
and Lau WY: Hepatic resection versus transcatheter arterial
chemoembolization for the treatment of hepatocellular carcinoma
with portal vein tumor thrombus. Cancer. 22:Feb 22–2012.(Epub ahead
of print). View Article : Google Scholar
|
|
15.
|
Park JW, Koh YH, Kim HB, Kim HY, An S,
Choi JI, Woo SM and Nam BH: Phase II study of concurrent
transarterial chemoembolization and sorafenib in patients with
unresectable hepatocellular carcinoma. J Hepatol. 56:1336–1342.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Sansonno D, Lauletta G, Russi S, Conteduca
V, Sansonno L and Dammacco F: Transarterial chemoembolization plus
sorafenib: a sequential therapeutic scheme for HCV-related
intermediate-stage hepatocellular carcinoma: a randomized clinical
trial. Oncologist. 17:359–366. 2012. View Article : Google Scholar
|
|
17.
|
Bruix J and Sherman M; Practice Guidelines
Committee, American Association for the Study of Liver Diseases:
Management of hepatocellular carcinoma. Hepatology. 42:1208–1236.
2005. View Article : Google Scholar
|
|
18.
|
Kudo M and Okanoue T; Japan Society of
Hepatology: Management of hepatocellular carcinoma in Japan:
consensus-based clinical practice manual proposed by the Japan
Society of Hepatology. Oncology. 72:2–15. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Llovet JM, Di Bisceglie AM, Bruix J,
Kramer BS, Lencioni R, Zhu AX, Sherman M, Schwartz M, Lotze M,
Talwalkar J and Gores GJ; Panel of Experts in HCC-Design Clinical
Trials: Design and endpoints of clinical trials in hepatocellular
carcinoma. J Natl Cancer Inst. 100:698–711. 2008. View Article : Google Scholar
|
|
20.
|
National Cancer Institute: Common
terminology criteria for adverse events, version 3.0. http://ctepcancergov/reporting/ctc.htmluri
Published August 9, 2006. Accessed August 2010.
|
|
21.
|
Niu ZJ, Ma YL, Kang P, Ou SQ, Meng ZB, Li
ZK, Qi F and Zhao C: Transarterial chemoembolization compared with
conservative treatment for advanced hepatocellular carcinoma with
portal vein tumor thrombus: using a new classification. Med Oncol.
December 27–2011.(Epub ahead of print).
|
|
22.
|
Luo J, Guo RP, Lai EC, Zhang YJ, Lau WY,
Chen MS and Shi M: Transarterial chemoembolization for unresectable
hepatocellular carcinoma with portal vein tumor thrombosis: a
prospective comparative study. Ann Surg Oncol. 18:413–420. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Chung GE, Lee JH, Kim HY, Hwang SY, Kim
JS, Chung JW, Yoon JH, Lee HS and Kim YJ: Transarterial
chemoembolization can be safely performed in patients with
hepatocellular carcinoma invading the main portal vein and may
improve the overall survival. Radiology. 258:627–634. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Welker MW and Trojan J: Anti-angiogenesis
in hepatocellular carcinoma treatment: current evidence and future
perspectives. World J Gastroenterol. 17:3075–3081. 2011.PubMed/NCBI
|
|
25.
|
Leelawat K, Laisupasin P, Kiatdilokrut A,
Pongtongpool T, Narong S, Samkhumphim N and Ket-Horm S: The effect
of doxorubicin on the changes of serum vascular endothelial growth
factor (VEGF) in patients with hepatocellular carcinoma after
transcatheter arterial chemoembolization (TACE). J Med Assoc Thai.
91:1539–1543. 2008.
|
|
26.
|
Wang B, Xu H, Gao ZQ, Ning HF, Sun YQ and
Cao GW: Increased expression of vascular endothelial growth factor
in hepatocellular carcinoma after transcatheter arterial
chemoembolization. Acta Radiol. 49:523–529. 2008. View Article : Google Scholar
|
|
27.
|
Niizeki T, Sumie S, Torimura T, Kurogi J,
Kuromatsu R, Iwamoto H, Aino H, Nakano M, Kawaguchi A, Kakuma T and
Sata M: Serum vascular endothelial growth factor as a predictor of
response and survival in patients with advanced hepatocellular
carcinoma undergoing hepatic arterial infusion chemotherapy. J
Gastroenterol. March 1–2012.(Epub ahead of print).
|
|
28.
|
Llovet JM, Pena C, Lathia C, Shan M,
Meinhardt G and Bruix J; on behalf of the SHARP Investigators Study
Group: Plasma biomarkers as predictors of outcome in patients with
advanced hepatocellular carcinoma. Clin Cancer Res. 18:2290–2300.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Zhu AX, Duda DG, Sahani DV and Jain RK:
HCC and angiogenesis: possible targets and future directions. Nat
Rev Clin Oncol. 8:292–301. 2011. View Article : Google Scholar : PubMed/NCBI
|