Introduction
The mortality and morbidity of acute pancreatitis
(AP) generally depend on the severity of the disease, however, the
mechanisms regulating its severity are poorly understood. Numerous
studies have investigated the processes that regulate the severity
of AP using murine AP models (1).
Mild edematous AP may resolve either spontaneously or after
conservative therapy, but severe hemorrhagic pancreatitis may cause
multiple organ failure, leading to a high mortality rate (2). The pathophysiology underlying severe
acute pancreatitis (SAP) is not well-understood.
Nardostachys jatamansi (NJ) is widely used as
a bitter tonic and anti-spasmodic (3). The NJ root contains various
sesquiterpenes, including jatamansic acid, and jatamansones,
lignans and neolignans. The aqueous extract of the NJ root has been
used to treat mental disorders, insomnia and blood disorders
(3). We previously reported that
NJ is effective in protecting against inflammatory challenges
(4–6), particularly against cerulein-induced
edematous mild AP (4). At
relatively low doses (in line with doses of molecular inhibitors),
NJ protected against, and induced recovery from, mild edematous AP.
NJ also reduced cytokines, neutrophil infiltration and digestive
enzymes. However, the effects of NJ on CDE-induced hemorrhagic
severe necrotic AP have not been examined.
The present study was designed to investigate the
effects of NJ on CDE-induced SAP. To achieve this, we examined
histological changes in the pancreas as well as neutrophil
infiltration, digestive enzyme production and cytokine release. We
also measured the regulating mechanisms, including
mitogen-activated protein kinases (MAPKs).
Materials and methods
Materials
Avidin-peroxidase, Tris-HCl, NaCl,
hexadecyltrimethylammonium bromide, ethionine and
tetramethylbenzidine were purchased from Sigma-Aldrich (St. Louis,
MO, USA). Anti-mouse tumor necrosis factor (TNF)-α, interleukin
(IL)-1β and IL-6 antibodies and recombinant TNF-α, IL-1β and IL-6
were purchased from R&D Systems (Minneapolis, MN, USA).
Anti-phospho-extracellular signal-regulated kinases (ERK) 1/2,
anti-phospho-c-Jun N-terminal kinases (JNK) and anti-phospho-p38
were purchased from Cell Signaling Technology (Beverly, MA, USA).
Anti-inhibitory κ-Bα (Iκ-Bα), ERK 1/2, JNK, p38 and β-actin
antibodies were purchased from Santa Cruz Biotechnology Inc. (Santa
Cruz, CA, USA).
Plant materials
NJ was purchased from a standard commercial source
(Omni Herb, Seoul, Korea). The herb identity was confirmed at the
Korean drug test laboratory. The NJ was prepared by decocting 100 g
of dried herb with 1 l of boiling distilled water for approximately
2 h. The aqueous extract was frozen at −80°C then freeze-dried to
form a 6.59-g powder. The yield of the extract was 6.59%. The
powder was then rehydrated with distilled water, filtered and the
filtrates were stored at 4°C until use.
Animals
All experiments were performed according to methods
approved by the Animal Care Committee of Wonkwang University.
Female C57BL/6 mice (aged 3–4 weeks) were purchased from Orient Bio
(Sungnam, KyungKiDo, Republic of Korea). All animals were bred and
housed in standard shoebox cages in a climate-controlled room with
an ambient temperature of 23±2°C and a 12-h light-dark cycle. The
animals were fed a standard laboratory diet and water ad
libitum for seven days prior to random assignment to
experimental groups. The mice were fed the
choline/methionine-deficient diet (Harland Teklad Madison, WI, USA)
supplemented with 0.5% DL-ethionine for three days. To ensure equal
exposure by all animals, the diet was replaced with fresh CDE every
24 h. Following CDE administration, animals were provided with a
normal diet for four days in order to estimate the 7-day mortality
rate. The mice were then sacrificed, and the blood and pancreas
were obtained. The blood samples were used to determine serum
amylase, lipase and cytokine levels. For histological examination
and scoring, the entire pancreas was rapidly removed from each
mouse and fixed in formalin. Three portions of each pancreas were
stored at −80°C for later measurement of tissue myeloperoxidase
(MPO) activity as an indicator of neutrophil sequestration and for
real-time reverse-transcription polymerase chain reaction (RT-PCR)
measurements. NJ was dissolved in saline, then administrated orally
ad libitum via water for seven days. There was no
significant difference in the consumption of saline and
NJ-containing saline.
Measurement of amylase and lipase
The arterial blood samples were obtained 6 h after
induction of pancreatitis for the measurement of serum amylase and
lipase levels. The mice were anesthetized with an intraperitoneal
injection of ketamine (80 mg/kg) and xylazine (4 mg/kg). Following
anesthetization, blood was aspirated from the heart into a syringe.
Serum amylase was measured using ADIVA 1650 (Bayer, Leverkusen,
Germany). Serum lipase level was measured using a Cobas Mira
apparatus (Roche, Basel, Switzerland).
Enzyme-linked immunosorbent assay
(ELISA)
An ELISA for TNF-α, IL-1β and IL-6 (R&D Systems)
was carried out in duplicate in 96-well plates (Nunc, Denmark)
coated with 100 µl of anti-mouse TNF-α, IL-1β or IL-6 monoclonal
antibodies (1.0 µg/ml) in phosphate-buffered saline (PBS) at pH 7.4
following an overnight incubation at 4°C. The plates were washed in
PBS containing 0.05% Tween-20 and blocked with PBS containing 10%
FBS for 2 h. After additional washes, the standards and serum were
added to the plates and incubated at room temperature for 3 h. The
wells were then washed and 0.2 µg/ml of biotinylated anti-mouse
TNF-α, IL-1β or IL-6 was added to each well and incubated at room
temperature for 1 h. The wells were washed, avidinperoxidase was
added and the plates were incubated for 30 min at room temperature.
The wells were washed again and 3,3′,5,5′-tetramethylbenzidine
substrate was added. Color development was measured at 450 nm using
an automated microplate ELISA reader. Standard curves were obtained
for each sample by using serial dilutions of recombinant TNF-α,
IL-1β and IL-6.
Messenger RNA (mRNA) expression
The mRNA transcripts were analyzed by RT-PCR in
mouse pancreatic tissue. The total RNA was isolated from the mouse
pancreata using TRIzol (Invitrogen, Carlsbad, CA, USA) and
subjected to reverse transcription using SuperScript II RT
(Invitrogen). TaqMan quantitative RT-PCR was performed using a
LightCycler 2.0 system according to the manufacturer’s instructions
(Roche, Basel, Switzerland). Each reaction was performed in
triplicate and a control reaction (without reverse transcription)
was performed. All samples were analyzed for expression of the gene
of interest and the results were normalized to those of the
housekeeping mRNA, hypoxanthine-guanine phosphoribosyltransferase
(HPRT). Arbitrary expression units were calculated by dividing the
expression of the gene of interest by that of HPRT. The
forward, reverse and probe oligonucleotide primers for Multiplex
Real-Time TaqMan PCR were as follows: mouse TNF-α (forward,
5′-TCTCTTCAAGGGACAAGGCTG-3′; reverse, 5′-ATAGCAAATCGGCTGACGGT-3′;
probe, 5′-CCCGACTACGTGCTCCTCACCCA-3′), mouse IL-1β [forward,
5′-TTGACGGACCCCAAAAGAT-3′; reverse, 5′-GAAGCTGGATGCTCTCATCTG-3′;
universal probe, M15131.1 (Roche Applied Science)], mouse IL-6
[forward, 5′-TTCATTCTCTTTGCTCTTGAATTAGA-3′; reverse,
5′-GTCTGACCTTTAGCTTCAAATCCT-3′; universal probe, M20572.1 (Roche
Applied Science)].
Estimation of MPO activity
Neutrophil sequestration in the pancreas was
quantified by measuring tissue MPO activity. The tissue samples
were thawed, homogenized in 20 mM phosphate buffer (pH 7.4) and
centrifuged (15,000 rpm, 10 min, 4°C). The pellet was resuspended
in 50 mM phosphate buffer (pH 6.0) containing 0.5%
hexadecyltrimethylammonium bromide. The suspension was subjected to
four cycles of freezing and thawing and was further disrupted by
sonication for 40 sec. The sample was then centrifuged (15,000 rpm,
5 min, 4°C) and the supernatant was used for the MPO assay. The
reaction mixture consisted of the supernatant, 1.6 mM
tetramethylbenzidine, 80 mM sodium phosphate buffer (pH 5.4) and
0.3 mM hydrogen peroxide. This mixture was incubated at 37°C for
110 sec, the reaction was terminated with 2 mol/l
H2SO4 and the absorbance was measured at 450
nm.
Western blotting
The pancreatic tissues were retrieved from storage
at −80°C and homogenized in RIPA lysis buffer. Whole-cell lysates
were obtained by boiling the homogenates in sample buffer [62.5 mM
Tris-HCl, pH 6.8, 2% sodium dodecyl sulfate (SDS), 20% glycerol and
10% 2-mercaptoethanol]. Lysate proteins were then separated by 10%
SDS-polyacrylamide gel electrophoresis and transferred to PVDF
membranes. The membrane was blocked with 5% skimmed milk in
PBS-Tween-20 for 1 h at room temperature and then incubated with
anti-phospho-ERK 1/2, anti-phospho-JNK, anti-phospho-p38 and
anti-Iκ-Bα antibodies. After four washes in PBS-Tween-20, the blot
was incubated with the secondary antibody for 1 h.
Antibody-specific proteins were visualized by an enhanced
chemiluminesence detection system according to the manufacturer’s
instructions (Amersham Corp.).
Statistical analysis
The results are expressed as the mean ± SEM of
independent experiments. Independent one-way ANOVAs were used to
analyze the statistical significance of the results between or
among groups. All statistical analyses were performed using SPSS
version 10.0 statistical analysis software (SPSS, Chicago, IL,
USA). P<0.05 was considered to indicate a statistically
significant result.
Results
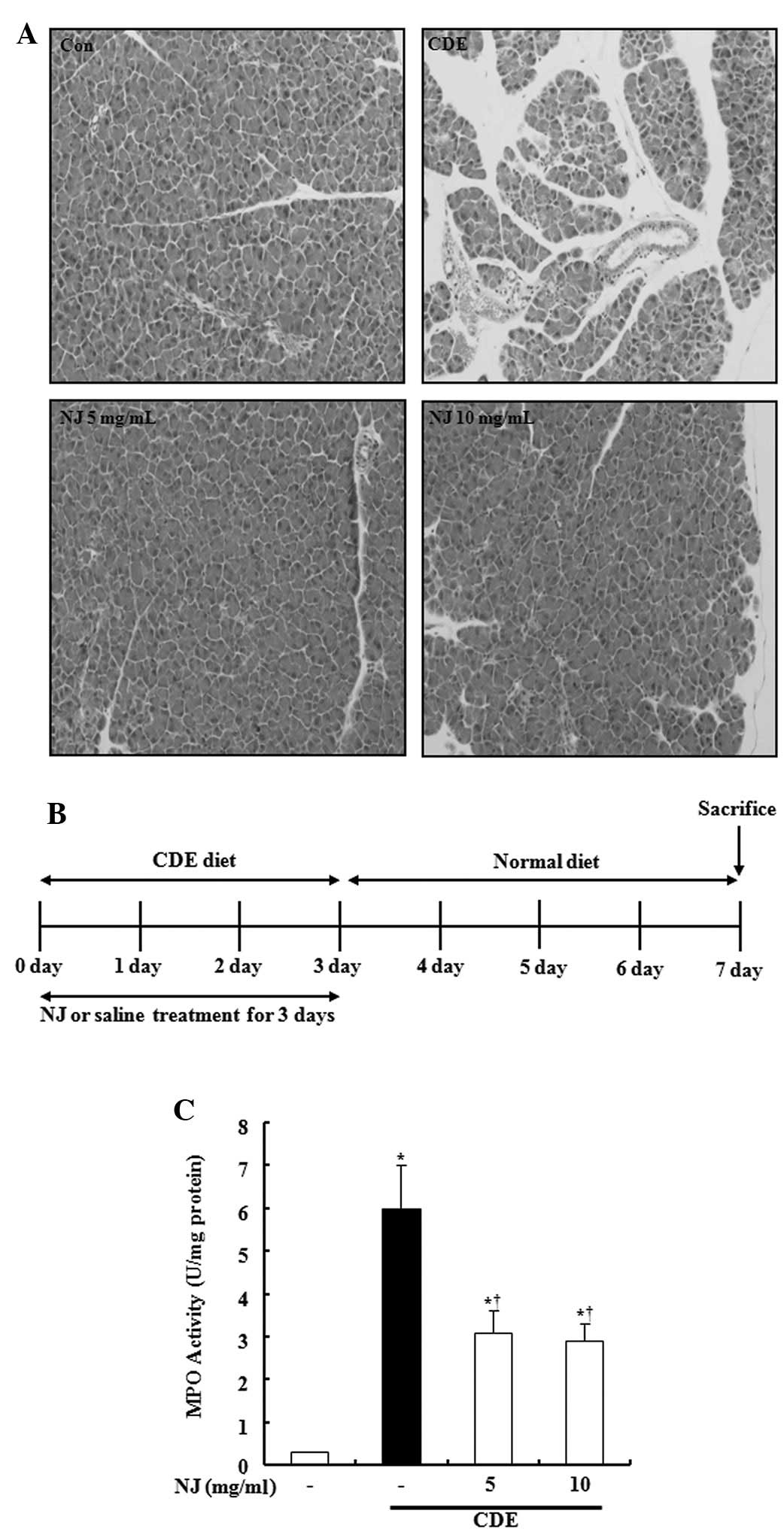
NJ attenuated the severity of CDE-induced
pancreatic damage
To examine the effect of NJ on the development and
severity of AP, mice were co-treated with NJ (5 or 10 mg/ml) and
CDE-induced SAP. The severity of CDE-induced pancreatitis was
assessed by histological examination. Pancreatic sections obtained
seven days after the onset of SAP (CDE for three days and normal
diet for four days, Fig. 1B)
revealed the extent of the tissue injury. There was inflammatory
cell infiltration of the pancreas and interstitial edema in SAP
mice. However, treatment with NJ (5 or 10 mg/ml) attenuated the
severity of pancreatitis (Fig.
1A). We also investigated the amount of neutrophil infiltration
into the pancreas by assessing the MPO activity. As hypothesized,
pre-treatment with NJ significantly inhibited the MPO activity
(Fig. 1C).
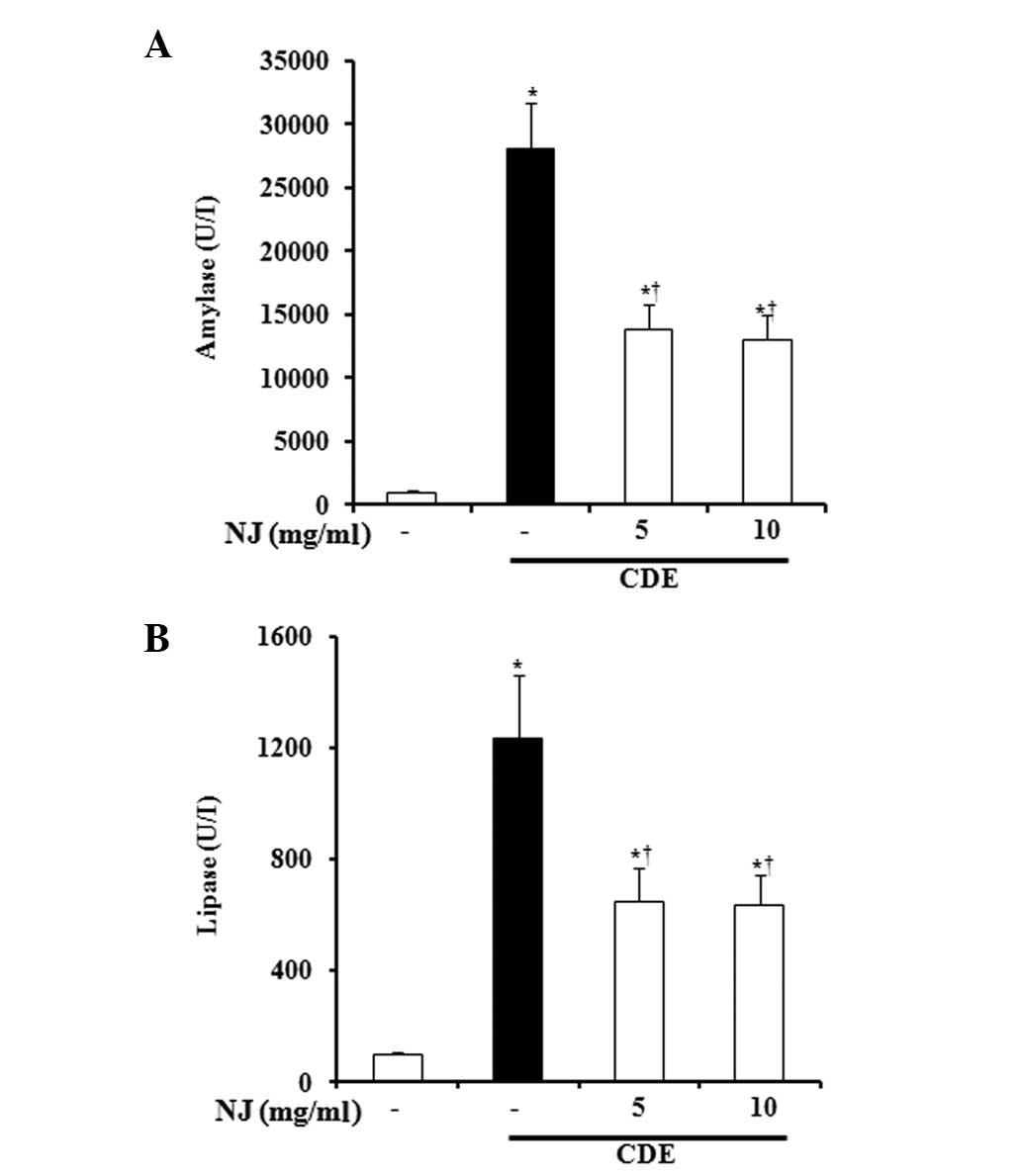
NJ significantly inhibited secretion of
serum amylase and lipase
During AP, the digestive pro-enzymes are converted
into their active forms, leading to acinar cell death. Therefore,
amylase and lipase secretion into the serum signals the initiation
of AP. We assessed SAP severity by measuring enzyme production.
During CDE-induced SAP, amylase and lipase levels in the serum were
significantly increased. However, NJ (5 or 10 mg/ml) significantly
reduced the serum levels of amylase and lipase (Fig. 2A and B).
NJ reduced serum cytokine levels and
pancreatic cytokine expression
It has been reported that ILs and TNFs increase
during AP (7,8). Therefore, we examined cytokine levels
in the serum and pancreas. The serum and pancreatic TNF-α, IL-1β
and IL-6 levels were increased in mice with CDE-induced SAP. NJ
treatment significantly reduced the levels of TNF-α, IL-1β, and
IL-6 in the serum and pancreas (Fig.
3A and B).
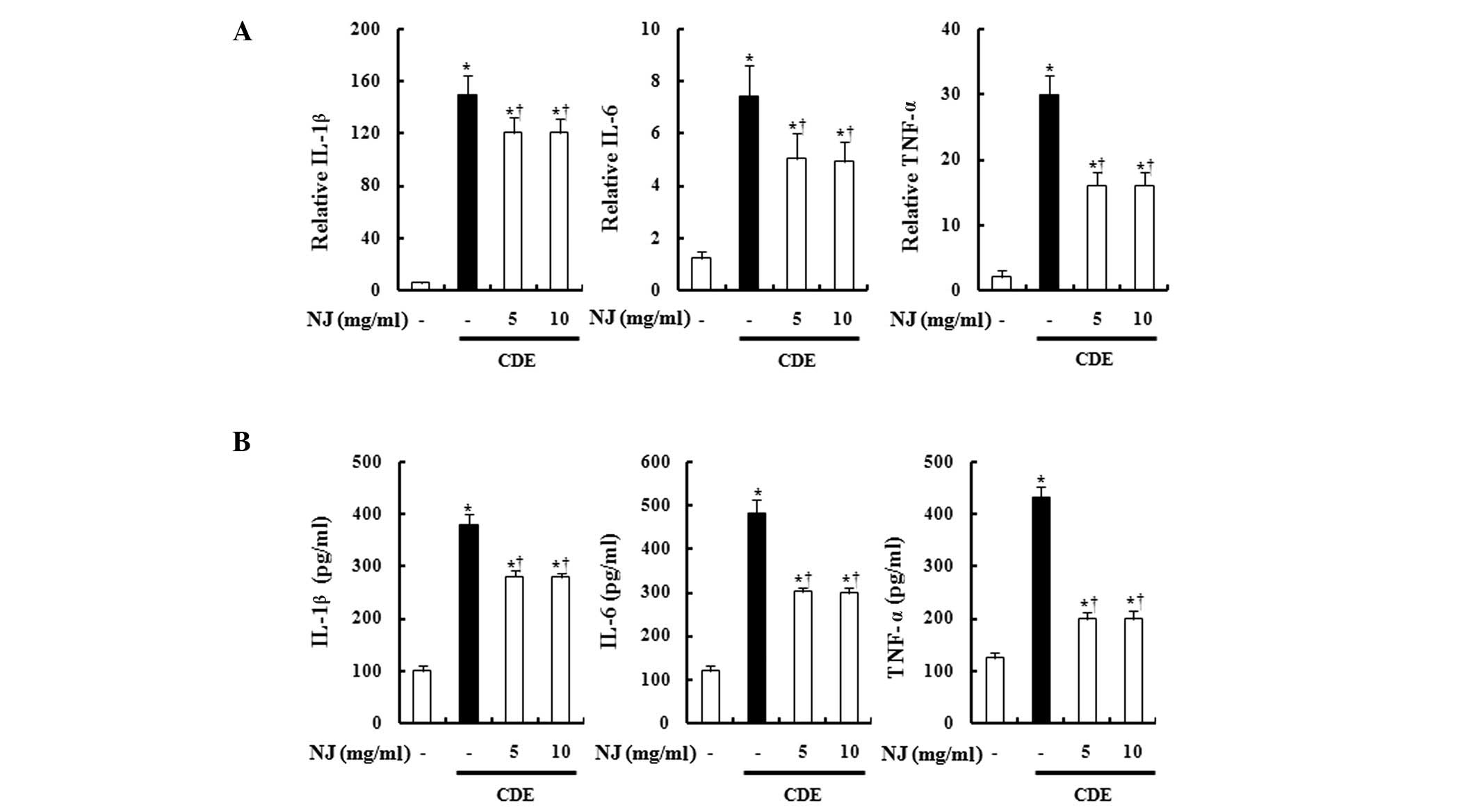 | Figure 3Effect of NJ on TNF-α, IL-1β and IL-6.
To examine production of TNF-α, IL-1β and IL-6 (A) serum levels and
(B) mRNA levels in the pancreas were measured by ELISA and
real-time RT-PCR, respectively. Data are represented as the means ±
SEM (n=6 in each group). *P<0.05 vs. control group
and †P<0.05 vs. CDE-induced SAP mice. P<0.05 was
considered to indicate a statistically significant result. The
results were similar in 3 additional experiments. NJ,
Nardostachys jatamansi; CDE, choline-deficient diet
supplemented with ethionine; TNF, tumor necrosis factor; IL,
interleukin; mRNA, messenger RNA; ELISA, enzyme-linked
immunosorbent assay; RT-PCR, reverse-transcription polymerase chain
reaction; SAP, severe acute pancreatitis. |
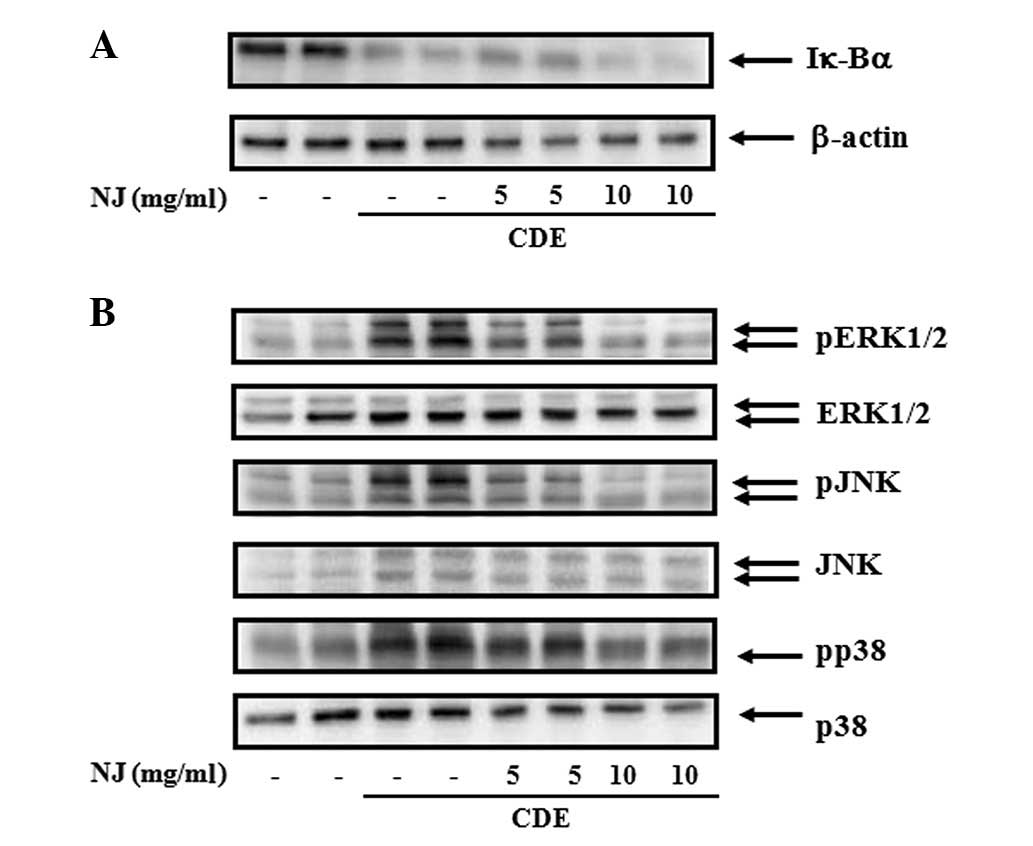
NJ inhibited activation of pancreatic
MAPKs during CDE-induced SAP
We assessed the effect of NJ on the activation of
NF-κB (Fig. 4A) and MAPKs
(Fig. 4B) in the pancreas. NF-κB
activation was assessed by Iκ-Bα degradation (9). The CDE caused Iκ-Bα degradation,
indicating an activation of NF-κB. NJ treatment did not inhibit
Iκ-Bα degradation, suggesting that the anti-inflammatory effect of
NJ was not associated with NF-κB (Fig.
4A). MAPKs were also activated during SAP. NJ treatment
significantly reduced SAP-induced activation of MAPKs (Fig. 4B).
Discussion
In the present study, we investigated the protective
effects of NJ on diet-induced SAP. NJ inhibited the CDE-induced
pancreatic damage markedly and reduced the digestive enzyme
secretion and cytokine productions significantly. NJ treatment also
reduced MAPK activation in a dose-dependent manner. Our results
show that NJ may be a candidate for the treatment of SAP.
In Figs. 1 and
2, CDE caused significant
pancreatic inflammation and hyper-stimulation of amylase and
lipase. When this model was first introduced, it was considered
that a CDE inhibited the biosynthesis of lecithins, a major
membrane constituent, and caused a blockage of the exocytosis of
zymogen granules, leading to intracellular enzyme accumulation,
acinar cell injury and enzyme leakage into the interstitium/blood,
resulting in auto-digestion of the pancreas by the activated
enzymes (10). The
hyper-stimulated enzymes would attack the acinar cells, then the
injured cells secrete the pro-inflammatory cytokines (11). In this study, NJ treatment
inhibited CDE-induced pancreatic damage and digestive enzymes
(Figs. 1 and 2), which indicates that the action of NJ
is mediated by the inhibition of digestive enzymes.
In the present study, NJ treatment inhibited the
pancreatic and serum IL-1β, IL-6 and TNF-α levels (Fig. 3). The production of
pro-inflammatory cytokines, including IL-1, IL-6 and TNF-α, has now
been shown in the majority of animal models of pancreatitis
(12–14). Also, the degree of cytokine
elevation correlates well with the severity of organ inflammation
and destruction. Investigations into the origin of these peptides
have demonstrated that intra-pancreatic cytokine levels reach
concentrations several-fold higher than corresponding systemic
levels, thus providing evidence that IL-1, IL-6 and TNF-α are
produced within the pancreas during acute pancreatitis (12). Therefore, the inhibition of
pro-inflammatory cytokines is critical to reduce the severity of
SAP.
NF-κB and MAPKs play key roles in regulating the
cytokines involved in acute inflammatory pancreatic diseases
(15,16). The abnormal activation of NF-κB and
MAPKs may promote the transcription of pro-inflammatory factors,
including TNF-α, IL-1β and IL-6. In an extracellular signaling
loop, TNF-α, IL-1β and IL-6 also activate NF-κB and MAPKs, further
promoting inflammatory reactions. In the present study, mice with
SAP showed increased TNF-α, IL-1β and IL-6 release via NF-κB and
MAPK activation. NJ treatment did not inhibit NF-κB activation, but
did inhibit activation of MAPKs, consequently inhibiting cytokine
release. These data suggest that the effective pathway of NJ is via
MAPKs and not NF-κB (Fig. 4).
Our results indicate that NJ has protective effects
on SAP by inhibiting MAPK pathways, thereby inhibiting TNF-α, IL-1β
and IL-6 production. NJ treatment also reduced neutro-phil
infiltration into the pancreas and reduced levels of serum enzymes.
Our findings suggest that NJ may be a candidate for SAP
treatment.
Acknowledgements
This study was supported by Wonkwang
University in 2010.
References
|
1.
|
Foitzik T, Hotz HG, Eibl G and Buhr HJ:
Experimental models of acute pancreatitis: are they suitable for
evaluating therapy? Int J Colorectal Dis. 15:127–135. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Geokas MC, Baltaxe HA, Banks PA, Silva J
Jr and Frey CF: Acute pancreatitis. Ann Intern Med. 103:86–100.
1985. View Article : Google Scholar
|
|
3.
|
Bose BC, Vijayvargiya Y and Bhatnagar JN:
Nardostachys Jatamansi DC: a phyto-chemical study of its
active constituents. Indian J Med Sci. 11:799–802. 1957.
|
|
4.
|
Bae GS, Park HJ, Kim DY, Song JM, Kim TH,
Oh HJ, Yun KJ, Park RK, Lee JH, Shin BC, Sim HJ, Hong SP, Song HJ
and Park SJ: Nardostachys jatamansi protects against
cerulein-induced acute pancreatitis. Pancreas. 39:520–529. 2010.
View Article : Google Scholar
|
|
5.
|
Bae GS, Seo SW, Kim MS, Park KC, Koo BS,
Jung WS, Cho GH, Oh HC, Yun SW, Kim JJ, Kim SG, Hwang SY, Song HJ
and Park SJ: The roots of Nardostachys jatamansi inhibits
lipopolysaccharide-induced endotoxin shock. J Nat Med. 65:63–72.
2011.
|
|
6.
|
Song MY, Bae UJ, Lee BH, Kwon KB, Seo EA,
Park SJ, Kim MS, Song HJ, Kwon KS, Park JW, Ryu DG and Park BH:
Nardostachys jatamansi extract protects against
cytokine-induced beta-cell damage and streptozotocin-induced
diabetes. World J Gastroenterol. 16:3249–3257. 2010. View Article : Google Scholar
|
|
7.
|
Bhatia M, Brady M, Shokuhi S, Christmas S,
Neoptolemos JP and Slavin J: Inflammatory mediators in acute
pancreatitis. J Pathol. 190:117–125. 2000. View Article : Google Scholar
|
|
8.
|
Mota RA, Sánchez-Bueno F, Saenz L,
Hernández-Espinosa D, Jimeno J, Tornel PL, Martínez-Torrano A,
Ramírez P, Parrilla P and Yélamos J: Inhibition of poly(ADP-ribose)
polymerase attenuates the severity of acute pancreatitis and
associated lung injury. Lab Invest. 85:1250–1262. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Barnes PJ: Anti-inflammatory actions of
glucocorticoids: molecular mechanisms. Clin Sci (Lond). 94:557–572.
1998.PubMed/NCBI
|
|
10.
|
Lombardi B, Estes LW and Longnecker DS:
Acute hemorrhagic pancreatitis (massive necrosis) with fat necrosis
induced in mice by DL-ethionine fed with a choline-deficient diet.
Am J Pathol. 79:465–480. 1975.PubMed/NCBI
|
|
11.
|
Steer M: Pancreatitis severity: who calls
the shots? Gastroenterology. 122:1168–1172. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Norman JG, Franz MG, Fink GS, Messina J,
Fabri PJ, Gower WR and Carey LC: Decreased mortality of severe
acute pancreatitis after proximal cytokine blockade. Ann Surg.
221:625–634. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Hughes CB, Gaber LW, Mohey el-Din AB,
Grewal HP, Kotb M, Mann L and Gaber AO: Inhibition of TNF alpha
improves survival in an experimental model of acute pancreatitis.
Am Surg. 62:8–13. 1996.PubMed/NCBI
|
|
14.
|
Perides G, Weiss ER, Michael ES,
Laukkarinen JM, Duffield JS and Steer ML: TNF-alpha-dependent
regulation of acute pancreatitis severity by Ly-6C(hi) monocytes in
mice. J Biol Chem. 286:13327–13335. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Denham W, Yang J, Wang H, Botchkina G,
Tracey KJ and Norman J: Inhibition of p38 mitogen activate kinase
attenuates the severity of pancreatitis-induced adult respiratory
distress syndrome. Crit Care Med. 28:2567–2572. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Kim TH, Bae GS, Oh HJ, Kim MS, Park KC,
Koo BS, Kim BJ, Yang YS, Park DE, Lee JH, Seo SW, Shin YK, Yun KJ,
Sohn DH, Kim HJ, So HS, Park RK, Song HJ and Park SJ:
2′,4′,6′-Tris(methoxymethoxy) chalcone (TMMC) attenuates the
severity of cerulein-induced acute pancreatitis and associated lung
injury. Am J Physiol Gastrointest Liver Physiol. 301:G694–G706.
2011.
|