Introduction
Blunt chest trauma is involved in approximately 1 of
3 acute trauma admissions to the hospital, and pulmonary contusion
(PC) is an independent risk factor for the development of acute
lung injury (ALI), acute respiratory distress syndrome (ARDS) and
ventilator-associated pneumonia (VAP). Moreover, PC is the major
cause of mortality after blunt chest trauma (1). Clinically, it is common that patients
with approximately the same pulmonary contusion area have different
manifestations, which may be relevant to the complex condition of
the injured patients. Inflammatory molecular mechanisms, which are
unable to be demonstrated by imaging tools, may be involved in the
pathophysiology of PC. Research on the pathophysiology of PC is
helpful for early diagnosis and therapy, reducing mortality and
morbidity. Various models for PC reported in the literature utilize
large animals such as canines, swine and monkeys. Although more
analogous to the human, these models are costly. Even worse, the
lack of molecular probes and other cell- and mediator-specific
reagents, which are much more widely available for small animals
such as mice and rats, limits the widespread use of large animal
models in the study of PC pathophysiology. Most rodent models for
PC in the literature are unilateral PC models and are complicated
by the coexistence of myocardial contusion, which is likely to have
an effect on the interpretation of the experimental results. The
bilateral PC model designed by Raghavendran et al (2) is less likely to cause myocardial
contusion, however, the experimental platform is complex and
relatively costly. The correlation between pathophysiological
changes and contusion lesions in images in rat models has not been
well established. In this study, we aimed to develop a modified
feasible rat model for bilateral contusion and to determine the
most suitable injury energy in order to further reduce the
possibility of myocardial contusion, which will provide a simple
and reliable animal model for the further study of PC.
Materials and methods
Experimental animals
Adult Sprague-Dawley rats (n=36; 300–350 g; male)
(Shanghai SLAC Laboratory Animal, Co., Ltd.) were equally divided
into 4 groups. PC was induced with different injury energies in
these 36 rats. Another 9 rats comprised the comparative group.
Principles of energy production and
control
The rat model for bilateral PC was induced by
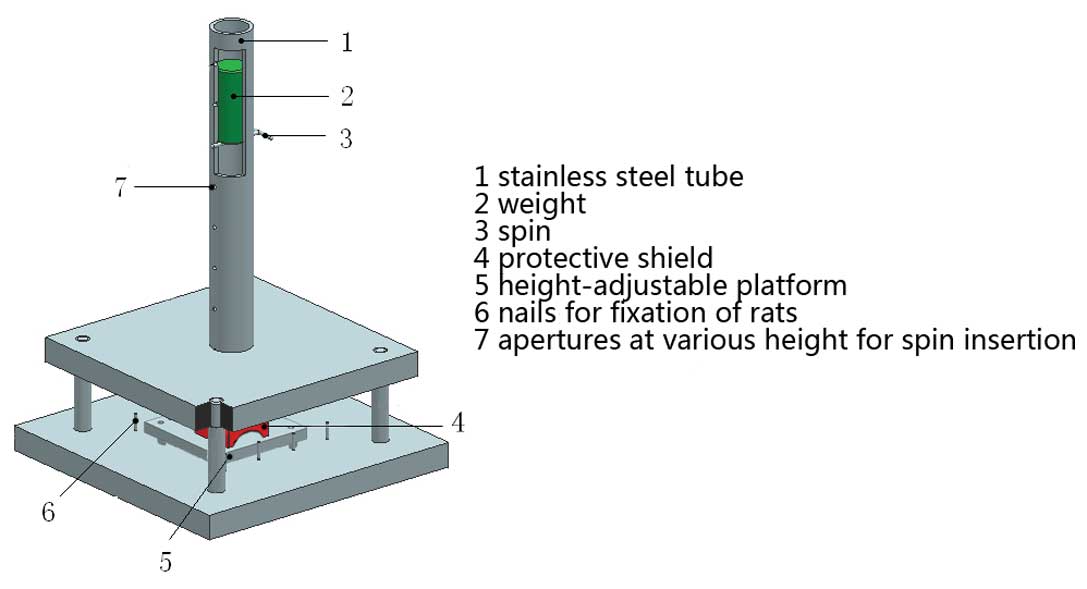
high-fall energy. The experimental device is illustrated in
Fig. 1. A hollow aluminum
cylindrical weight was dropped freely from various heights
(controlled by a spin) through a vertical lubricated stainless
steel tube onto a protective shield resting on the dorsum of the
rats, which was fixed on a height-adjustable steel platform. The
injury energy was calculated using the following equation: E = mgh,
where m is the mass of the aluminum weight (kg), g is the
gravitational acceleration (10 m/s2 to facilitate
calculation) and h is the height of spin above the protective
shield (m). A key feature of the model was the arch protective
shield made of plexiglass. The shield is placed between the
undersurface of the vertical stainless steel tube and the bilateral
posterolateral chest wall of the rat by adjustment of the height of
the platform (Fig. 1). The
specially designed arch on the undersurface of the shield averts
direct contact between the rat spine, scapulae and the aluminum
weight and directs the high-fall energy to the bilateral
posterolateral chest wall of the rat. The friction throughout the
study was not recorded.
Method for reproducing the model
The rats were anesthetized with pentobarbital (40
mg/kg) injected peritoneally. The third thoracic vertebra and the
eighth vertebra were marked superficially. The anesthetized rats
were put on the platform in a prone position. The height of the
platform was adjusted so as to place the protective shield in
between the undersurface of the vertical stainless steel tube and
the bilateral posterolateral chest wall of the rat. The spin was
adjusted to allow the aluminum cylindrical weight (0.1 kg ×3) to
fall freely onto the protective shield. Various heights and masses
were adopted to generate 4 groups of injury energy of 2.1, 2.4, 2.7
and 3.0 J. Each group contained 9 rats. The injury energy of any
group with the mortality of rats exceeding 20% was defined as
lethal energy. Additional rats (n=9) were treated as the control
group.
Measurement of the contusion
After the rats regained consciousness naturally,
free activity in the cage was permitted and water was offered.
After injury (4 h), the rats were anesthetized again with the
method documented above and underwent high resolution computed
tomography (CT) examination. The pulmonary opacity area, regarded
as the PC lesion, on each slice of spiral CT scan was documented
and three-dimensionally reconstructed by Advantage Workstation AW
4.3 computer software (GE Healthcare, Waukesha, WI, USA), so that
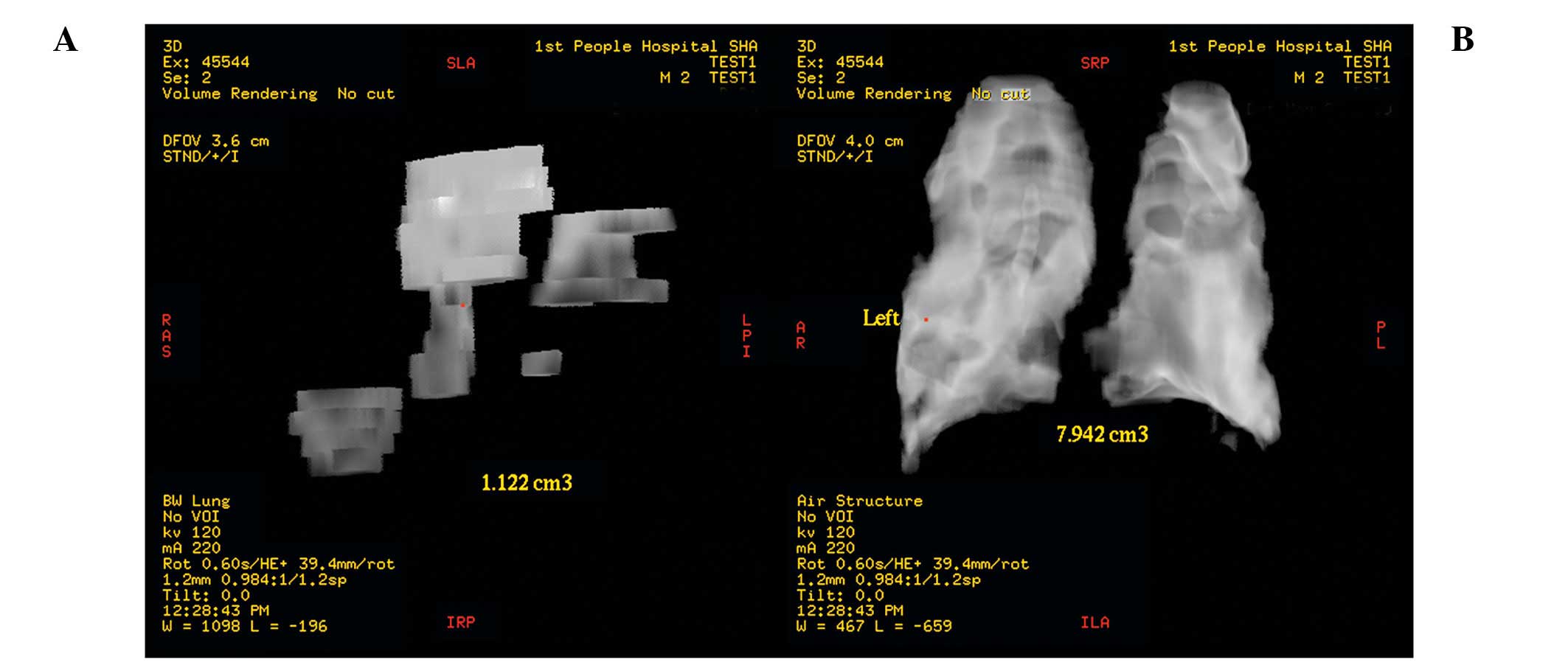
the precise contusion volume could be measured (Fig. 2A). The bilateral pulmonary fields
were then also reconstructed and the pulmonary volume was also
measured (Fig. 2B). Total
contusion volume for both pulmonary fields was expressed as a
percentage of total pulmonary volume. Special attention was paid to
the possibility of bone fracture and thoracic and peritoneal
bleeding.
Blood gas analysis
After CT examination, all of the living rats were
administered 100% oxygen for 5 min. Then, a mid-line ventral
incision was made to expose the descending aorta, from which 0.5 ml
arterial blood was drawn into a heparinized syringe, followed by
analysis with an ABL5 blood gas analyzer (Radiometer America,
Westlake, OH, USA). The arterial oxygen partial pressure
(PaO2) was documented. During dissection, special
attention was paid to the possibility of bone fracture and thoracic
and peritoneal bleeding.
Histological assessment
After blood gas analysis, the rats from the group
receiving maximal sublethral energy were sacrificed by transection
of the abdominal inferior vena cava. The bilateral lungs and the
heart were removed. The lung tissue where the opacity area was the
most evident in the CT scan was sliced and immersed in 1% formalin
together with the cardiac tissue. The tissue was then stained with
hematoxylin and eosin (H&E) and viewed using optical
microscopy. Histological assessment of cardiac tissue included
evaluation of all four chambers of the heart in multiple sections.
Special attention was paid to the findings at the cardiac apex,
which was most likely to suffer from contrecoup injury. For those
rats dying from the injury, autopsy was performed to determine the
cause of death. The same experienced pathologist was blinded to the
histological assessment.
Statistical analysis
Statistical Product and Service Solutions (SPSS)
13.0 software was used to perform all statistical calculations.
Unless otherwise stated, mean values and standard deviations are
reported. For the comparison between means, a one-way analysis of
variance (ANOVA) was used. For further intergroup pairwise
comparisons, ANOVA with Bonferroni’s post hoc test was used. In the
case of categorical variables, a Chi-square or a Fisher’s exact
test was used when appropriate. Scatter-dot figure was drawn based
on PaO2 and contusion volume percentage. Pearson’s
correlation was used to analyze the relationship between the two
parameters and the linear regression equation was fitted. The
regression coefficient was checked by the Student’s t-test. For all
statistical analyses, P-values of <0.05 were considered to
indicate statistical significance.
Results
Mortality and determination of the
maximal sublethal injury energy
The mortality in the 3.0 J group was as high as
33.3% (3/9), which exceeded the criterion of lethal energy
(Table I). Autopsy showed that the
cause of death was rib fracture and thoracic bleeding, which was
also the cause of death for the only case of mortality in group 2.4
J. Thus, 2.7 J was regarded as the maximal sublethal energy in this
model.
 | Table IMortality rate of each energy group
(n=9). |
Table I
Mortality rate of each energy group
(n=9).
| Group | Number of deaths | Mortality (%) |
|---|
| 2.1 J | 0 | 0.0 |
| 2.4 J | 1 | 11.1 |
| 2.7 J | 0 | 0.0 |
| 3.0 J | 3 | 33.3 |
Comparison of PaO2 and
contusion volume percentage between groups
The PaO2 and contusion volume percentage
were different among all groups and achieved significance (Table II). Upon further intergroup
pairwise comparisons, the PaO2 and contusion volume
percentage was not significantly different between groups 2.1 and
2.4 J. The difference in PaO2 between groups 2.7 and 3.0
J did not reach significance. Compared with those in the groups
with lower energy, the rats in group 2.7 J manifested larger
contusion lesion and more severe hypoxemia, which basically met the
diagnostic criterion for acute respiratory distress syndrome
(ARDS).
 | Table IIComparison in PaO2 and
contusion volume percentage between groups. |
Table II
Comparison in PaO2 and
contusion volume percentage between groups.
| Control | 2.1 J | 2.4 J | 2.7 J | 3.0 J | P-value |
|---|
| PaO2
(mmHg) | 397.22±20.99a | 238.78±11.57 | 220.83±16.33b | 193.93±16.68d | 166.23±25.53f | <0.001 |
| Contusion volume
percentage | 0.41±0.85a | 4.46±1.08 | 5.88±1.16c | 10.50±0.96e | 15.90±1.84g | <0.001 |
Relationship between PaO2 and
contusion volume percentage
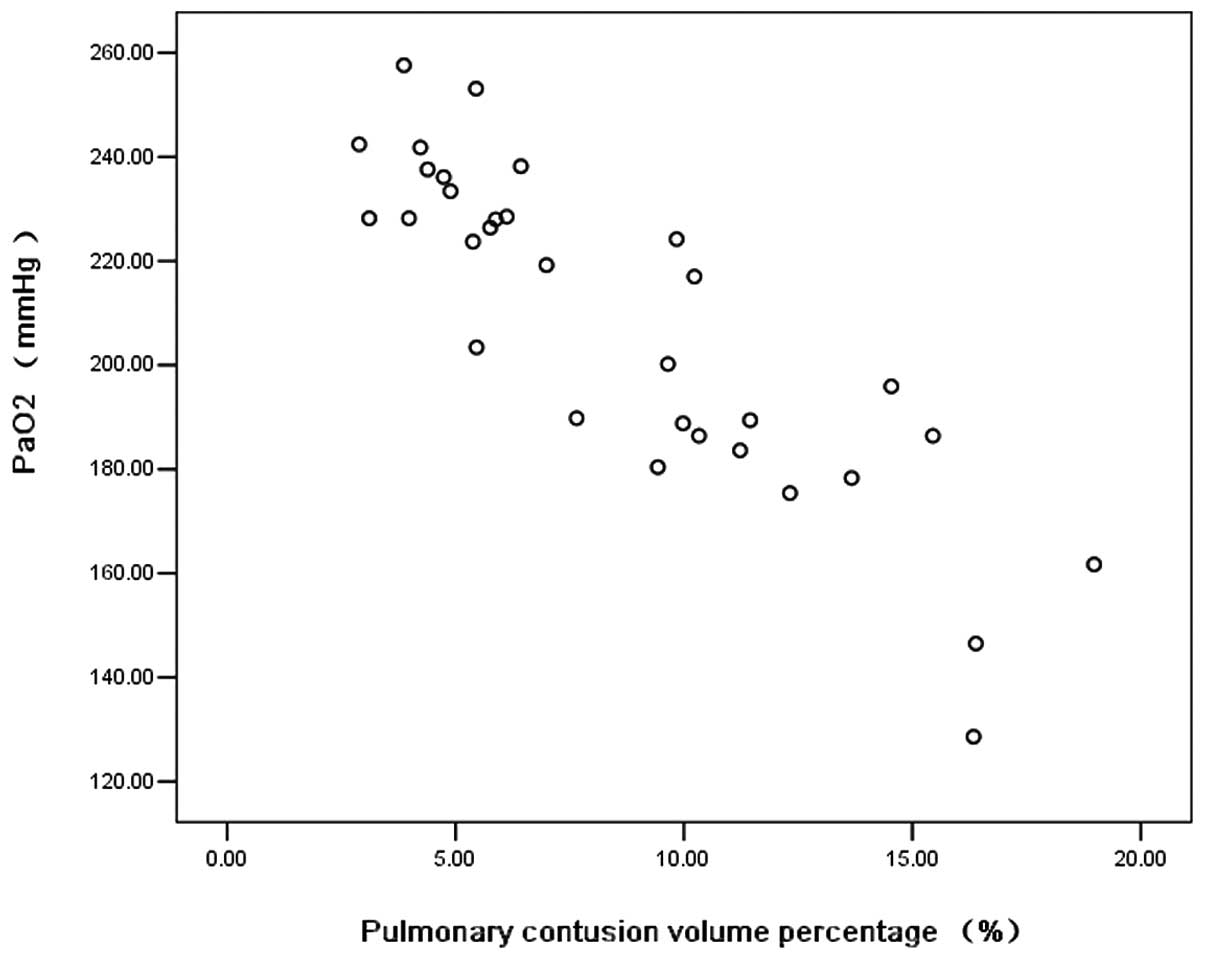
Pearson’s correlation showed that there was
basically a negative correlation between PaO2 and
contusion volume percentage (R2=0.762, Fig. 3). The linear regression equation
was fitted, the efficacy of which was determined by the Student’s
t-test (Table III): y =−6.18x +
261.57, where y represents PaO2 and x represents
contusion volume percentage shown by three dimension (3D)CT.
 | Table IIILinear regression coefficienta. |
Table III
Linear regression coefficienta.
| Variable | β | SE | Sβ | t-value | P-value |
|---|
| Constant | 261.57 | 6.12 | - | 42.75 | <0.001 |
| Contusion volume
percentage | −6.18 | 0.63 | −0.87 | −9.79 | <0.001 |
Histological assessment
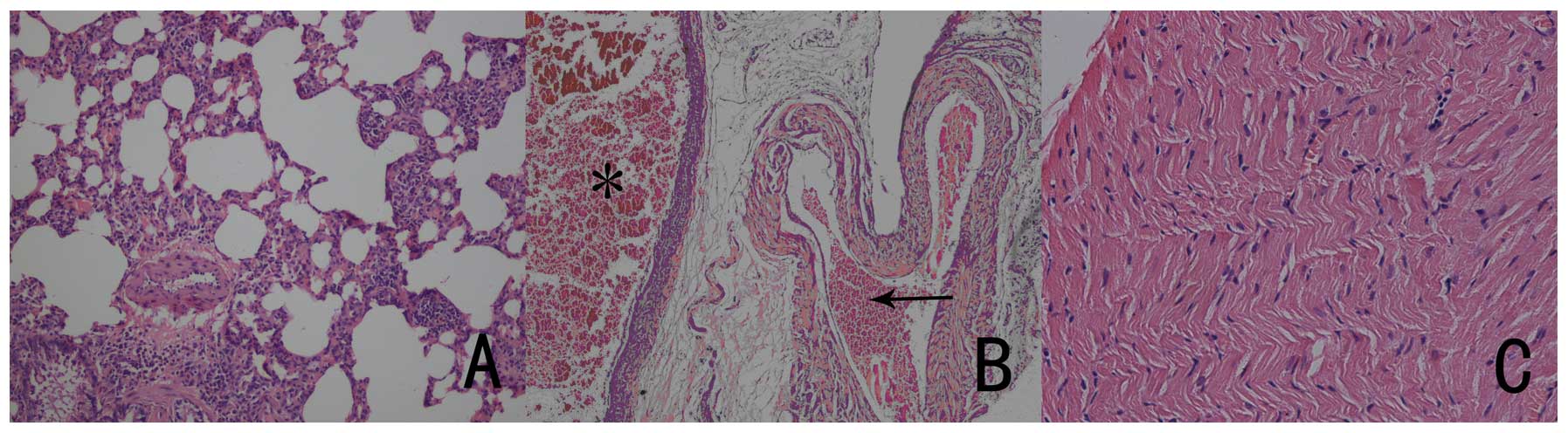
Pathological examination 4 h after injury showed
numerous neutrophils together with several monocytes and
lymphocytes apparent in the air space with pulmonary parenchyma and
interstitial edema, and still red blood cell infiltration in the
alveolar space; these observations were compatible with a diagnosis
of PC (Fig. 4A). Arterial and
venous congestion was noted in some areas (Fig. 4B). There was no significant
evidence of cardiac muscle disruption, edema and bleeding in the
multiple heart sections (Fig.
4C).
Discussion
The earliest animal models for PC were reported in
the 1960’s, when Border et al (3) induced PC in canines using a weight
dropped on a shielded chest. The constancy of lungs was
compromised, and the pathology examination revealed atelectasis,
consolidation and air trapping, which confirmed the diagnosis of
PC. Nichols et al (4)
modified the model based on the work of Border by sliding steel
bars with a plate onto the chest wall of canines. They observed the
progression of PC in dogs and revealed that hypoxemia reached a
peak at 24 h and was improved by 48 h. Other large animal models
for PC such as swine and monkey have been developed (5). Although more similar to PC in humans,
these large animal models are much more costly and lack molecular
probes and other cell-and mediator-specific reagents, compared with
rodent models. Thus, rodent models are widely used in the study of
PC.
To date, several mature and reproducible rodent
models for PC have been reported in the literature. Knoferl et
al (6) developed the first
murine model for PC with a unilateral chest impact using an
ultrasonic blast wave. Hoth et al (7) published the results of another closed
chest murine model, where an electrical cortical impactor was used
to deliver a target energy level producing a uniform contusion to
the right lung. These experimental platforms were found to be
complex. Wang et al (8)
induced combined cardiac and pulmonary contusion using a swing
pendulum with energy ranging from 1.7 to 6.0 J. This model had
advantages such as easy design and controllable energy, however,
myocardial contusion inevitably had an impact on the interpretation
of the results of hypoxemia. Moreover, the leading cause of murine
mortality in their study was blunt myocardial contusion.
Raghavendran et al (2)
induced PC in anesthetized rats by dropping a weight onto a
precordial protective shield to direct the impact force away from
the heart and toward the lungs. Their experimental platform was
suspended on Teflon guides, which was costly and relatively
complex. However, compared with the animal models mentioned above,
their models were easily reproducible, and injury energy was
controllable. Moreover, the models were regarded as isolated PC
models. Our model was created on the basis of Raghavendran’s work.
The posterolateral chest wall of the anesthetized rat was wedged
into the under surface of the arch shield by adjusting the height
of the base on which the rat was fixed, so that the injury energy,
which was dependent on the mass and height of the weight, could be
transferred to the posterolateral chest wall of the rat. The rat
was in a prone position, which increased the distance between the
injury region and the heart and hence further reduced the risk of
myocardial contusion. Contrecoup injury was once a concern in this
model, but biopsy confirmed the diagnosis of bilateral PC and ruled
out the existence of myocardial contusion. The model also used an
impact induction method that was highly relevant to not only motor
vehicle accident injury, but also to high fall injury, both of
which are the most common causes of clinical blunt thoracic trauma.
The value for the acceleration of gravity was taken to be 10
m/sec2 to facilitate calculation.
The energy in our model was higher than that in
Raghavendran’s model. The elasticity of the posterolateral ribs of
the rats is not as good as that of the anterolateral ribs.
Therefore, a certain energy level induced a smaller contusion
lesion size in our model when compared with the model of
Raghavendran. Less elasticity is likely to facilitate rib fracture
caused by high-fall energy, which was a major concern in our model.
We prospectively chose 20% as the mortality limit to define
sublethal PC injury energy. To direct the injury to the thorax and
avoid neck and abdominal injury, we marked the third thoracic
vertebra and the eighth thoracic vertebra prior to injury. The
specially designed arch shield lowered the risk of vertebral and
scapular fracture and mediastinal organ injury. Mortality in the
3.0 J group exceeded the limit, and the deaths of the rats were
relevant to rib fracture and subsequent thoracic bleeding. which
demonstrated that the energy was too high. However, energy <3.0
J is considered safe.
The major data collected in this study included
PaO2 and PC volume percentage demonstrated by 3DCT.
PaO2, which reflects the severity of hypoxemia, is the
most manifest pathophysiological alteration in PC and is essential
in experimental and clinical research on PC. Imaging is clinically
the most common method used for the diagnosis of PC. Although
expedient and common, chest plain reontography may overlook lesions
shortly after injury. Research (9)
which compared the results of plain chest radiography and
pathologic results from autopsy found that plain chest radiography
had a sensitivity of only 34%, which would be even lower if the
film was taken shortly after injury (10). CT, when compared with plain film,
was reported to have a high sensitivity of ∼100% (11). Its advantages rely on the strict
correlation between CT density and the lung physical density,
allowing a quantification of lung compartments with different
degrees of aeration. Therefore, as far as early diagnosis is
concerned, chest CT is the optimal tool. Estimation of contusion
volume based on opacity area in bilateral lung fields on each slice
is feasible. However, as for quantification, this method relies on
visual estimation. Therefore, inter-reader and intra-reader
variation may result in poor reproducibility in the quantification
of PC volume. Attenuation-defined measurement has been reported
(12). This method considered
hydrothorax and atlectasis which were also likely to impair gas
exchange. However, variation in the attenuation in different injury
circumstances also made the definition of the damaged lung field
difficult, and it was of no use in isolated PC models. PC volume
percentage quantification demonstrated by 3DCT is accurate and
repeatable, and thoracic bleeding, organ injury and bone fracture
can be observed on the CT image simultaneously. A prospective
research study (13) demonstrated
that PC volume percentage quantification demonstrated by 3DCT was
an independent predictor for ARDS development after PC. Thus, in
this study, it was PaO2 and PC volume percentage
demonstrated by 3DCT that were chosen as indictors for assessing
the size of PC lesions and evaluating the severity of PC. Given the
fact that there is probably pulmonary exudative lesions of various
causes in ‘healthy’ rats which would mimick PC lesions in 3DCT
imaging, we treated another 9 rats as controls to obtain data on
the background of pulmonary exudation in ‘healthy’ rats.
A previous study (14) illustrated that clinical
PaO2/FiO2 was not linearly correlated to PC
volume percentage as demonstrated by 3DCT, which was explained by
the fact that the PaO2/FiO2 ratio may be
influenced by numerous factors such as the state of consciousness
and systolic blood pressure. In this model, PaO2 and PC
volume percentage were basically negatively correlated (Fig. 3, R2=0.762), which
illustrated that 76.2% of the change in PaO2 was
explained by a change in contusion volume percentage. That is,
hypoxemia in this study reflected the severity and distribution of
PC. Hence, compared with clinical PC which is accompanied by other
injuries, PC induced by this model was regarded as simple and
specific.
PC is an evolving lesion. Animal experiments and
clinical research illustrate that hypoxemia in PC is serious 4 h
after injury, may last for 24 h and improves by 48 h (2). We chose 4 h as the interval between
injury and data collection, which minimized the impact of pulmonary
infection, suptum retention and atlectasis on the results.
Cardiac output and other variables of cardiac
dysfunction were not measured in the present study, since these are
not specific to blunt cardiac trauma. The hypoxemia and pulmonary
hemodynamic changes (specifically acute reactive pulmonary
hypertension) associated with PC have been shown in many animal
studies to seriously affect cardiac function (15). Multiple biopsies of cardiac muscles
demonstrated the existence of spotted congestion change without
apparent myocardial rupture, which is relevant to pulmonary
hemodynamic changes during PC.
Finally, we focused on hypoxemia, distribution of
lesions on imaging and the pathological findings of PC.
Quantification of pulmonary inflammation and comparison with past
models in the literature were lacking. However, this is a pilot
study. After determining the maximal sublethal energy of this
model, we will carry out a quantificative study on pulmonary
inflammation after PC using this model in subsequent research.
In conclusion, the method documented in this study,
which is easy to duplicate, results in satisfactory isolated
bilateral pulmonary contusion in rats, and thus 2.7 J can be
regarded as the maximal sublethal injury energy. The severity of
hypoxemia reflected the distribution of PC lesions to a great
extent.
References
|
1.
|
Raghavendran K, Notter RH, Davidson BA,
Helinski JD, Kunkel SL and Knight PR: Lung contusion: inflammatory
mechanisms and interaction with other injuries. Shock. 32:122–130.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Raghavendran K, Davidson BA, Woytash JA,
Helinski JD, Marschke CJ, Manderscheid PA, Notter RH and Knight PR:
The evolution of isolated, bilateral lung contusion from blunt
chest trauma in rats: cellular and cytokine responses. Shock.
24:132–138. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Border JR, Hopkinson BR and Schenk WG Jr:
Mechanisms of pulmonary trauma: an experimental study. J Trauma.
8:47–62. 1968. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Nichols RT, Pearce HJ and Greenfield LJ:
Effects of experimental pulmonary contusion on respiratory exchange
and lung mechanics. Arch Surg. 96:723–730. 1968. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Davis KA, Fabian TC, Ragsdale DN, et al:
Endogenous adenosine and secondary injury after chest trauma. J
Trauma. 49:892–898. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Knoferl MW, Liener UC, Seitz DH, Perl M,
Bruckner UB, Kinzl L and Gebhard F: Cardiopulmonary, histological,
and inflammatory alterations after lung contusion in a novel mouse
model of blunt chest trauma. Shock. 19:519–525. 2003. View Article : Google Scholar
|
|
7.
|
Hoth JJ, Hudson WP, Brownlee NA, Yoza BK,
Hiltbold EM, Meredith JW and McCall CE: Toll-like receptor 2
participates in the response to lung injury in a murine model of
pulmonary contusion. Shock. 28:447–452. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Wang ND, Stevens MH, Doty DB and Hammond
EH: Blunt chest trauma: an experimental model for heart and lung
contusion. J Trauma. 54:744–748. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Schild HH, Strunk H, Weber W, et al:
Pulmonary contusion: CT vs. plain radiograms. J Comput Assist
Tomogr. 13:417–420. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Tyburski JG, Collinge JD, Wilson RF and
Eachempati SR: Pulmonary contusions: quantifying the lesions on
chest X-ray films and the factors affecting prognosis. J Trauma.
46:833–838. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Caironi P, Carlesso E and Gattinoni L:
Radiological imaging in acute lung injury and acute respiratory
distress syndrome. Semin Respir Crit Care Med. 27:404–415. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Darly M, Miller PR, Carr JJ, et al:
Traumatic pulmonary pathology measured with computed tomography and
a semiautomated analytic method. Clin Imaging. 32:346–354. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Wang S, Ruan Z, Zhang J, et al: The value
of pulmonary contusion volume measurement with three-dimensional
computed tomography in predicting acute respiratory distress
syndrome development. Ann Thorac Surg. 92:1977–1983. 2011.
|
|
14.
|
Miller PR, Croce MA, Bee TK, Qaisi WG,
Smith CP, Collins GL and Fabian TC: ARDS after pulmonary contusion:
accurate measurement of contusion volume identifies high-risk
patients. J Trauma. 51:223–228. 2001.PubMed/NCBI
|
|
15.
|
Moomey CB Jr, Fabian TC, Croce MA, et al:
Cardiopulmonary function after pulmonary contusion and partial
liquid ventilation. J Trauma. 45:283–290. 1998. View Article : Google Scholar : PubMed/NCBI
|