Introduction
ATP-binding cassette (ABC) transporters, which act
as efflux pumps, play a role in drug pharmacokinetics (1). It has been revealed that ABC
transporters are expressed in various tissues, including the liver,
kidneys, small intestine and brain (2). ABC transporters are also considered
to be one of the main causes of multidrug resistance of anticancer
drugs and are a major issue in the clinical setting (3).
Although numerous findings have previously been
acquired with regard to the expression patterns of ABC transporters
in typical tissues, including the liver, kidneys, small intestine
and brain, information on the expression levels in blood cells
remains inadequate (4). As for
solid cancer cells, the relatively abundant expression and
functional mechanisms of ABC transporters, including multidrug
resistance 1 (MDR1), multi-drug resistance-associated protein 1
(MRP1) and BCRP, have been previously reported (3). In addition, the solute carrier
protein (SLC) transporters (e.g. PEPT1 and OATP1B3), which play a
functional role as influx pumps for drugs, are also expressed in
solid cancer cells in excess (5–7). The
clarification of expression levels of drug transporters in blood
cancer cells would provide critical background data for developing
new drugs for blood cancer, and examination of the affinity of
drugs to transporters would provide useful data on multiple drug
resistance.
Methotrexate (MTX) is currently used as a
therapeutic drug for acute leukemia and chronic myelogenous
leukemia, and is reported to be actively transported into normal
and cancer cells (8). MTX is a
typical substrate of MRP transporters (e.g. MRP1, 2 and 4)
(9,10), and the efflux transport of MTX via
MRP transporters expressed on the blood cell membrane may be
functioning to a certain extent.
Although there is insufficient information on MRP4
expression in normal rat blood cells at present, the possibility of
the efflux function of MRP4/5 in normal rat erythrocytes has
previously been suggested (11).
In addition, the contribution of MRP4 as the efflux pump in bone
marrow, spleen, thymus and the gastrointestinal tract was suggested
in an in vivo study using Mrp4-knockout mice (12). In the present study, the relative
mRNA expression levels of ABC transporters in human blood cancer
cell lines were measured. Secondly, MTX and an MRP inhibitor
(MK-571) were coadministered to normal rats to investigate the
contribution of MRP4 in normal blood cells and other tissues by
measuring the MTX concentrations.
MTX is mainly excreted in the urine both in rats
(13) and humans (14). Accordingly, side effects of MTX
would be expected if the inhibitors of MRP transporters were
concomitantly administered, which would cause inhibition of the
renal clearance of MTX at the renal proximal tubule. In the present
in vivo study using rats, to avoid the inhibitory effect
against the renal clearance of MTX, MK-571 was selected as the
concomitant drug possessing inhibitory potency for MRP
transporters, which demonstrates a typical bile-excretion
pharmacokinetic property (15).
The conversion of MTX to 7-hydroxy-MTX has been reported as a main
metabolic pathway of MTX in animals and humans (16,17).
As the reported serum concentration of 7-hydroxy-MTX was
significantly lower than that of MTX following intravenous (i.v.)
bolus administration of MTX to rats (17), the influence of MTX metabolites in
the present in vivo study may not be so large.
Materials and methods
RNA extraction and cDNA synthesis
Human blood cancer cell lines, KU812 (chronic
myelocytic leukemia), KG-1 (acute myelocytic leukemia), U937
(lymphoma) and RPMI 1788 (normal blood cell line derived from a
healthy subject), were obtained from the Health Science Research
Resources Bank (Osaka, Japan). Total RNA extraction from the cells
was carried out according to the manufacturer’s instructions using
the RNAqueous kit (Ambion, Austin, TX, USA). First-strand cDNA
synthesis was performed with the Reverse Transcription system
(Roche, Mannheim, Germany). cDNA derived from healthy human liver
was purchased from BioChain (Newark, CA, USA).
Real-time polymerase chain reaction
(RT-PCR)
The obtained cDNA was diluted with water and 10 μl
was used for amplification. Parameter-specific primer sets
optimized for the LightCycler (RAS) for the measurement of human
transporters [MDR1 (ABCB1), BCRP (ABCG2), MRP1 (ABCC1), MRP2
(ABCC2), MRP3 (ABCC3), MRP4 (ABCC4), MRP5 (ABCC5), PEPT1 (SLC15A1)
and OATP1B3 (SLCO1B3)], human cytochrome P450s (CYPs: CYP1A2,
CYP2A6, CYP2B6, CYP2C9, CYP2C19, CYP2D6 and CYP3A4) and
housekeeping genes [human glyceraldehyde 3-phosphate dehydrogenase
(GAPDH) and human TATA binding protein (TBP)] were developed by and
purchased from Search-LC GmbH (Heidelberg, Germany). The PCR was
performed with the LightCycler FastStart DNA SYBR Green kit (RAS)
according to the manufacturer’s instructions and as described
previously (18). To control for
the specificity of the amplification products a melting curve
analysis was performed and no amplification of unspecific products
was observed. The data of two independent analyses for each sample
and parameter were averaged. The copy number was normalized by the
two housekeeping genes, GAPDH and TBP. As the relative expression
levels obtained from the two housekeeping genes were almost
identical, the copy number is represented as the number of
transcripts per 103 copies of GAPDH.
Materials
The 3H-labeled MTX ([3H]MTX)
disodium salt [specific activity, 21.0 Ci/mmol, 1.0 mCi/23.8 μg/ml,
ethanol:water (4:6) solution] was purchased from Moravek
Biochemicals (Brea, CA, USA). The MK-571 sodium salt hydrate was
purchased from Sigma Aldrich (Tokyo, Japan).
Animals
Male Sprague-Dawley (SD) rats aged 6 to 7 weeks and
weighing between 230 and 260 g were purchased from Charles River
Japan, Inc. (Shiga, Japan) for the in vivo study. Animals
were housed at least 7 days prior to experiments under controlled
environmental conditions (23±2°C, 60±10% humidity and a 12-h
light/12-h dark cycle) and fed a commercial food diet (CRF-1,
Oriental Yeast, Tokyo, Japan) with water available ad
libitum. The experimental protocols and procedures were
reviewed and approved by the Animal Ethics Committee of Otsuka
Pharma Ltd.
Preparation of dosing solution
Appropriate quantities of [3H]MTX were
diluted with water or MK-571 solution (2.5 mg/ml in water) to
adjust the specific activity required for the dose preparation (0.1
mCi/kg).
Time course of the plasma and tissue
concentrations of [3H]MTX following i.v. bolus
administration to rats
[3H]MTX (2.38 μg/kg, 4.76 nmol/kg) was
administered i.v. as a bolus into the tail vein to two groups of
rats. The animals in one group received [3H]MTX only,
the other group was concomitantly administered i.v. MK-571 (1
mg/kg, 1.94 μmol/kg). Following drug administration, the blood was
drawn from the inferior vena cava with a heparinized syringe. The
sampling times for venous blood were 0.25, 1 and 4 h following
administration. After blood sampling from each animal, the liver,
kidneys, spleen, thymus, whole brain (cerebrum, cerebellum and
medulla oblongata), bone marrow and mesenteric lymph nodes were
dissected, then washed with saline and stored in a freezer (−20°C)
until sample preparation.
Sample collection and preparation
Approximately 2 ml of blood was transferred to
polypropylene tubes and was rapidly centrifuged for 10 min at 1,800
× g at 4°C to obtain plasma. A portion of blood was encapsulated in
a hematocrit (Ht) capillary (n=1) and centrifuged for 5 min at
10,500 × g at room temperature, and the Ht-value was measured by Ht
reader. The residual blood was divided into two portions; whole
blood sample (100 μl) for radioactivity measurement and the
specimen for blood cell separation (remaining blood). For blood
cell separation, an equivalent amount of PBS (−) (pH 7.4;
Invitrogen, Carlsbad, CA, USA) was added to 2 ml of blood, and a
layer (4 ml) of the diluted blood was put over 3 ml
Lympholyte®-Mammal (Cedarlane, Burlington, NC, USA)
using a large Pasteur pipette with as little agitation as possible
at the interface. Following centrifugation for 20 min at 800 × g at
room temperature, the cells (lymphocyte, monocyte and erythrocyte
fractions) were carefully removed from the interface. The tissues
(approximately 100 mg or less), with the exception of the kidneys
and whole brain, were transferred directly to a glass vial and used
for the preparation of radioactivity counting samples. The kidneys
and whole brain were homogenized with 2 volumes of saline using a
polytron homogenizer (Kinematica, Bohemia, NY, USA), and 0.1 ml of
homogenates (precise weight) was used for the preparation of the
radioactivity counting samples. A 1-ml portion of the tissue
solubilizer (Nacalai Tesque, Kyoto, Japan) was added to each
sample, with the exception of plasma, and the resulting mixture was
treated with a tissue-dissolving apparatus (Biomerit, Sekisui
Medical, Tokyo, Japan) for approximately 30 min. The solubilized
sample was mixed with 15 ml of Hionic-Fluor (PerkinElmer, San Jose,
CA, USA) for radioactivity measurement. The plasma was mixed with 1
ml of water and 15 ml of Ultima Gold (PerkinElmer) and the
radioactivity was counted.
Liquid scintillation counter
analysis
The radioactivity of samples was counted using a
liquid scintillation counter (Packard, St. Paul, MN, USA). The
counting efficiency was corrected by the external standard method.
The background value (dpm) was determined by measuring the
scintillation cocktail alone.
Distribution ratio to erythrocytes
The blood cell distribution ratio (%) was calculated
from the radioactivity concentrations in the blood (Cb) and plasma
(Cp) as well as the hematocrit value (Ht) using the following
equation: Blood cell distribution ratio (%) = [1 − Cp/Cb × {(100 −
Ht)/100}] × 100.
Statistical analysis
In the in vivo study using rats, data are
expressed as mean values ± SD. The comparison between the MTX alone
group and the MTX and MK-571 concomitant group for every time point
was carried out by Student’s t-test. P<0.05 was considered to
indicate a statistically significant result.
Results
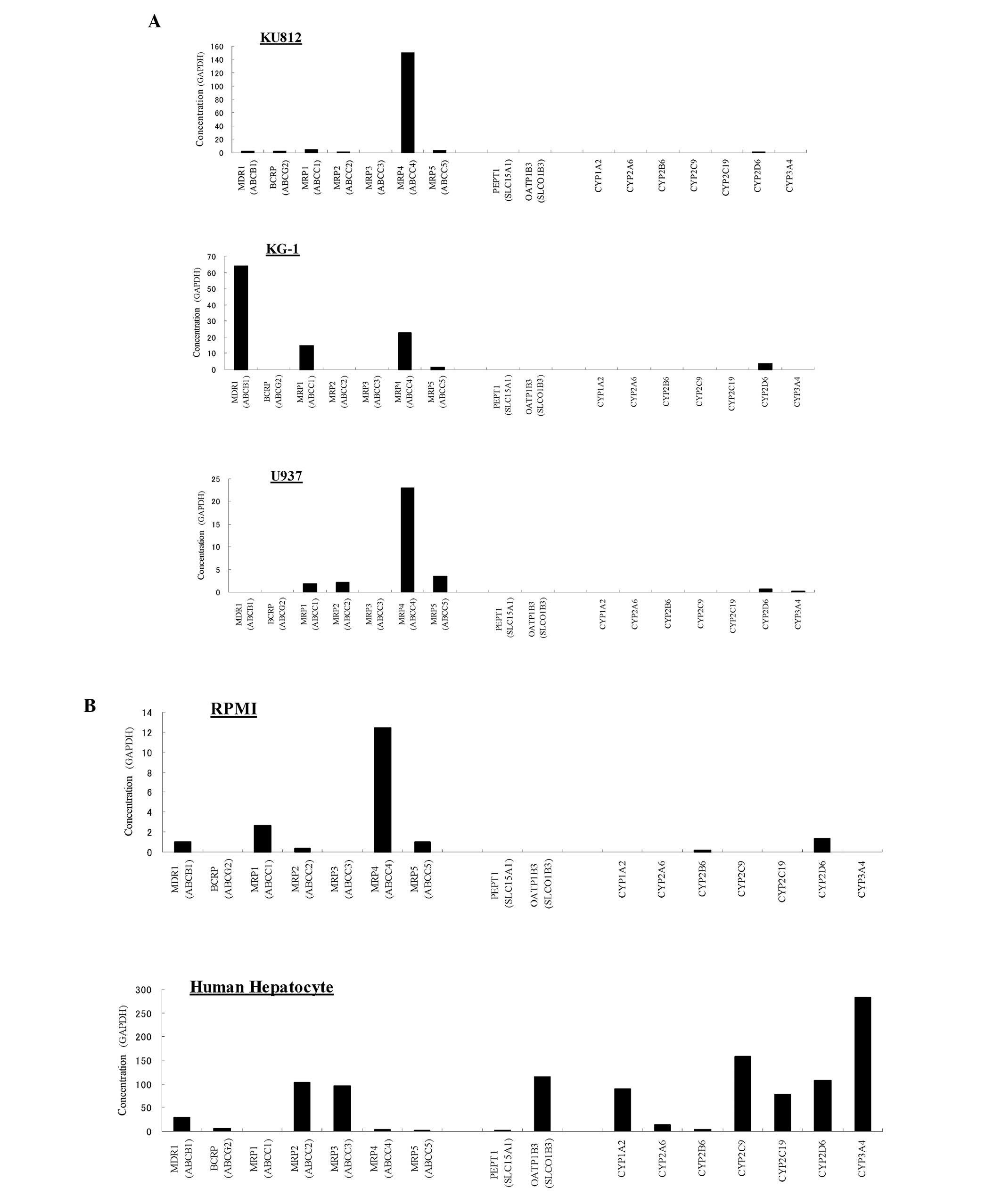
mRNA expression level of typical drug
transporters in human blood cancer cell lines
The mRNA expression levels of typical ABC
transporters (MDR1, BCRP and MRP1/2/3/4/5) and of SLC transporters
(PEPT1 and OATP1B3) in human blood cancer cell lines were analyzed
by RT-PCR. The mRNA levels of major human CYPs (CYP1A2, CYP2A6,
CYP2B6, CYP2C9, CYP2C19, CYP2D6 and CYP3A4) were measured as the
reference data. The mRNA expression levels of the transporters and
the CYPs in human hepatocytes were also measured as a control. As a
result, the expression level of MRP4 was extremely high (151 copies
of transcript/103 copies of GAPDH) by comparison with
those of other transporters in the chronic myelocytic leukemia cell
line KU812 (Fig. 1A and C). The
numbers of transcripts for the other transporters in KU812 were
below 4 copies/103 GAPDH. In the acute myelocytic
leukemia cell line KG-1, MDR1 expression level was the highest (64
copies/103 GAPDH), followed by those of MRP4 (23
copies/103 GAPDH) and MRP1 (15 copies/103
GAPDH; Fig. 1A and C).
Additionally, MRP4 expression level was the highest in the lymphoma
cell line U937 (23 copies/103 GAPDH) and the blood cell
line derived from a healthy subject RPMI 1788 (12
copies/103 GAPDH; Fig.
1A–C). Relatively low expression levels of MRP1/2/5 in the U937
cell line, and of MDR1, MRP1/2/5 in the RPMI 1788 cells were
observed (Fig. 1A–D). The relative
mRNA expression levels of the typical human transporters and human
CYPs in the normal human hepatocytes were almost the same as those
previously reported (19–21). Neither of the two major human SLC
transporters (PEPT1 and OATP1B3) was detected in any of the blood
cell line in the present study (Fig.
1A, B and D). With regard to the human CYPs, low expression
levels of CYP2B6 in U937 and RPMI 1788, and of CYP2D6 in KU812,
KG-1, U937 and RPMI 1788 were observed (Fig. 1A and B).
Inhibitory effects of MK-571 on MTX
distribution in rats
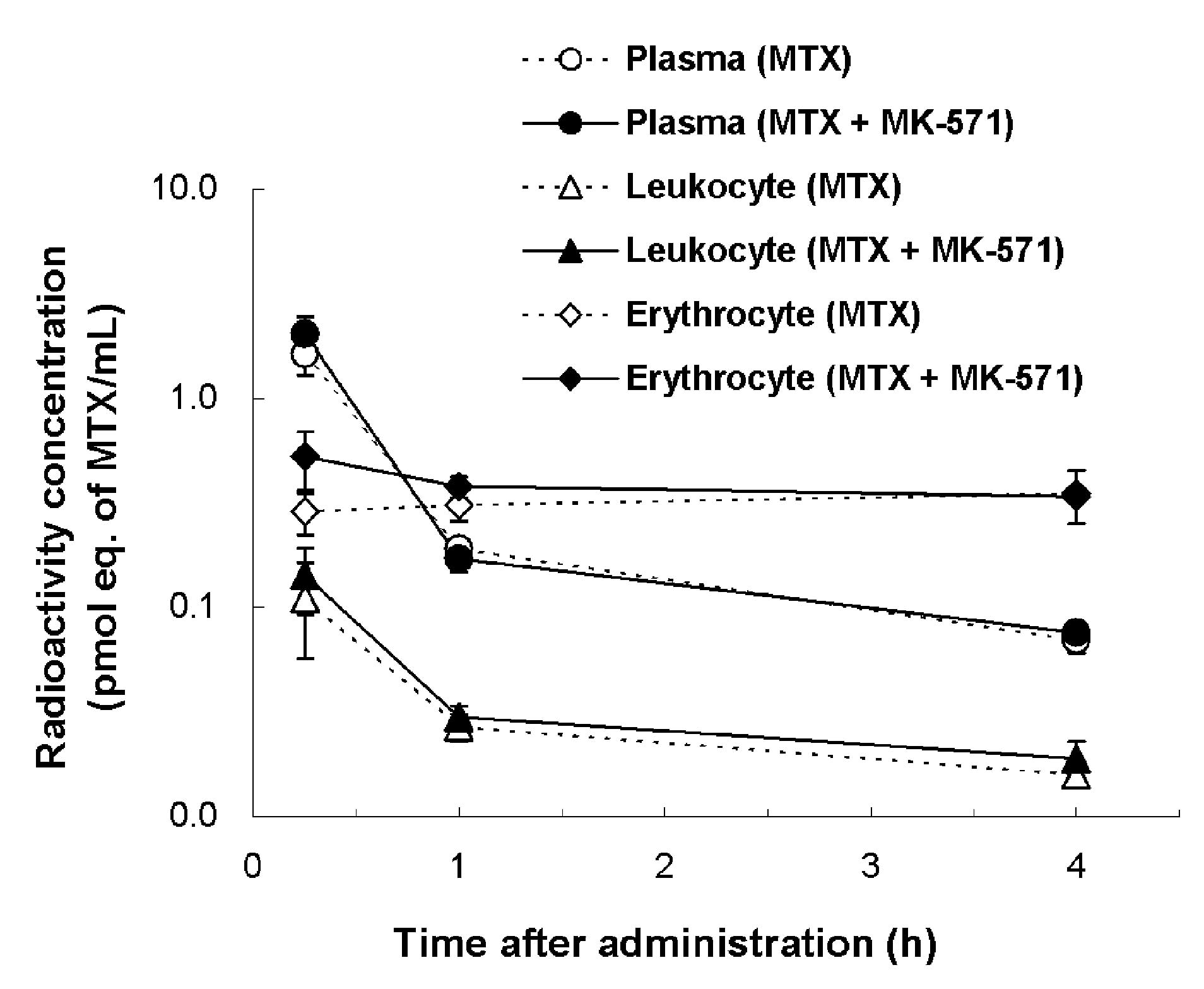
Following the [3H]MTX i.v. bolus
administration to rats, no significant difference in the plasma,
leukocyte and erythrocyte concentrations was observed between the
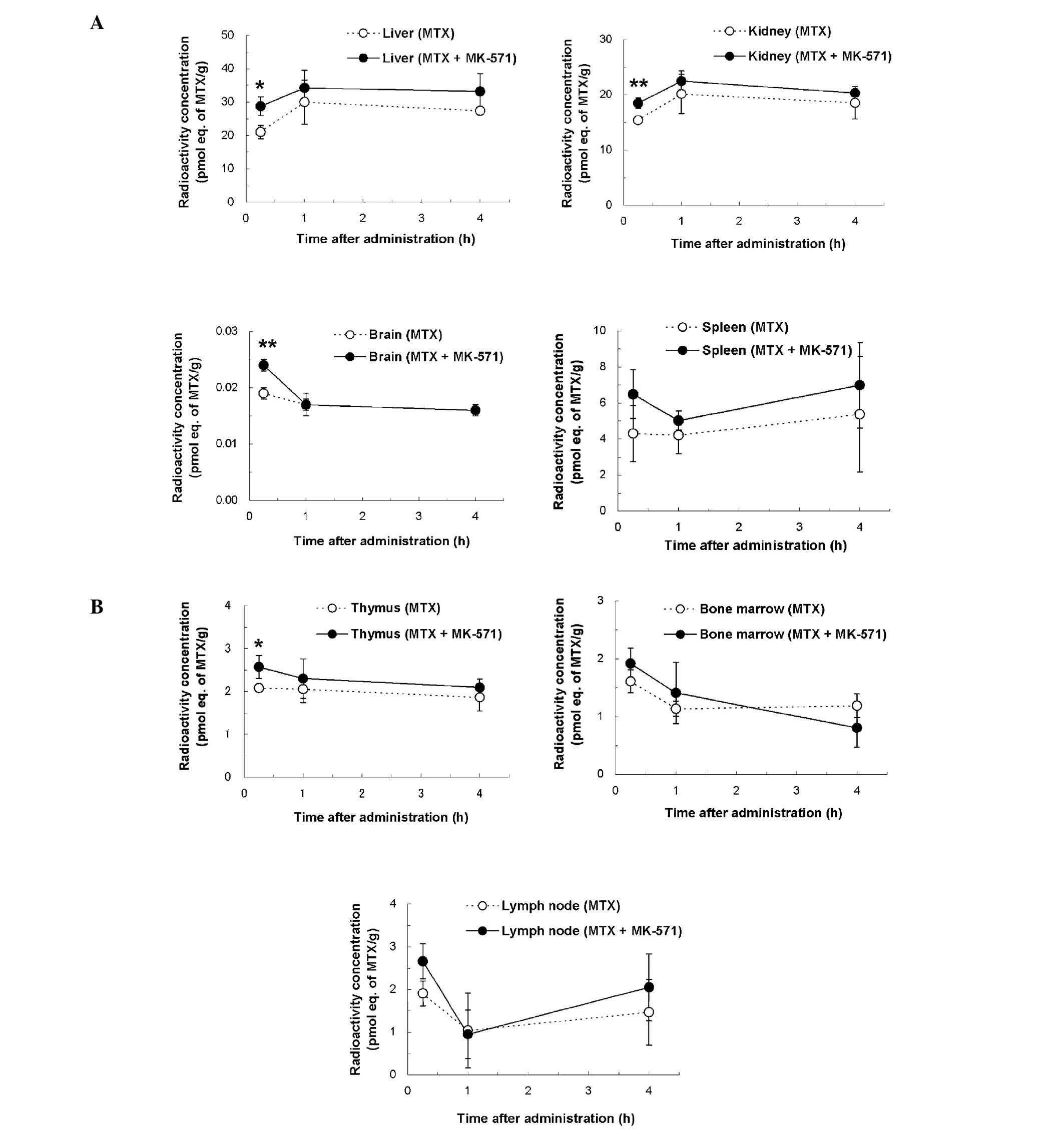
MTX alone and the MTX and MK-571 concomitant groups (Fig. 2). By contrast, a slight difference
was observed in other tissues. The MTX concentrations in the liver,
kidneys, brain and thymus at 15 min after i.v. administration in
the concomitant group revealed, respectively, 1.4 (P<0.05), 1.2
(P<0.01), 1.3 (P<0.01) and 1.2 times (P<0.05) higher
values compared with those of the MTX alone group (Fig. 3). The Kp values (tissue
concentration/plasma concentration) of the concomitant group showed
slightly higher values compared with those of the MTX alone group
at 15 min after administration in erythrocytes (1.4 times,
P<0.001), spleen (1.3 times, P<0.05) and thymus (1.2 times,
P<0.05), respectively (Fig. 4).
With regard to the distribution ratios to blood cells, no
significant difference was observed between the two groups. At each
time point, the mean values of the distribution ratios to blood
cells in the MTX alone and the concomitant group were respectively
21.9±3.7 and 24.4±4.6% (15 min after administration), 63.7±6.6 and
67.2±1.8% (1 h) and 83.5±0.5 and 82.6±1.4% (4 h).
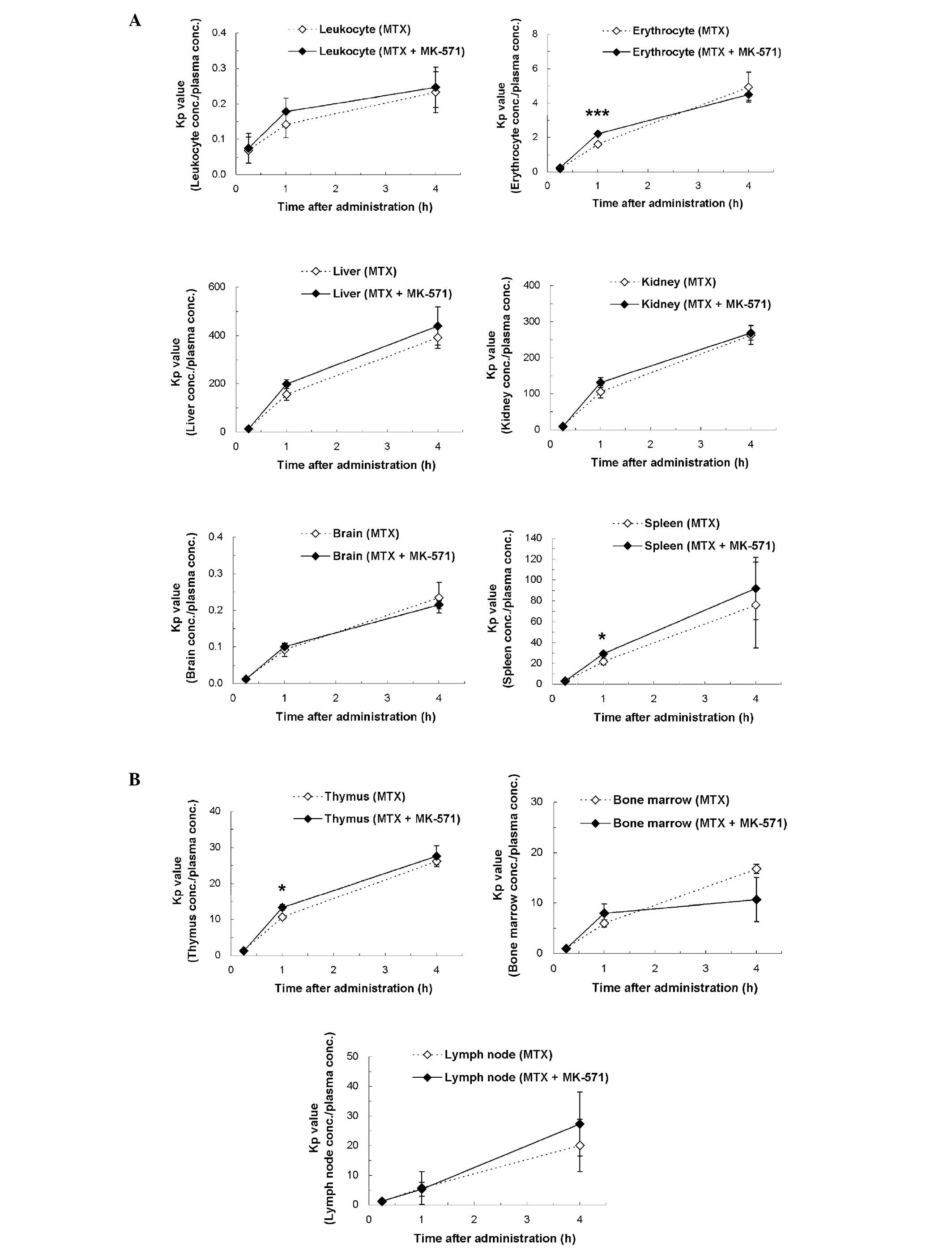 | Figure 4Kp values of radioactivity after i.v.
bolus administration of [3H]MTX alone or concomitant
administration of MTX and MK-571 to rats. Kp values (tissue
concentration/plasma concentration) of leukocytes, erythrocytes,
the liver, kidneys, brain and spleen, thymus, bone marrow and lymph
node between the two administration groups were compared. Each
point represents the mean ± SD of three animals. Significantly
higher values in the concomitant administration of MTX and MK-571
(*P<0.05, ***P<0.001) by Student’s
t-test. (A) Leukocytes, erythrocytes, liver, kidneys, brain and
spleen; (B) thymus, bone marrow and lymph node. |
Discussion
We set out to determine whether MRP4 is expressed
and functions as an efflux transporter in blood cancer cells.
Although direct involvement of the MRP4 function in blood cancer
cells could not be evaluated in this study, the results revealed
excessive expression of MRP4 in the chronic myelocytic leukemia
cell line, KU812. Additionally, MRP4 expression in the normal blood
cell line derived from a healthy subject, RPMI 1788, was relatively
high. The phenomena associated with rodent MRP4 function have
recently been suggested in normal erythrocytes of rats and in
normal tissues (e.g. spleen and thymus) of mice (11,12).
At present, there is insufficient data for the expression and the
function for MRP4 linking cancer cells with normal blood
cells/tissues. However, due to the possibility that a slight efflux
function of MRP4 was suggested in normal blood cells/tissues of
rats in the present study, the possibility of a functional
contribution of MRP4 particularly in cancer cells with high MRP4
expression, e.g. KU812 was suggested.
To demonstrate the efficacy of anticancer drugs,
direct contact between the target cancer cells and anticancer drugs
at an effective drug concentration is usually required. For
anti-blood cancer drugs, appropriate delivery of drugs to cancer
cells (including atypical leukocytes and lymphocytes) that
circulate through blood systemically, or bone marrow cells is
considered to become a trigger of drug efficacy. A recent study
(22) suggested that OCT1 mediates
the uptake of selected anticancer drugs into lymphoma cells. It has
been revealed that PEPT1 and OATP1B3 are abundantly expressed as
uptake transporters in solid cancer cells. As no expression of
PEPT1 and OATP1B3 was detected in blood cancer cell lines in the
present study, a difference in the expression patterns of the
influx transporters between solid cancer cells and blood cancer
cells was suggested.
As for the efflux transporters, ABC transporters are
considered to be one of the main causes of multidrug resistance to
anticancer drugs, and the involvement of MDR1, BCRP and MRP1 is
mainly reported at present as a drug-resistant mechanism in cancer
cells (3,20). Strategies in the clinical setting
for overcoming drug resistance caused by multidrug resistance
proteins have been suggested (23). One of the proposed strategies is
the use of agents that inhibit multidrug resistance proteins. If
the expression levels of objective transporters are significantly
different between cancer cells and normal cells, a selective
approach may be adopted without unwanted adverse phenomena. For
instance, the susceptibility of cancer cells to anticancer drugs
would be expected through the use of specific uptake and/or efflux
inhibition via the corresponding transporters. A previous study has
revealed that the level of MRP4 expression in resistant strains of
hepatocellular cancer is relatively high (24). Taking these findings together with
the result that MRP4 expression in KU812 was extremely high by
comparison with those of other typical ABC transporters in the
present study, it was suggested that MRP4 may partly contribute to
the multidrug resistance properties of cancer cells, especially for
anticancer drugs that show a relatively high affinity to MRP4, e.g.
MTX (10).
MTX is currently used as a therapeutic drug for
acute leukemia and chronic myelogenous leukemia. As MTX is a
typical substrate of MRP transporters, efflux transport of MTX via
MRP transporters expressed in blood cells and tissues may be
functional to a certain extent. In the case of concomitant
administration of MTX and MK-571, a specific inhibitor of MRP
transporters, an increase in the intracellular concentration of MTX
may occur. Although no large difference was observed between the
MTX alone and the MTX and MK-571 concomitant groups in the present
in vivo study using rats, the Kp values of the concomitant
group showed significantly high values (P<0.05) compared with
those of the MTX alone group in erythrocytes, spleen and thymus
samples. For the other tissues, slightly higher concentrations in
the concomitant group were also observed. As the involvement of MRP
transporters in MTX concentrations in blood cells and certain
tissues was examined using normal animals in the present in
vivo study, a more notable inhibitory effect of MK-571 would be
expected in certain types of cancer cells (e.g. KU812) in which the
MRP4 expression is abundant. Referring to the results that the
unbound fraction of plasma MK-571 in rats was <0.5% (15) following single i.v. bolus
administration, the plasma unbound concentration of MK-571 in the
present study was presumed to be 0.008 nM (0.16 nM as the total
concentration) at most as the concentration at 15 min after
administration. MK-571 is recognized as a specific inhibitor of MRP
transporters, including MRP1 (25), MRP2 (26,27)
and MRP4 (28). As the reported Ki
values of MK-571 for these transporters were 0.6 μM (MRP1), 13.1 to
26.4 μM (MRP2) and 10 μM (IC50; MRP4), the exposure
concentration of MK-571 in the present study may be too low to show
sufficient inhibitory effects. Although increasing the dosing
concentration of MK-571 in the in vivo study is restricted
by its solubility, higher exposure of MK-571 in blood cells and
tissues may lead to an increase in the intracellular concentration
of MTX caused by MRP4 inhibition.
In the development of anticancer drugs for blood
cancer, KU812, KG-1 and U937 cell lines are generally used in
preclinical pharmacology studies as mice xenograft models.
Accordingly, the influence of MRP4, which showed high expression in
these blood cancer cell lines, cannot be disregarded since the
contribution ratio of transporters is usually prescribed by
affinity to objective drugs and the expression amount in the target
locus. Numerous studies on MRP4 function in the liver, kidneys and
brain have been performed (29–31).
In addition, recent reports have indicated the efflux function of
MRP4 in the spleen and thymus (12). Furthermore, the drug interaction
between valproic acid and carbapenem antibiotics in the clinical
setting is reported to be partly explained by MRP4, i.e. the
inhibition of efflux transport of valproic acid from erythrocytes
by the MRP4 inhibitor, e.g. panipenem (11,32).
Through further examination of the function of MRP4 and its tissue
localization, avoidance of drug-drug interaction in the clinical
setting, and the enhancement of the potency of objective drugs may
be expected.
References
|
1.
|
International Transporter Consortium;
Giacomini KM, Huang SM, Tweedie DJ, Benet LZ, Brouwer KL, Chu X,
Dahlin A, Evers R, Fischer V, et al: Membrane transporters in drug
development. Nat Rev Drug Discov. 9:215–236. 2010. View Article : Google Scholar
|
|
2.
|
Kusuhara H and Sugiyama Y: Role of
transporters in the tissue-selective distribution and elimination
of drugs: transporters in the liver, small intestine, brain and
kidney. J Control Release. 78:43–54. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Chen ZS and Tiwari AK: Multidrug
resistance proteins (MRPs/ABCCs) in cancer chemotherapy and genetic
diseases. FEBS J. 278:3226–3245. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Köck K, Grube M, Jedlitschky G, Oevermann
L, Siegmund W, Ritter CA and Kroemer HK: Expression of adenosine
triphosphate-binding cassette (ABC) drug transporters in peripheral
blood cells: relevance for physiology and pharmacotherapy. Clin
Pharmacokinet. 46:449–470. 2007.PubMed/NCBI
|
|
5.
|
Nakanishi T, Tamai I, Sai Y, Sasaki T and
Tsuji A: Carrier-mediated transport of oligopeptides in the human
fibrosarcoma cell line HT1080. Cancer Res. 57:4118–4122.
1997.PubMed/NCBI
|
|
6.
|
Gonzalez DE, Covitz KM, Sadee W and Mrsny
RJ: An oligo-peptide transporter is expressed at high levels in the
pancreatic carcinoma cell lines AsPc-1 and Capan-2. Cancer Res.
58:519–525. 1998.PubMed/NCBI
|
|
7.
|
Abe T, Unno M, Onogawa T, Tokui T, Kondo
TN, Nakagomi R, Adachi H, Fujiwara K, Okabe M, Suzuki T, et al:
LST-2, a human liver-specific organic anion transporter, determines
methotrexate sensitivity in gastrointestinal cancers.
Gastroenterology. 120:1689–1699. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Bleyer WA: The clinical pharmacology of
methotrexate: new applications of an old drug. Cancer. 41:36–51.
1978. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Zelcer N, Reid G, Wielinga P, Kuil A, van
der Heijden I, Schuetz JD and Borst P: Steroid and bile acid
conjugates are substrates of human multidrug-resistance protein
(MRP) 4 (ATP-binding cassette C4). Biochem J. 371:361–367. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
El-Sheikh AA, van den Heuvel JJ,
Koenderink JB and Russel FG: Interaction of nonsteroidal
anti-inflammatory drugs with multidrug resistance protein (MRP)
2/ABCC2- and MRP4/ABCC4-mediated methotrexate transport. J
Pharmacol Exp Ther. 320:229–235. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Ogawa K, Yumoto R, Hamada N, Nagai J and
Takano M: Interaction of valproic acid and carbapenem antibiotics
with multidrug resistance-associated proteins in rat erythrocyte
membranes. Epilepsy Res. 71:76–87. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Belinsky MG, Guo P, Lee K, Zhou F, Kotova
E, Grinberg A, Westphal H, Shchaveleva I, Klein-Szanto A, Gallo JM
and Kruh GD: Multidrug resistance protein 4 protects bone marrow,
thymus, spleen, and intestine from nucleotide analogue-induced
damage. Cancer Res. 67:262–268. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Takeuchi A, Masuda S, Saito H, Doi T and
Inui K: Role of kidney-specific organic anion transporters in the
urinary excretion of methotrexate. Kidney Int. 60:1058–1068. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Freeman MV: The fluorometric measurement
of the absorption, distribution and excretion of single doses of
4-amino-10-methyl pteroylglutamic acid (amethopterin) in man. J
Pharmacol Exp Ther. 122:154–162. 1958.PubMed/NCBI
|
|
15.
|
Tocco DJ, deLuna FA, Duncan AE, Hsieh JH
and Lin JH: Interspecies differences in stereoselective protein
binding and clearance of MK-571. Drug Metab Dispos. 18:388–392.
1990.PubMed/NCBI
|
|
16.
|
Bore P, Bruno R, Lena N, Favre R and Cano
JP: Methotrexate and 7-hydroxy-methotrexate pharmacokinetics
following intravenous bolus administration and high-dose infusion
of methotrexate. Eur J Cancer Clin Oncol. 23:1385–1390. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Bremnes RM, Slordal L, Wist E and Aarbakke
J: Formation and elimination of 7-hydroxymethotrexate in the rat in
vivo after methotrexate administration. Cancer Res. 49:2460–2464.
1989.PubMed/NCBI
|
|
18.
|
Giese NA, Raykov Z, DeMartino L, Vecchi A,
Sozzani S, Dinsart C, Cornelis JJ and Rommelaere J: Suppression of
metastatic hemangiosarcoma by a parvovirus MVMp vector transducing
the IP-10 chemokine into immunocompetent mice. Cancer Gene Ther.
9:432–442. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Shimada T, Yamazaki H, Mimura M, Inui Y
and Guengerich FP: Interindividual variations in human liver
cytochrome P-450 enzymes involved in the oxidation of drugs,
carcinogens and toxic chemicals: studies with liver microsomes of
30 Japanese and 30 Caucasians. J Pharmacol Exp Ther. 270:414–423.
1994.
|
|
20.
|
Kool M, de Haas M, Scheffer GL, Scheper
RJ, van Eijk MJ, Juijn JA, Baas F and Borst P: Analysis of
expression of cMOAT (MRP2), MRP3, MRP4, and MRP5, homologues of the
multidrug resistance-associated protein gene (MRP1), in human
cancer cell lines. Cancer Res. 57:3537–3547. 1997.PubMed/NCBI
|
|
21.
|
Langmann T, Mauerer R, Zahn A, Moehle C,
Probst M, Stremmel W and Schmitz G: Real-time reverse
transcription-PCR expression profiling of the complete human
ATP-binding cassette transporter superfamily in various tissues.
Clin Chem. 49:230–238. 2003. View
Article : Google Scholar
|
|
22.
|
Gupta S, Wulf G, Henjakovic M, Koepsell H,
Burckhardt G and Hagos Y: Human organic cation transporter 1 is
expressed in lymphoma cells and increases the susceptibility to
irinotecan and paclitaxel. J Pharmacol Exp Ther. 341:16–23. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Borowski E, Bontemps-Gracz MM and
Piwkowska A: Strategies for overcoming ABC-transporters-mediated
multidrug resistance (MDR) of tumor cells. Acta Biochim Pol.
52:609–627. 2005.PubMed/NCBI
|
|
24.
|
Wakamatsu T, Nakahashi Y, Hachimine D,
Seki T and Okazaki K: The combination of glycyrrhizin and
lamivudine can reverse the cisplatin resistance in hepatocellular
carcinoma cells through inhibition of multidrug
resistance-associated proteins. Int J Oncol. 31:1465–1472.
2007.
|
|
25.
|
Jedlitschky G, Leier I, Buchholz U,
Barnouin K, Kurz G and Keppler D: Transport of glutathione,
glucuronate, and sulfate conjugates by the MRP gene-encoded
conjugate export pump. Cancer Res. 56:988–994. 1996.PubMed/NCBI
|
|
26.
|
Chen ZS, Kawabe T, Ono M, Aoki S, Sumizawa
T, Furukawa T, Uchiumi T, Wada M, Kuwano M and Akiyama SI: Effect
of multidrug resistance-reversing agents on transporting activity
of human canalicular multispecific organic anion transporter. Mol
Pharmacol. 56:1219–1228. 1999.
|
|
27.
|
Tang F, Horie K and Borchardt RT: Are MDCK
cells transfected with the human MRP2 gene a good model of the
human intestinal mucosa? Pharm Res. 19:773–779. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Reid G, Wielinga P, Zelcer N, De Haas M,
Van Deemter L, Wijnholds J, Balzarini J and Borst P:
Characterization of the transport of nucleoside analogue drugs by
the human multidrug resistance proteins MRP4 and MRP5. Mol
Pharmacol. 63:1094–1103. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Hasegawa M, Kusuhara H, Adachi M, Schuetz
JD, Takeuchi K and Sugiyama Y: Multidrug resistance-associated
protein 4 is involved in the urinary excretion of
hydrochlorothiazide and furosemide. J Am Soc Nephrol. 18:37–45.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Ose A, Ito M, Kusuhara H, Yamatsugu K,
Kanai M, Shibasaki M, Hosokawa M, Schuetz JD and Sugiyama Y:
Limited brain distribution of
[3R,4R,5S]-4-acetamido-5-amino-3-(1-ethylpropoxy)-1-cyclohexene-1-carboxylate
phosphate (Ro 64-0802), a pharmacologically active form of
oseltamivir, by active efflux across the blood-brain barrier
mediated by organic anion transporter 3 (Oat3/Slc22a8) and
multidrug resistance-associated protein 4 (Mrp4/Abcc4). Drug Metab
Dispos. 37:315–321. 2009.
|
|
31.
|
Chai J, Luo D, Wu X, Wang H, He Y, Li Q,
Zhang Y, Chen L, Peng ZH, Xiao T, et al: Changes of organic anion
transporter MRP4 and related nuclear receptors in human obstructive
cholestasis. J Gastrointest Surg. 15:996–1004. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Mori H, Takahashi K and Mizutani T:
Interaction between valproic acid and carbapenem antibiotics. Drug
Metab Rev. 39:647–657. 2007. View Article : Google Scholar : PubMed/NCBI
|