Introduction
Advances in diagnostic techniques have led to an
increased incidence of small and early-stage gastric cancers
(1,2). The incidence of early gastric cancer
is >40% (3,4) and patients with early gastric cancer
have an extremely favorable prognosis following curative treatment,
with 5-year survival rates of >90% (4–6). The
incidence of lymph node metastases originating from mucosal and
submucosal lesions in early gastric cancer has been reported to be
3 and 20%, respectively (7); thus,
standard gastrectomy with extensive lymphadenectomy may be
inappropriate for such populations with regard to the quality of
life (QOL) (8).
Endoscopic resection (ER), including endoscopic
mucosal resection (EMR) and endoscopic submucosal dissection (ESD),
may be the optimal treatment for early gastric cancer in terms of
improving the QOL of the patient. However, ER has several problems;
it occasionally fails to completely remove the cancerous lesion and
pathological examination of the resected specimen may reveal a
potentially high risk of lymph node metastases that does not meet
the criteria for curative ER (9).
Pathologists are occasionally unable to accurately evaluate the
margin status following ER due to the burn effect and mechanical
damage. Furthermore, even if exposed tumor cells are observed in
the ER margin, residual tumor cells are not always detected in the
surgically resected specimens (10,11).
Thus, the selection of patients who require radical gastrectomy
following incomplete ER for early gastric cancer is difficult.
In order to establish significant indications for
radical gastrectomy in patients with ER margins positive for
residual tumor cells, we reviewed the clinicopathological
characteristics of patients who underwent incomplete ER for early
gastric cancer. We also examined the predictive value of the
pathological extent of the tumor invasion in the ER margins
positive for residual tumor cells in the surgically resected
specimens.
Materials and methods
Patients
Patients (n=758) with early gastric cancer underwent
gastrectomy (n=207) or ESD (n=551) at the Departments of Surgery or
Internal Medicine at the National Defense Medical College Hospital
between 2005 and 2009. Since 2005, we have regarded the following
features as indications for ESD according to the gastric cancer
treatment guidelines in Japan (9):
i) presence of differentiated-type carcinoma limited to the mucosal
layer and ii) absence of ulceration or ulcer scars in the depressed
type or irrespective of macroscopic type. A single-channel
endoscope (GIF-H260; Olympus, Tokyo, Japan) was used in patients
under conscious sedation. Lesions were marked beyond the margins
using a conventional needle knife (needle papillotome; MTW
Endoscopy, Wesel, Germany). A solution of 0.25% sodium hyaluronate
in normal saline solution containing 0.001% epinephrine and 0.002%
indigo carmine was injected into the submucosal layer and a
circumferential incision was made to include the markings. Lesions
were dissected using an insulation-tripped electrosurgical knife
(EMR Knives; MTW Endoscopy) to curatively exfoliate tumors through
the submucosal layer. ESD specimens were spread out, pinned on a
flat cork and fixed in formalin solution. The size of the
specimens, the size and shape of the tumor and the margins were
recorded on a schematic diagram. Fixed materials were sectioned
serially at 2-mm intervals parallel to a line that included the
closest resection margin of the specimens (12).
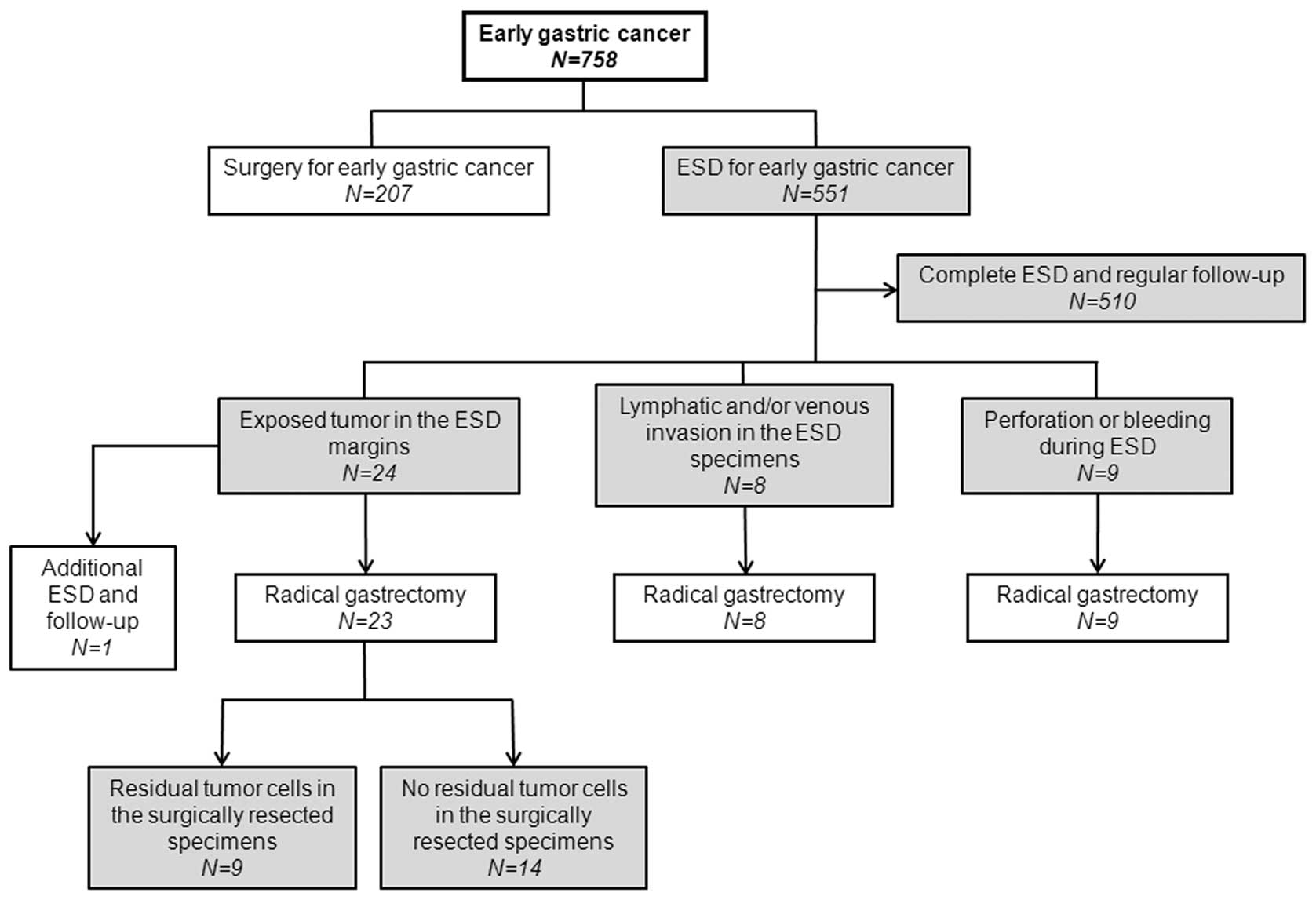
Of the 551 patients who underwent ESD, 510 patients
underwent complete ESD and regular follow-up. The remaining 41
patients underwent incomplete ESD, of which 40 underwent additional
radical gastrectomy (Fig. 1). One
patient whose ESD specimen showed exposed tumor cells in the
vertical margin underwent additional ESD and intensive follow-up
due to severe liver cirrhosis.
The clinicopathological findings of the patients
were evaluated according to the Japanese Classification of Gastric
Carcinoma (JCGC) published by the Japanese Gastric Cancer
Association (12).
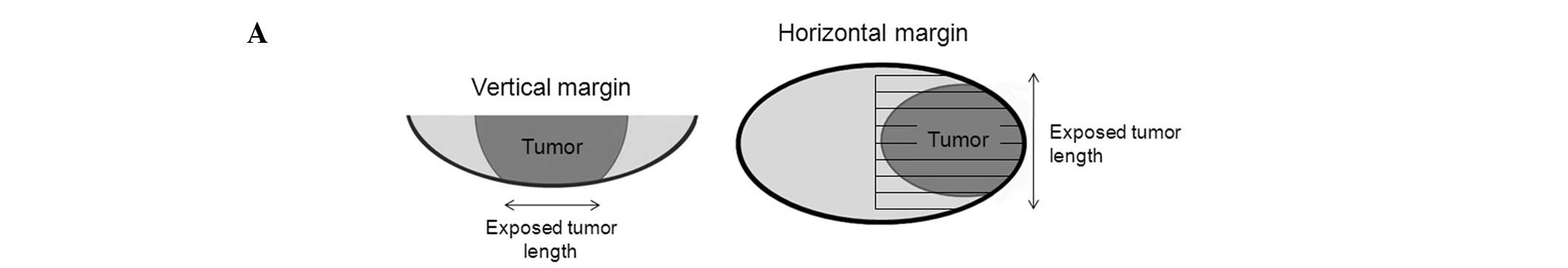
We measured the horizontal and/or vertical length of
the exposed tumor in the ESD margins of 23 patients with ER margins
positive for tumor cells. In the horizontal margin, we calculated
the number of tumor-positive sections of a 2-mm width (horizontal
lengths were calculated by the number of tumor-positive sections ×
2; Fig. 2A). In the vertical
margin, we microscopically measured the distance between the ends
of the exposed tumor with a scale. The highest of these values was
considered to be the vertical length of the exposed tumor. A
representative microscopic image with a scale is shown in Fig. 2B. Pathological examination and
measurement of the length in the ESD margins were evaluated by an
author (S.O.), who was blinded to the pathological findings of the
surgically resected specimens.
Statistical analysis
The data are expressed as mean ± SD. The
Mann-Whitney U test or the Chi-square test was used to compare the
two groups. The ability of the clinicopathological data (including
the length of the exposed tumor in the ESD margins, venous invasion
and lymphatic invasion) to distinguish between the presence and
absence of residual tumor cells in the surgically resected
specimens was assessed using the area under the receiver operating
characteristic (AUROC) curve. These data were analyzed using the
MedCalc version 9 statistical software package (MedCalc software,
Mariakerke, Belgium). P<0.05 was considered to indicate a
statistically significant result.
Results
Demographic data of the patients who underwent
incomplete ESD for early gastric cancer are shown in Table I. The mean age of the patients who
underwent incomplete ESD was 70.3±6.5 years and the mean maximum
tumor size was 25.9±15.5 mm. Reasons for performing an incomplete
ESD were as follows: accidental perforation or bleeding during ESD
(9 patients), lymphatic and/or venous invasion in the ESD specimens
(8 patients) and exposed tumor cells in the ESD margins (23
patients; Fig. 1). Radical
gastrectomy was performed in 23 patients due to a diagnosis of
having exposed tumor cells in the vertical and/or horizontal
margins of the ESD specimens. Thirteen patients had exposed tumor
cells only in the vertical margin, 6 patients only in the
horizontal margin and 4 patients in both margins (Table II). Twenty patients underwent en
bloc resection and the remaining 3 patients underwent piecemeal
resection. Of 17 lesions with exposed tumor cells in the vertical
margins of the ESD specimens, only 3 (17.6%) had residual tumor
cells in the corresponding site of the surgically resected
specimens. By contrast, of 10 lesions with exposed tumor cells in
the horizontal margins of the ESD specimens, 8 (80.0%) had residual
tumor cells in the corresponding site of the surgically resected
specimens. In the vertical margins of the surgically resected
specimens, the length of the exposed tumor in patients with
residual tumor cells was 5.7±4.0 mm, which was significantly
greater than that in patients without residual tumor cells (1.2±1.6
mm; Table III). In contrast to the
vertical margins, no differences were observed in the lengths of
the exposed tumors between patients with residual tumor cells in
the horizontal margins of the surgically resected specimens and
those without. No differences were observed with respect to age,
gender, tumor location, tumor depth, lymphatic or venous invasion
and lymph node metastases between patients with residual tumor
cells in the surgically resected specimens and those without.
 | Table IDemographic data of patients who
underwent incomplete ESD for early gastric cancer. |
Table I
Demographic data of patients who
underwent incomplete ESD for early gastric cancer.
| Feature | n (%) or mean ±
SD |
|---|
| Number | 41 |
| Age (years) | 70.3±6.5 |
| Gender | |
| Male | 33 (80.5) |
| Female | 8 (19.5) |
| Tumor depth | |
| Mucosal
invasion | 13 (31.7) |
| SM1 | 10 (24.4) |
| SM2 | 16 (39.0) |
| MP | 2 (4.9) |
| Tumor location | |
| U | 12 (29.3) |
| M | 9 (22.0) |
| L | 20 (48.8) |
| Gross type | |
| Elevated | 21 (51.2) |
| Depressed | 20 (48.8) |
| Maximum tumor size
(mm) | 25.9±15.5 |
| Histological
classification | |
|
Well-differentiated | 23 (56.1) |
| Moderately
differentiated | 9 (22.0) |
| Poorly
differentiated | 2 (4.9) |
| Papillary | 4 (9.8) |
| Carcinoid | 2 (4.9) |
| Mucinous | 1 (2.4) |
| Lymphatic
invasion | |
| Yes | 23 (56.1) |
| No | 18 (43.9) |
| Venous invasion | |
| Yes | 28 (68.3) |
| No | 13 (31.7) |
| Lymph node
metastasis | |
| Positive | 4 (9.8) |
| Negative | 37 (90.2) |
| Surgical
procedure | |
| Distal
gastrectomy | 22 (53.7) |
| PPG | 8 (19.5) |
| Proximal
gastrectomy | 6 (14.6) |
| Total
gastrectomy | 4 (9.8) |
| No surgery | 1 (2.4) |
| Open surgery | 25 (62.5) |
| Laparoscopic
surgery | 15 (37.5) |
 | Table IIClinicopathological findings in
patients who underwent radical gastrectomy due to being diagnosed
with, or suspected of having, exposed tumor cells in the vertical
and/or horizontal margins of the ESD specimens. |
Table II
Clinicopathological findings in
patients who underwent radical gastrectomy due to being diagnosed
with, or suspected of having, exposed tumor cells in the vertical
and/or horizontal margins of the ESD specimens.
| | | | | | | | Horizontal margin
| Vertical margin
| | |
|---|
| Patient | Age (years) | Gender | Location | Gross type | Histological
type | En bloc
resection | Maximum tumor size
(mm) | Diagnosis | Exposed tumor length
(mm) | Diagnosis | Exposed tumor length
(mm) | Tumor depth | Residual tumor in
surgically resected specimens |
|---|
| 1 | 67 | Male | M | Depressed | Tub1 | Yes | 10 | Positive | 2 | Positive | 8 | MP | HM, VM |
| 2 | 75 | Female | L | Elevated | Pap | No | 27 | Positive | 8 | Negative | 0 | Muc I | HM |
| 3 | 79 | Male | L | Depressed | Tub1 | No | 25 | Positive | 8 | Negative | 0 | SM1 | HM |
| 4 | 68 | Female | L | Depressed | Tub2 | Yes | 30 | Negative | 0 | Positive | 2 | SM1 | - |
| 5 | 65 | Male | L | Elevated | Tub1 | Yes | 10 | Negative | 0 | Suspected | 0 | SM1 | - |
| 6 | 72 | Male | L | Elevated | Pap | Yes | 26 | Positive | 14 | Negative | 0 | SM2 | - |
| 7 | 59 | Female | M | Elevated | Tub1 | Yes | 70 | Positive | 55 | Negative | 0 | Muc I | HM |
| 8 | 55 | Male | M | Depressed | Tub2 | Yes | 32 | Negative | 0 | Suspected | 0 | SM1 | - |
| 9 | 63 | Female | M | Elevated | Carcinoid | Yes | 9 | Negative | 0 | Positive | 1 | SM2 | - |
| 10 | 69 | Male | M | Elevated | Tub1 | No | 20 | Positive | 8 | Negative | 0 | Muc I | HM |
| 11 | 70 | Female | L | Elevated | Muc | Yes | 38 | Positive | 10 | Positive | 8 | SM2 | HM, VM |
| 12 | 72 | Male | L | Depressed | Tub1 | Yes | 19 | Positive | 2 | Negative | 0 | Muc I | HM |
| 13 | 73 | Male | L | Elevated | Tub1 | Yes | 67 | Negative | 0 | Positive | 6 | SM2 | - |
| 14 | 70 | Male | L | Depressed | Tub2 | Yes | 16 | Negative | 0 | Suspected | 0 | SM2 | - |
| 15 | 73 | Male | U | Depressed | Tub1 | Yes | 18 | Positive | 5 | Positive | 1 | SM1 | HM |
| 16 | 66 | Male | L | Elevated | Tub1 | Yes | 19 | Negative | 0 | Suspected | 0 | Muc I | - |
| 17 | 73 | Male | M | Depressed | Tub1 | Yes | 30 | Negative | 0 | Positive | 3 | SM1 | - |
| 18 | 75 | Male | L | Elevated | Por | Yes | 8 | Negative | 0 | Positive | 3 | SM2 | - |
| 19 | 80 | Male | U | Depressed | Tub1 | Yes | 22 | Negative | 0 | Positive | 3 | SM1 | - |
| 20 | 65 | Male | U | Elevated | Tub1 | Yes | 14 | Positive | 4 | Positive | 1 | Muc I | VM |
| 21 | 65 | Male | L | Depressed | Tub2 | Yes | 41 | Negative | 0 | Positive | 1 | SM2 | - |
| 22 | 61 | Male | L | Depressed | Tub2 | Yes | 16 | Negative | 0 | Positive | 2 | SM1 | - |
| 23 | 72 | Male | U | Elevated | Tub1 | Yes | 19 | Negative | 0 | Positive | 1 | Muc I | - |
 | Table IIILength of the residual tumor in the
ESD margins. |
Table III
Length of the residual tumor in the
ESD margins.
| A, Exposed tumor
length in the horizontal margins of the ESD specimens (n=10). |
|
| Mean ± SD (mm) | P-value |
|
| Surgical specimen
in the lateral margin | | |
| Positive for
residual tumor cells (n=8) | 12.3±17.5 | 0.8104 |
| Negative for
residual tumor cells (n=2) | 9.0±7.1 | |
|
| B, Exposed tumor
length in the vertical margins of the ESD specimens (n=17). |
|
| Mean ± SD (mm) | P-value |
|
| Surgical specimen
in the vertical margin | | |
| Positive for
residual tumor cells (n=3) | 5.7±4.0 | 0.0103 |
| Negative for
residual tumor cells (n=14) | 1.2±1.6 | |
In the univariate analysis, no parameter was
associated with the incidence of residual tumor cells in the
horizontal margins of the surgically resected specimens. On the
other hand, the length of the exposed tumor in the vertical margins
of the ESD specimens was significantly associated with the
incidence of residual tumor cells in the vertical margins of the
surgically resected specimens (Table
IV). The length of the exposed tumor in the vertical margins of
the ESD specimens was found to be the most reliable parameter for
distinguishing between the surgically resected specimens that were
positive and negative for residual tumor cells in terms of AUROC,
although we could not find such differences in the horizontal
margins of the ESD specimens. When the cut-off value for the length
of the exposed tumor in the vertical margins of ESD specimens was
set to >3 mm, the sensitivity, specificity and positive and
negative predictive values were 0.67, 0.95, 0.67 and 0.95,
respectively (Table V).
 | Table IVUnivariate analysis and the AUROC
curve of the factors associated with the incidence of residual
tumor cells in the surgically resected specimens. |
Table IV
Univariate analysis and the AUROC
curve of the factors associated with the incidence of residual
tumor cells in the surgically resected specimens.
| A, Horizontal
margin | | | | |
|
| Hazard Ratio | 95% CI | P-value | AUROC |
|
| Age, 1-year
increments | 0.98 | 0.914–1.046 | 0.5131 | 0.656 |
| Gender (male) | 1.31 | 0.339–5.093 | 0.6930 | 0.524 |
| Tumor location
(U) | 1.54 | 0.325–7.314 | 0.5860 | 0.687 |
| Tumor size (≥20.0
mm) | 0.92 | 0.259–3.259 | 0.8963 | 0.562 |
| Gross type
(elevated) | 1.39 | 0.390–4.936 | 0.6130 | 0.548 |
| Tumor depth | | | | |
| SM2 or
deeper | 1.22 | 0.315–4.727 | 0.7732 | 0.762 |
| Nodal involvement
(compared with N0) | | | | |
| N1 | 0.84 | 0.198–3.598 | 0.8182 | 0.562 |
| Lymphatic invasion
(yes) | 12.00 | 0.489–294.59 | 0.1281 | 0.687 |
| Venous invasion
(yes) | 1.67 | 0.537–5.168 | 0.3763 | 0.562 |
| Exposed tumor
length (mm) | 1.02 | 0.892–1.163 | 0.7875 | 0.562 |
|
| B, Vertical
margin | | | | |
|
| Hazard Ratio | 95% CI | P-value | AUROC |
|
| Age, 1 year
increments | 1.03 | 0.845–1.249 | 0.7872 | 0.552 |
| Gender (male) | 0.60 | 0.039–9.156 | 0.7133 | 0.542 |
| Tumor location
(U) | 1.67 | 0.109–25.434 | 0.7133 | 0.542 |
| Tumor size (≥20.0
mm) | 1.40 | 0.145–13.569 | 0.7715 | 0.542 |
| Gross type
(elevated) | 0.17 | 0.013–2.160 | 0.1704 | 0.708 |
| Tumor depth | | | | |
| SM2 or
deeper | 6.00 | 0.463–77.753 | 0.1704 | 0.708 |
| Nodal involvement
(compared with N0) | | | | |
| N1 | 0.46 | 0.096–2.212 | 0.3324 | 0.750 |
| Lymphatic invasion
(yes) | 0.71 | 0.254–1.994 | 0.5176 | 0.708 |
| Venous invasion
(yes) | 5.00 | 0.419–59.660 | 0.2032 | 0.667 |
| Exposed tumor
length (mm) | 2.34 | 1.005–5.429 | 0.0488 | 0.865 |
 | Table VThe sensitivity, specificity and
positive and negative predictive values of being positive for
residual tumor cells in the surgically resected specimens according
to the length of the exposed tumors in the vertical ESD
margins. |
Table V
The sensitivity, specificity and
positive and negative predictive values of being positive for
residual tumor cells in the surgically resected specimens according
to the length of the exposed tumors in the vertical ESD
margins.
| Length of exposed
tumor (mm) | Sensitivity | Specificity | PPV | NPV |
|---|
| >0 | 1.00 | 0.53 | 0.25 | 1.00 |
| >1 | 0.67 | 0.68 | 0.25 | 0.93 |
| >3 | 0.67 | 0.95 | 0.67 | 0.95 |
| >6 | 0.67 | 1.00 | 1.00 | 0.95 |
Recurrence was not observed in any patient following
curative surgery during a mean follow-up period of 36.9 (range
11–70) months.
Discussion
In Japan, the indications for ER in early gastric
cancer patients include well- or moderately differentiated
adenocarcinoma restricted to the mucosal layer without ulceration
(9,13,14);
more recently, these indications have been extended (13,14).
The indication for additional gastrectomy following incomplete ER
is to remove the residual cancer cells at the site of the ER and/or
the potentially metastatic regional lymph nodes. The risks for
residual tumors or lymph node metastases following an incomplete ER
have been extensively discussed in previous studies (11,15,16).
Song et al reported that the residual tumor rate in the
surgically resected specimens of patients with tumor-positive ER
margins was >70% in a Korean multicenter study (17). Residual tumors in the surgically
resected specimens have been found in 5.8–63.0% of patients with
tumor-positive horizontal margins and in 35–50% of patients with
tumor-positive vertical margins in the ESD specimens (11,15,18).
These findings are consistent with our results, suggesting that the
remaining patients who were pathologically diagnosed with
tumor-positive ER margins theoretically did not require radical
gastrectomy, except for a potentially high risk of lymph node
metastases.
To the best of our knowledge, no study has used the
extent of tumor invasion in the ER margins to predict the presence
of residual tumor cells in the surgically resected specimens. In
this study, we demonstrated that the length of the exposed tumor in
the vertical ESD margins was an exclusive parameter that could
predict the presence of residual tumor cells in the surgically
resected specimens. Only 1 of 14 patients (7.1%) with ≤1 mm of
exposed tumor in the vertical ESD margins had residual tumor cells
in the surgically resected specimen (Table II). We evaluated the sensitivity,
specificity and positive and negative predictive values using
serial cut-off values around the inflection points on the receiver
operating characteristic (ROC) curve for the length of the exposed
tumors in the vertical ESD margins. When the cut-off value was set
to >3 mm, the specificity was 0.95. Therefore, patients with
exposed tumors >3 mm in length in the vertical ESD margins
should be considered as candidates for additional radical
gastrectomy. In patients with exposed tumors of ≤3 mm in length in
the vertical ESD margins, the optimal treatment should be
determined on the basis of risks for surgery and general
anesthesia.
Although a tumor-positive horizontal margin is not
required for the evaluation of tumor extent, the residual tumor
rate was higher in patients with tumor-positive horizontal margins
than in those with tumor-positive vertical margins. Indeed, 80% of
lesions with exposed tumor cells in the horizontal margins of the
ESD specimens had residual tumor cells in the surgically resected
specimens. Although repeat ER may not be feasible due to the
increased complication rate resulting from scar formation or
thinning of the gastric wall following the first ER procedure
(10), it appears to be relatively
safe in the case of tumor-positive horizontal ER margins compared
with the tumor-positive vertical ER margins (19). In this study, the horizontal ER
margins were not identified as predictive factors for residual
tumors, but the vertical ER margins were. Therefore, additional ER
or gastrectomy should be considered when exposed tumors are
observed in the horizontal ER margins regardless of the length of
the these tumors (11).
As the indications for ER have been extended, the
number of patients who undergo incomplete ER should increase. In
this study, we did not have a large enough sample size of patients
with ER margins positive for tumor cells, which is the limitation
of this study, and it is necessary to conduct a multicenter
prospective randomized study in order to verify our results.
In conclusion, for early gastric cancer, the
measurement of the length of the exposed tumor in the ER margins,
especially in the vertical margins, is a simple procedure that is
able to determine whether an additional surgical intervention is
necessary, except for a potentially high risk of lymph node
metastases. Although the indication of additional surgery is
generally decided not only by the tumor-positive ER margin but also
by other pathological factors, this method may be used to prevent
unnecessary surgery in patients with early gastric cancer,
especially in high-risk patients for whom general anesthesia is not
suitable.
References
|
1.
|
Kim JJ, Lee JH, Jung HY, Lee GH, Cho JY,
Ryu CB, Chun HJ, Park JJ, Lee WS, Kim HS, et al: EMR for early
gastric cancer in Korea: a multicenter retrospective study.
Gastrointest Endosc. 66:693–700. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Roukos DH: Current advances and changes in
treatment strategy may improve survival and quality of life in
patients with potentially curable gastric cancer. Ann Surg Oncol.
6:46–56. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Lee HJ, Yang HK and Ahn YO: Gastric cancer
in Korea. Gastric Cancer. 5:177–182. 2002. View Article : Google Scholar
|
|
4.
|
Tsujimoto H, Sugasawa H, Ono S, Ichikura
T, Yamamoto J and Hase K: Has the accuracy of preoperative
diagnosis improved in cases of early-stage gastric cancer? World J
Surg. 34:1840–1846. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Itoh H, Oohata Y, Nakamura K, Nagata T,
Mibu R and Nakayama F: Complete ten-year postgastrectomy follow-up
of early gastric cancer. Am J Surg. 158:14–16. 1989.PubMed/NCBI
|
|
6.
|
Lee HJ, Kim YH, Kim WH, Lee KU, Choe KJ,
Kim JP and Yang HK: Clinicopathological analysis for recurrence of
early gastric cancer. Jpn J Clin Oncol. 33:209–214. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Sano T, Kobori O and Muto T: Lymph node
metastasis from early gastric cancer: endoscopic resection of
tumour. Br J Surg. 79:241–244. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Ichikura T, Morita D, Uchida T, Okura E,
Majima T, Ogawa T and Mochizuki H: Sentinel node concept in gastric
carcinoma. World J Surg. 26:318–322. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Nakajima T: Gastric cancer treatment
guidelines in Japan. Gastric Cancer. 5:1–5. 2002. View Article : Google Scholar
|
|
10.
|
Oka S, Tanaka S, Kaneko I, Mouri R, Hirata
M, Kanao H, Kawamura T, Yoshida S, Yoshihara M and Chayama K:
Endoscopic submucosal dissection for residual/local recurrence of
early gastric cancer after endoscopic mucosal resection. Endoscopy.
38:996–1000. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Jung H, Bae JM, Choi MG, Noh JH, Sohn TS
and Kim S: Surgical outcome after incomplete endoscopic submucosal
dissection of gastric cancer. Br J Surg. 98:73–78. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Japanese Gastric Cancer Association:
Japanese Classification of Gastric Carcinoma, 2nd English edition.
Gastric Cancer. 1:10–24. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Yamao T, Shirao K, Ono H, Kondo H, Saito
D, Yamaguchi H, Sasako M, Sano T, Ochiai A and Yoshida S: Risk
factors for lymph node metastasis from intramucosal gastric
carcinoma. Cancer. 77:602–606. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Gotoda T, Yanagisawa A, Sasako M, Ono H,
Nakanishi Y, Shimoda T and Kato Y: Incidence of lymph node
metastasis from early gastric cancer: estimation with a large
number of cases at two large centers. Gastric Cancer. 3:219–225.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Nagano H, Ohyama S, Fukunaga T, Seto Y,
Fujisaki J, Yamaguchi T, Yamamoto N, Kato Y and Yamaguchi A:
Indications for gastrectomy after incomplete EMR for early gastric
cancer. Gastric Cancer. 8:149–154. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Korenaga D, Orita H, Maekawa S, Maruoka A,
Sakai K, Ikeda T and Sugimachi K: Pathological appearance of the
stomach after endoscopic mucosal resection for early gastric
cancer. Br J Surg. 84:1563–1566. 1997. View Article : Google Scholar
|
|
17.
|
Song KY, Hyung WJ, Kim HH, Han SU, Cho GS,
Ryu SW, Lee HJ and Kim MC: Is gastrectomy mandatory for all
residual or recurrent gastric cancer following endoscopic
resection? A large-scale Korean multi-center study. J Surg Oncol.
98:6–10. 2008. View Article : Google Scholar
|
|
18.
|
Chung YS, Park DJ, Lee HJ, Kim SG, Jung
HC, Song IS, Kim WH, Lee KU, Choe KJ and Yang HK: The role of
surgery after incomplete endoscopic mucosal resection for early
gastric cancer. Surg Today. 37:114–117. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Etoh T, Ishikawa K, Shiromizu A, Yasuda K,
Inomata M, Shiraishi N and Kitano S: Clinicopathologic features and
treatment of residual early cancers after endoscopic mucosal
resection of the stomach. J Clin Gastroenterol. 40:801–805. 2006.
View Article : Google Scholar : PubMed/NCBI
|