Introduction
ALZET® osmotic pumps are implantable
pumps used for research in mice and other animals for the
continuous infusion of drugs or proteins at controlled rates from 1
day to 4 weeks, without the need for external handling. These pumps
are used for systemic administration by implantation subcutaneously
or intraperitoneally. Pumps have previously been used in a number
of studies on the effects of controlled delivery of a wide range of
experimental agents, including addictive drugs, steroids,
chemotherapeutic drugs, hormones, and antibodies or other proteins.
It is extremely important that compounds of any molecular
conformation are delivered predictably at controlled rates,
independent of their properties (1–4). In
the present study, the pumps were used to deliver plasminogen
activator inhibitor-1 (PAI-1) to successfully reduce tumor size in
SCID mouse in the past (5).
In this study, ALZET osmotic pumps were used on mice
with diabetic nephropathy. Various factors have been suggested in
the pathogenesis of diabetic nephropathy, including an increased
PAI-1 level in plasma. PAI-1 mediates diabetic nephropathy, which
is characterized by excessive accumulation of extracellular matrix
(ECM) in the kidney. Excessive PAI-1 inactivates tissue plasminogen
activator, which is one of the proteolytic enzymes in a cascade
responsible for ECM remodeling in the kidney. A decrease of PAI-1
in the kidney has been shown to arrest the progression of
nephropathy in experimental animals. This decrease was achieved
using inactive PAI-1R which increased clearance of wild-type PAI-1
in order to protect net proteolytic activity and ECM clearance
(6,7). However, this protein has a brief
half-life in vivo, therefore, high and frequent doses are
required for it to be effective. VLHL NS PAI-1 (8) with a long half-life of over 700 h
(Gln197Cys, Gly355Cys) inactivated by single point mutation
(Arg369Ala) was therefore used (9). We hypothesized that this protein is
likely to prevent nephropathy when used in the early stages of
diabetes and arrest its progression in advanced stages of this
disease.
VLHL NS PAI-1 was loaded into osmotic pumps to
deliver protein over a 2-week period in Dock7m +/+
Leprdb diabetic mice to observe whether it had any
effects on diabetes. All pumps containing VLHL NS PAI-1 were found
to be clogged and the majority of the buffer with the active
ingredient remained within the pumps while the control pumps
contained little, if any, buffer. Analyses of proteins in the pumps
suggests that the pumps were clogged by cellular material early in
the experiment.
Materials and methods
VLHL NS PAI-1
VLHL NS PAI-1 was expressed and purified as reported
previously (9). VLHL NS PAI-1 was
in an active conformation with traces of VLHL NS PAI-1 in latent
conformation. The purity of protein was determined as high as +95%
by densitometry (9).
Animals
Animals were purchased from The Jackson Laboratory
(TJL; Bar Harbor, ME, USA) and maintained according to TJL
recommendations. Mice homozygous for the diabetes spontaneous
mutation (Leprdb), strain name BKS. Cg-Dock7m
+/+ Leprdb/J (db/db), become obese at 3–4 weeks of age.
Elevation of plasma insulin begins at 10–14 days and elevation of
blood sugar at 4–8 weeks. Homozygous mutant mice are polyphagic,
polydipsic and polyuric. The severity of disease on this genetic
background leads to an uncontrolled rise in blood sugar, severe
depletion of the insulin-producing β-cells of the pancreatic islets
and death by 10 months of age. Exogenous insulin fails to control
blood glucose levels and gluconeogenic enzyme activity increases.
Peripheral neuropathy and myocardial disease are observed and wound
healing is delayed. An increased amount of PAI-1 in kidney and in
animal serum was also detected (6,7).
The db/db mouse is the model that develops
abnormalities in renal morphology and function that parallel those
in human nephropathy of type 2 diabetes (6). Following uninephrectomy at the 8th
week of age, a greatly accelerated development and progression of
diabetic nephropathy was reported. Increased levels of glucose were
observed between weeks 8 and 20 with an increase of PAI-1 in kidney
tissue. Uninephrectomized diabetic db/db mice developed nephropathy
by 20 weeks of age, manifested by expansion of the mesangium and
significant albuminuria (6,7).
At 20 weeks, animals had an intra-abdominal osmotic
pump implanted to administer the buffer (control group) or VLHL NS
PAI-1 (treatment group). The pumps were purchased from Durect
Corporation ALZET Osmotic Pumps (Cupertino, CA, USA). The treatment
group mice (n=6) were implanted with ALZET pump #2002 filled with
200 μl of VLHL NS PAI-1 (350 μg of protein). These
pumps are designed to deliver 0.5 μl/h over 16 days at a
total of ∼200 μl. Similarly, the control group mice (n=6)
were implanted with ALZET pump #1002 filled with 100 μl of
PBS. These pumps are designed to deliver 0.25 μl/h over 16
days for a total of ∼100 μl. At 22 weeks of age (14 weeks
post-uninephrectomy), the two groups of animals were placed in
metabolic cages for 24 h to collect 24-h urine samples. At the end
of 24 h, blood samples were collected via cardiac puncture under
anaesthesia and sacrificed via exsanguination.
Mass spectrometry-based proteomic
analysis
The mass spectrometry-based proteomic analysis was
performed at the Proteomics Resource Facility, Department of
Pathology (Ann Arbor, MI, USA) using multidimensional proteomic
identification technology. The data were searched against a mouse
database appended with human PAI-1 (UniProt accession,
#P05121).
Results and discussion
At the end of the experiments, the mice showed signs
of severe obesity and severe diabetes (data not shown). After
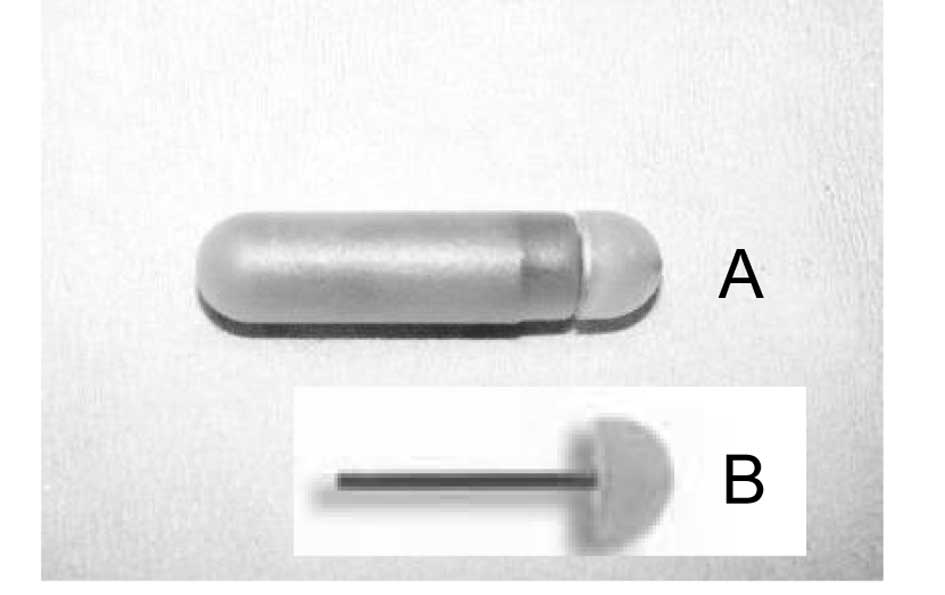
sacrifice it was observed that the tips of the flow moderator of
osmotic pumps in the treated animals were clogged by yellow cell
material. The control group was free of obstructing material. On
close examination it was noted that the tip plug was 1- to 3-mm
long and difficult to remove from the flow modulator (Fig. 1). In addition, in each pump from
the treatment group we collected 50–150 μl of clear liquid.
Almost no liquid remained in the pumps of the control group. The
collected liquid from two pumps of the treatment group was analysed
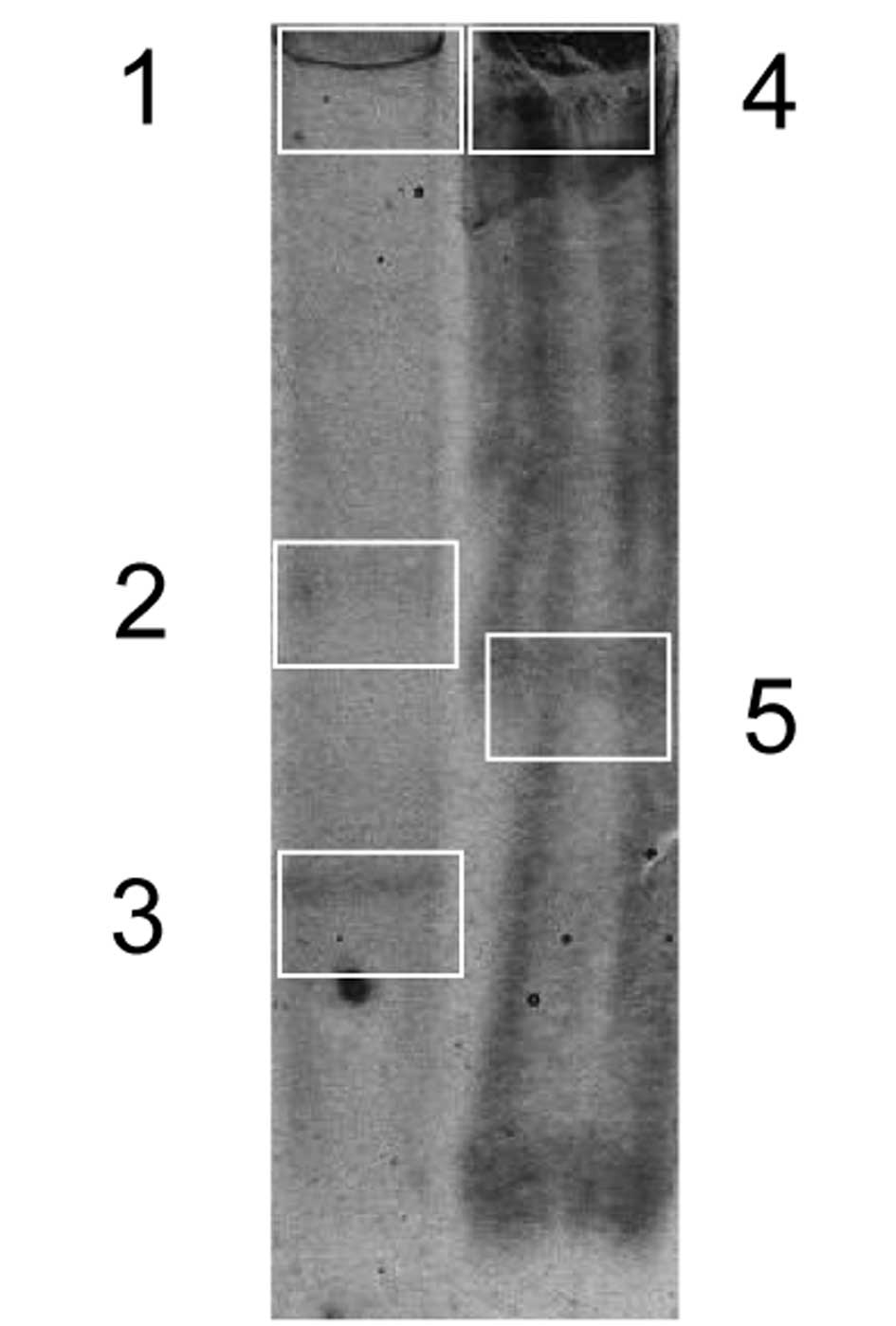
by SDS-PAGE electrophoresis. As shown in Fig. 2, numerous protein bands were
detected. Randomly chosen samples were cut from the SDS-PAGE gel
and sent for protein identification. Numerous proteins were
detected in various locations.
The dominant protein was VLHL NS PAI-1 identified as
wild-type human PAI-1 (Table I).
This protein was detected in all bands suggesting multiple
complexes with other proteins. It is also proof that VLHL NS PAI-1
remains in the pump, i.e., it was delivered as intended and the
pumps were blocked early. The other elevated proteins were
identified as tubulin, serpina 1a, serpina 1d and serum albumin.
These, and less abundant proteins, were of circulatory and cellular
origin (Table I).
ALZET® pumps operate at an osmotic pressure difference
between a compartment within the pump and the tissue environment in
which the pump is implanted may reach at least 0.5 atm (10). Thus, the stopper was relatively
rigid and could be made from adipose tissue abundant in the
intra-abdominal cavity of diabetic mice. Our initial hypothesis was
that VLHL NS PAI-1 attracted the cells into the tip of the flow
modulator of the osmotic pump. Plasminogen activator inhibitor has
been reported to be a motility factor (11,12)
and adhesion factor, however, inactive PAI-1 [P14 mutant
(Thr333Arg)] failed to enhance adhesion (13). Thus, the phenomenon remains
unexplained, and a literature search failed to yield any studies on
similar incidents. Additionally, the manufacturer was not aware of
such incidents.
 | Table IProteins detected in the SDS-PAGE
gel. |
Table I
Proteins detected in the SDS-PAGE
gel.
| UniProt # | Protein name | Probability | Line on gel | Relative
concentrationa |
|---|
| A0AUV1 | Histone H2A | 0.999 | 1,2 | |
| A1E281 | β-actin | 0.999 | 2 | |
| A2AL35 | Gelsolin, isoform
CRA_b | 0.999 | 2 | |
| A2CG44 | MAD homolog 3
(Drosophila) | 0.999 | 1,2 | |
| A5JUZ1 | Ubiqutin subunit
1 | 0.994 | 1,2 | |
| A8DUK0 | Hbbt1 | 0.999 | 1,2 | |
| A8DUV3 | α-globin | 0.999 | 2 | |
| B2RSN3 | Tubulin, β 2B | 0.999 | 1,4,5 | High |
| B7U582 | Heat shock protein
70-2 | 0.999 | 1,2,3 | |
| B7ZNJ1 | Fn1 protein | 0.999 | 1 | |
| B8JJM3 | Complement factor
B | 0.999 | 2 | |
| D3YTY9 | Putative
uncharacterized protein Kng1 | 0.999 | 3 | |
| D3YV43 | Putative
uncharacterized protein Rps3 | 0.999 | 4 | |
| D3YVC1 | Putative
uncharacterized protein Rps2 | 0.995 | 4 | |
| D3YVF4 | Putative
uncharacterized protein Rps14 | 0.999 | 4 | |
| D3YW44 | Putative
uncharacterized protein Gm5121 | 0.999 | 4 | |
| D3YW52 | Putative
uncharacterized protein Pzp | 0.999 | 1,2 | High |
| D3YYR8 | Putative
uncharacterized protein Trf | 0.999 | 2,3 | |
| D3Z0D8 | Putative
uncharacterized protein Rrm2 | 0.999 | 4 | |
| D3Z3P6 | Putative
uncharacterized protein ENSMUSP00000032206 | 0.994 | 1 | |
| D3Z451 | Putative
uncharacterized protein Gm4931 | 0.999 | 3 | |
| D3Z6U8 | Putative
uncharacterized protein Fmr1 | 0.999 | 4 | |
| P05121 | HUMAN plasminogen
activator inhibitor 1 | 0.999 | 1,2,3,4,5 | Very high |
| Q14AS7 | Serine (or cysteine)
peptidase inhibitor, clade A, member 3C | 0.999 | 3 | |
| Q2F3J4 | Truncated
ceruloplasmin | 0.999 | 2 | |
| Q3KQQ4 | Serpina1a
protein | 0.999 | 3 | High |
| Q3TGR2 | Putative
uncharacterized protein | 0.999 | 1 | |
| Q3TII3 | Elongation factor
1-α | 0.999 | 1,4 | |
| Q3TX45 | Gene name, Apoe;
putative uncharacterized protein | 0.999 | 1 | |
| Q3U9U3 | Gene name, Tubb6;
putative uncharacterized protein | 0.999 | 1,4,5 | High 4,5 |
| Q3UBS3 | Gene name, Hp;
putative uncharacterized protein | 0.999 | 2 | |
| Q3UID0 | Gene name, Smarcc2;
putative uncharacterized protein | 0.999 | 1 | |
| Q3UKP2 | Gene name, Hpx;
putative uncharacterized protein | 0.999 | 2,3 | High 3 |
| Q3UKX6 | Gene name, Apoa2;
putative uncharacterized protein | 0.999 | 1,2 | |
| Q3V2E0 | Gene name, Try5;
putative uncharacterized protein | 0.999 | 3 | |
| Q3V2G1 | Gene name, Apoa1;
putative uncharacterized protein | 0.999 | 1,2 | High 2 |
| Q543J5 | Serine (or cysteine)
peptidase inhibitor, antithrombin | 0.999 | 3 | Very high |
| Q546G4 | Serum albumin | 0.999 | 1,2,3,4,5 | |
| Q58E61 | Igh protein | 0.999 | 1 | |
| Q5FW91 | Tubulin, α 3B | 0.999 | 1,4,5 | High |
| Q5M9K1 | Transthyretin | 0.999 | 2,3 | |
| Q65ZL8 | VH7183-DSP2-JH3-CH1
protein | 0.999 | 1 | |
| Q80XP1 | Complement component
3 | 0.999 | 2 | |
| Q810I7 | Apoa4 protein | 0.999 | 1,2 | |
| Q8C4B1 | Gene name, Larp1b;
putative uncharacterized protein | 0.999 | 1 | |
| Q8K051 | Trip12 protein | 0.999 | 1 | |
| Q8VC41 | Serpina1d
protein | 0.999 | 2,3 | High 3 |
| Q9CZQ0 | Gene name, Nudt21;
putative uncharacterized protein | 0.999 | 2,3 | |
| Q9JHV2 | Lectin-related NK
cell receptor LY49T | 0.992 | 2 | |
| Q9Z1R9 | Trypsinogen 16 | 0.994 | 1,2 | |
Acknowledgements
This study was supported by grants
from Stranahan Endowment Fund for Oncological Research and
PharmaIP, LCC.
References
|
1
|
Babin MC, Ricketts K, Skvorak JP, Gazaway
M, Mitcheltree LW and Casillas RP: Systemic administration of
candidate antivesicants to protect against topically applied sulfur
mustard in the mouse ear vesicant model (MEVM). J Appl Toxicol.
20(Suppl 1): S141–S144. 2000. View Article : Google Scholar
|
|
2
|
Buffelli M, Busetto G and Cangiano A: The
use of in vivo direct drug application to assess neural regulation
of muscle properties. J Neurosci Methods. 106:113–120. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Knedla A, Riepl B, Lefèvre S, et al: The
therapeutic use of osmotic minipumps in the severe combined
immunodeficiency (SCID) mouse model for rheumatoid arthritis. Ann
Rheum Dis. 68:124–129. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tejeda M, Gaal D, Hullán L, et al:
Continuous administration of the somatostatin structural derivative
/TT-232/by subcutaneously implanted osmotic pump improves the
efficacy and potency of antitumor therapy in different mouse and
human tumor models. Anticancer Res. 28:2769–2774. 2008.
|
|
5
|
Swiercz R, Keck RW, Skrzypczak-Jankun E,
Selman SH and Jankun J: Recombinant PAI-1 inhibits angiogenesis and
reduces size of LNCaP prostate cancer xenografts in SCID mice.
Oncol Rep. 8:463–470. 2001.PubMed/NCBI
|
|
6
|
Huang Y, Border WA, Lawrence DA and Noble
NA: Mechanisms underlying the antifibrotic properties of
noninhibitory PAI-1 (PAI-1R) in experimental nephritis. Am J
Physiol Renal Physiol. 297:F1045–F1054. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Huang Y, Border WA, Yu L, Zhang J,
Lawrence DA and Noble NA: A PAI-1 mutant, PAI-1R, slows progression
of diabetic nephropathy. J Am Soc Nephrol. 19:329–338. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chorostowska-Wynimko J, Swiercz R,
Skrzypczak-Jankun E, Wojtowicz A, Selman SH and Jankun J: A novel
form of the plasminogen activator inhibitor created by cysteine
mutations extends its half-life: relevance to cancer and
angiogenesis. Mol Cancer Ther. 2:19–28. 2003. View Article : Google Scholar
|
|
9
|
Jankun J, Aleem AM, Specht Z, et al: PAI-1
induces cell detachment, downregulates nucleophosmin (B23) and
fortilin (TCTP) in LnCAP prostate cancer cells. Int J Mol Med.
20:11–20. 2007.PubMed/NCBI
|
|
10
|
Hjortsø E, Jordening H, Jensen H, Munck O
and Qvist J: The function of a continuous medication system in
subatmospheric pressure environment. Scand J Clin Lab Invest.
46:293–295. 1986.PubMed/NCBI
|
|
11
|
Kutz SM, Hordines J, McKeown-Longo PJ and
Higgins PJ: TGF-β1-induced PAI-1 gene expression requires MEK
activity and cell-to-substrate adhesion. J Cell Sci. 114:3905–3914.
2001.
|
|
12
|
Samarakoon R, Higgins SP, Higgins CE and
Higgins PJ: TGF-β1-induced plasminogen activator inhibitor-1
expression in vascular smooth muscle cells requires
pp60(c-src)/EGFR(Y845) and Rho/ROCK signaling. J Mol Cell Cardiol.
44:527–538. 2008.
|
|
13
|
Palmieri D, Lee JW, Juliano RL and Church
FC: Plasminogen activator inhibitor-1 and -3 increase cell adhesion
and motility of MDA-MB-435 breast cancer cells. J Biol Chem.
277:40950–40957. 2002. View Article : Google Scholar : PubMed/NCBI
|