Introduction
Prostate cancer (PCa) is the most common type of
cancer worldwide, and still ranks as the leading cause of death
among urological malignancies. One in six men will be diagnosed
with PCa during their lifetime and approximately 217,000 new cases
were diagnosed in the US in 2010 (1). PCa progression involves tumor cell
proliferation and infiltration into surrounding tissue and
induction of various adaptive pathophysiological and
pathomorphological processes in tissues that become involved in the
developing tumor stroma. However, the mechanism involved in the
development of PCa is still unclear.
Within the bone marrow stroma there exists a subset
of nonhematopoietic cells referred to as mesenchymal stem cells
(MSCs) (2–4). These cells can be expanded ex
vivo and induced in vitro or in vivo to
terminally differentiate into osteogenic, adipogenic, chondrogenic,
and myogenic lineages under appropriate conditions. In addition,
MSCs migrate to sites of injury and inflammation and tumors
(5,6). Phenotypically, MSCs are identified by
the absence of the CD34 and CD45 hematopoietic cell markers and are
positive for Thy-1 (CD90), endoglin (CD105), vascular cell adhesion
molecule-1 (VCAM-1/CD106), SH2 and SH3. MSCs express major
histocompatibility complex (MHC) class I but do not express MHC
class II, B7-1, B7-2, CD40, or CD40L molecules (7).
MSCs can be utilized as ‘tumor stromal cells’,
targeting invasive and metastatic malignant tumor cells (8). Djouad et al (9) also found that MSCs were associated
with side effects related to systemic immunosuppression favoring
tumor growth in vivo. It is therefore important to
investigate the factors related to the in vivo promotion of
tumor growth by MSCs and explore the safety of clinical
applications of MSCs. As the microenvironment of PCa is similar to
that of injured/stressed tissue (10,11),
it was hypothesized that PCa may provide a conducive environment
for the grafting of exogenously administered MSCs (12).
Therefore, in the present study, we aimed to
investigate the characteristics of MSCs obtained from bone marrow
or prostate tumors. Additionally, we investigated whether the
proliferation of grafted MSCs in the developing tumors is capable
of generating a significant fraction of tumor stroma.
Materials and methods
Animal subjects
Male mice (nu/nu) were purchased from the Animal
Production Area of the Fudan University Cancer Research Center. All
animal manipulations were carried out in accordance with Fudan
University guidelines under approved protocols. Male four-week-old
BALB/c mice (n=20) were maintained and bred under specific
pathogen-free conditions, and were divided into the various
experimental groups.
Cell lines and animal model
RM-1 cells were purchased from the Institute of Cell
Biology, Shanghai, China and cultured in DMEM supplemented with 10%
fetal bovine serum, 100 U/ ml penicillin, 100 g/ml streptomycin at
37°C in a humidified 5% CO2 incubator. BALB/c mice
(n=12) inoculated subcutaneously with RM-1 cells were used as a
model of prostate cancer whereas the control group comprised 8
BALB/c mice injected with physiological saline. The time and the
efficiency of cancer formation were measured.
Mesenchymal stem cell isolation and
culture
The methods described by Peister et al were
used (13). Briefly, MSCs were
extracted from the femur, tibia and humerus of normal mice (BMMSCs)
under axenic conditions by washing with PBS and filtration through
a 200-mesh sieve net. Prostate tumors were obtained within 15 days
following injection. Tumor tissue isolated from the mice with
prostate cancer was cut into 3 mm3 pieces. Prostate
tumor MSCs (PCa-MSCs) were obtained after filtration through a
200-mesh sieve net and centrifugation. CD105 cells separated using
magnetic beads were cultured in DMEM-LG medium containing 10% fetal
bovine serum.
In vitro efficacy experiments of
MSCs
MSCs are progenitors of skeletal tissue components
such as bone, cartilage and adipocytes. To ascertain the in
vitro differentiation ability of MSCs isolated from bone marrow
stroma and prostate tumor we induced differentiation using a
previously described method (14–16).
Growth ability of the BMMSCs and
PCa-MSCs
The growth ability of the two types of MSCs (BMMSCs
vs PCa-MSCs) was compared using growth curves. The RM-1 cell
concentration was adjusted to 1×107/ml with RPMI-1640.
The RM-1 cells were grown in 96-well culture plates (Nunc Inc.)
with 1x106/well density, to which different
concentrations (1:1, 1:2, 1:3, 1:4 and 1:5) of BMMSCs or PCa-MSCs
were added. The 96-well culture plates were cultured in DMEM/F12
medium (37°C, 5% CO2). The culture was terminated prior
to 12–16 h by adding 100 μl (0.5–1 μCi) tritium labeled thymidine
(3H-TdR) to each well. After the end of the culture, the cells were
collected on glass fiber filter paper for natural drying.
Scintillation counting (cpm) values were determined each minute
using a beta liquid scintillation counter. This experiment was also
performed with the control group (PBS).
Statistical analysis
All data are analyzed using the SigmaStat
statistical software (Jandel Scientific, San Rafael, CA, USA) and S
SigmaPlot (SPSS Inc. Chicago, IL, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
Cell culture
The results of the BMMSC culture were similar to
those of previous reports (17).
We therefore focused on the results for the PCa-MSCs culture.
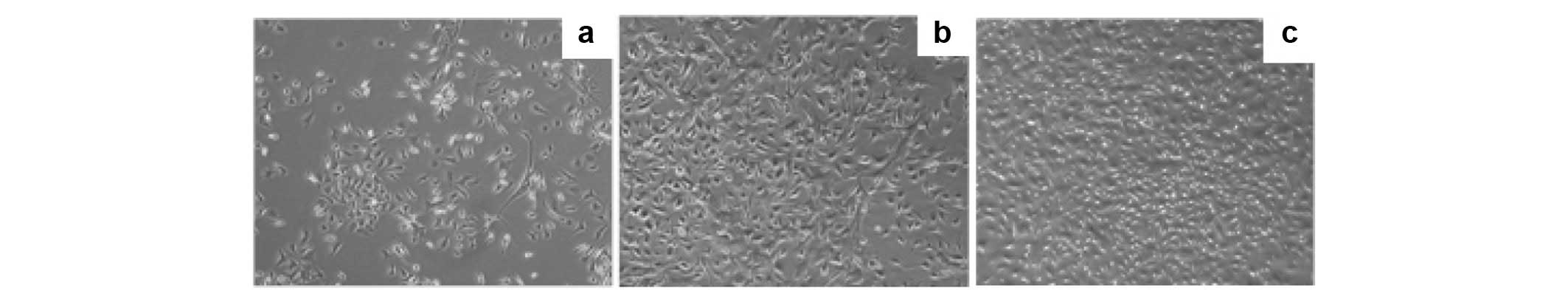
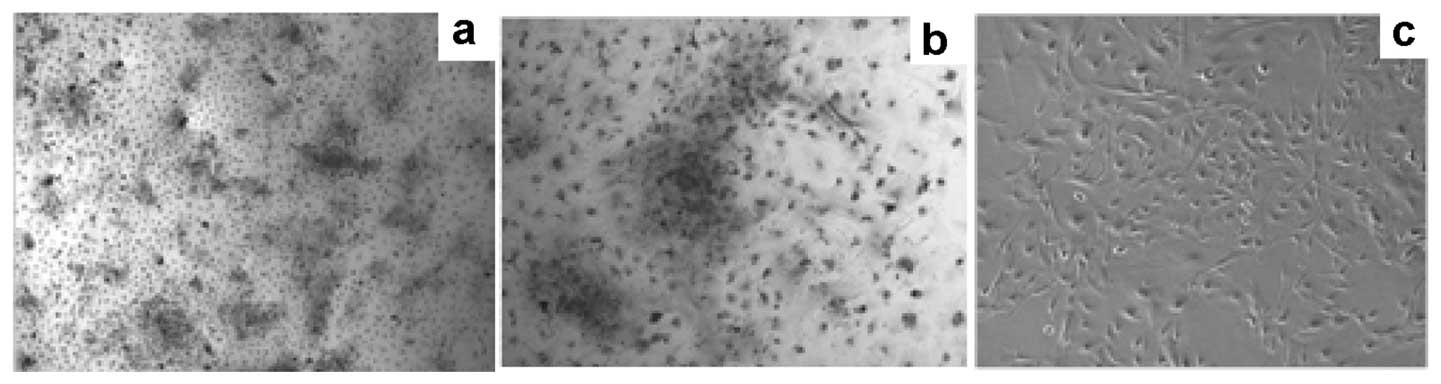
Fig. 1a demonstrates the cell
growth status 48 h after primary vaccination in the culture medium.
We used microscopy to observe the extent of cell growth and the
number of colonies, which were fusiform. The time required for 90%
confluence of the PCa-MSCs was markedly shorter than that of the
BMMSCs (Fig. 1b; 8–10 days vs.
12–14 days). PCa-MSCs were cultured, often with a mixture of
various cells, past 2–3 generations and became uniform in
morphology (Fig. 1c). As detected
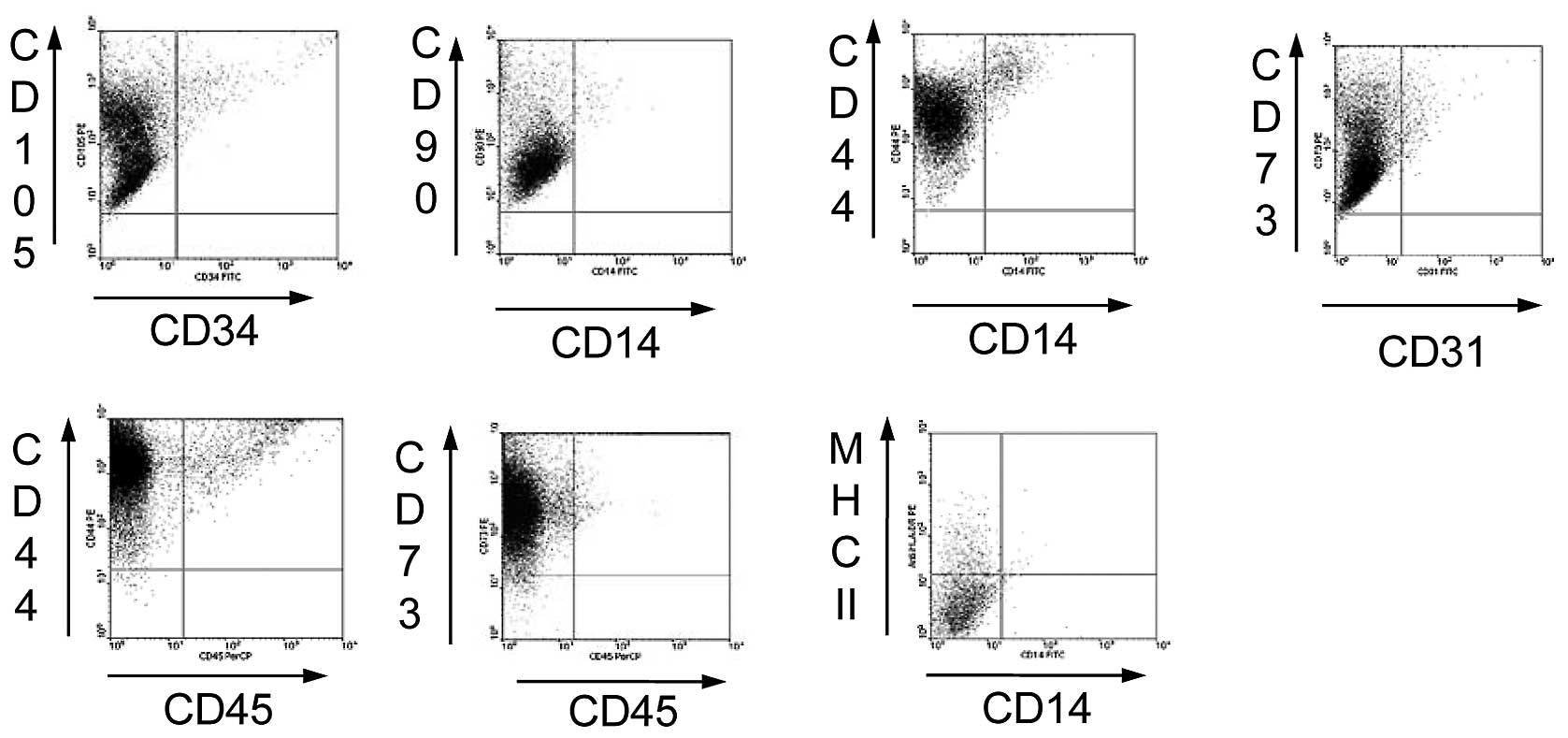
by flow cytometry, the P3 PCa-MSCs exhibited high expression of
CD44, CD73, CD90 and CD105, but were negative for CD14, CD34, CD45
and MHC-II. The PCa-MSCs were 95% homogeneous (Fig. 2).
Differentiation of PCa-MSCs
PCa-MSCs were induced to differentiate
into adipocytes (Oil red O)
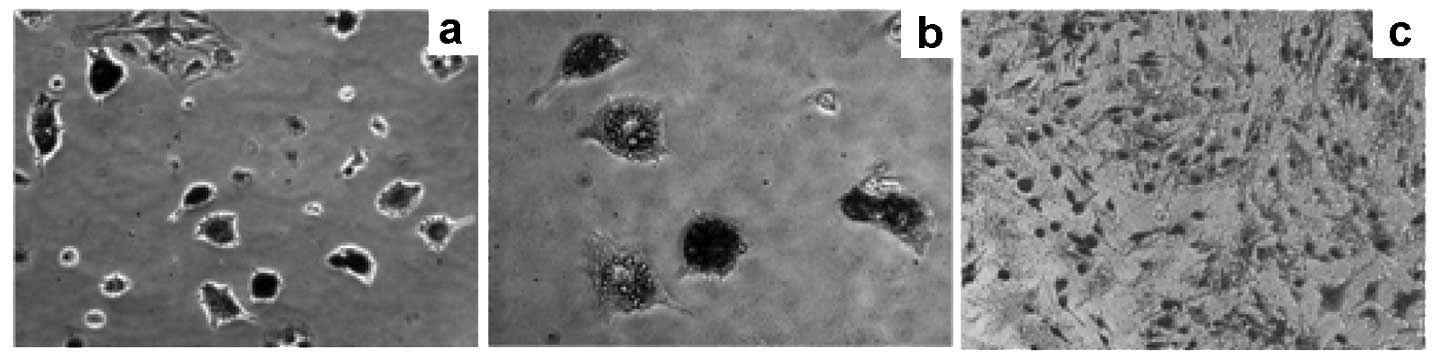
When induced by adipogenic medium, the PCa-MSCs
exhibiting a long spindle-shaped form gradually became oval or
round (Fig. 3a), with
intracytoplasmic refractive bright circular lipid droplets
(Fig. 3b). Lipid droplets
exhibited a brick-red color after staining with Oil red O while the
normal control cytoplasm was not stained (Fig. 3c).
PCa-MSCs were induced to differentiate
into bone cells (alkaline phosphatase)
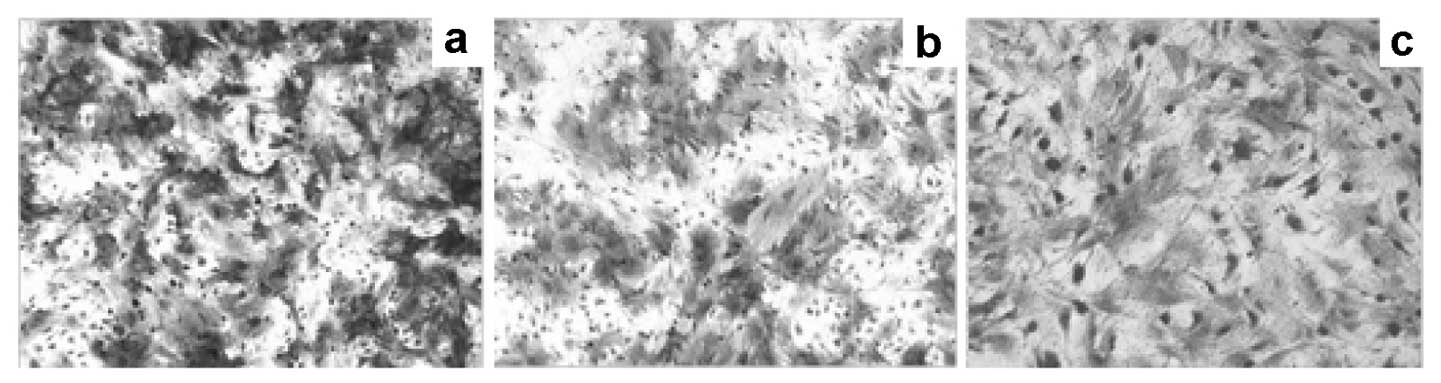
With osteogenic induction, the cell morphology of
PCa-MSCs changed from a spindle-shaped to a flat-shaped morphology
(Fig. 4a) and cells were observed
to be alkaline phosphatase-positive after 10 days (more red
alkaline grain-containing acid enzyme-positive granules in the
cytoplasm, Fig. 4b), whereas in
the normal controls, alkaline phosphatase expression was almost
negative (Fig. 4c).
PCa-MSCs were induced to differentiate
into bone cells (alizarin red)
The PCa-MSCs exposed to osteogenic induction with
0.1% alizarin red staining exhibited visible orange-red nodules and
a clear boundary of the mineralized nodules after 14 days (Fig. 5a and b), whereas normal control
cells showed negativity to alizarin red staining (Fig. 5c).
PCa-MSCs were induced to differentiate
into chondrocytes (toluidine blue)
After chondrogenic induction, the PCa-MSCs continued
to proliferate to form multiple cell nodules, in which cells were
polygonal or round in shape (Fig.
6a). We observed that the cells exhibited blue metachromasia in
the cytoplasm stained with toluidine blue 10 days after induction
(Fig. 6b). The normal control
cells were negative (Fig. 6c).
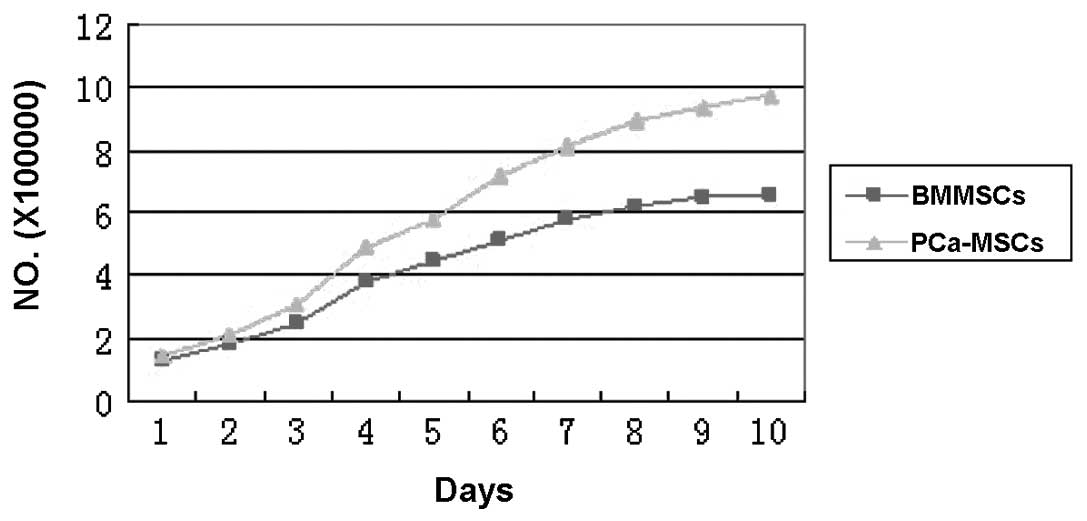
Proliferative activity and growth
ability of BMMSCs and PCa-MSCs
We found that the growth ability of PCa-MSCs was
markedly higher than that of BMMSCs. The growth curve of these two
cell types is shown in Fig. 7.
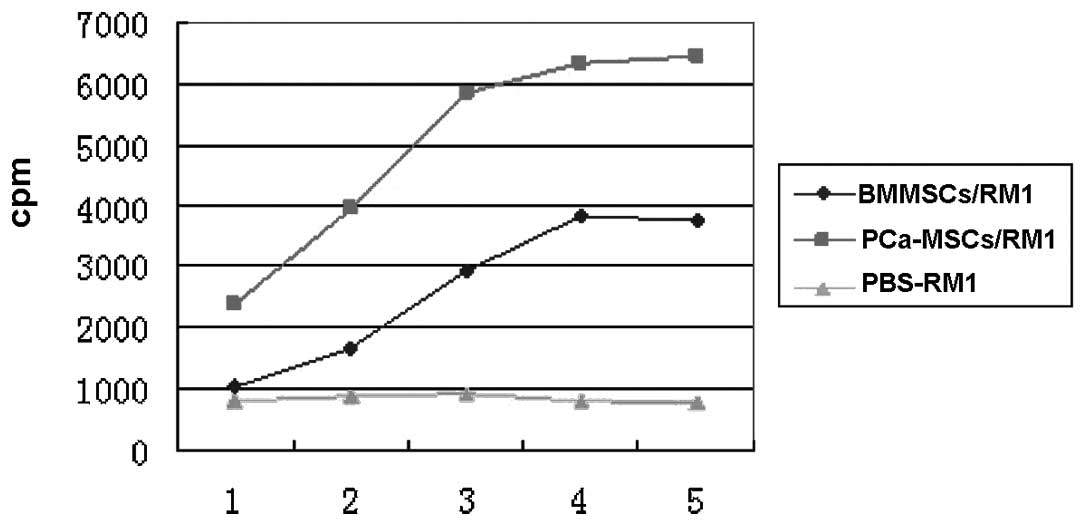
Based on the RM-1 cell proliferation experiments we found that the
proliferative activity of PCa-MSCs was also higher than that of the
BMMSCs (Fig. 8).
Discussion
In this study, we provide evidence that MSCs home to
subcutaneously implanted mouse prostate tumors, which was similar
to the results of previous studies, in which cells were observed in
the lung and liver. Our methods for PCa-MSC isolation and culture
were effective, since the cells showed a phenotype of
CD34−/CD45− [not hematopoietic cells
(18,19)],
CD44+/CD73+/CD105+/CD90+
[mesenchymal stromal cell and stem cell markers (20–22)]
and CD14−/MHC-II− [not endothelial progenitor
cells (21–23)]. The isolation of PCa-MSCs from
prostate tumors is an important aspect of our study. In fact, to
our knowledge, this is the first demonstration of PCa-MSCs obtained
from prostate tumors implanted in mice. Alternatively, human PCa,
similar to other cancers, requires the elaboration of mesodermal
elements, specifically endothelial cells and pericytes. It has been
suggested that MSCs are a main source of pericytes within the bone
marrow stroma (24,25); thus, MSCs may integrate into
prostate cancer to contribute to the mesenchymal elements of the
tumor. MSCs may localize to the tumor under physiological
conditions to assist with tissue repair. This results in a
microenvironment conducive to tumor growth.
Mesenchymal stem cells are a type of primary cell
that self-renew and have multiple differentiating potentials
(26). BMMSCs differentiate into
nerve cells, skeletal muscle cells, and vascular endothelial cells.
Our study also provides evidence that PCa-MSCs have differentiating
ability, which is consistent with other reports (14–16,17).
Collectively, these findings suggest that in the process of
prostate cancer development, MSCs may confer a potential
therapeutic advantage against bone metastases in PCa.
One aim of this study was to assess the
proliferative activity and growth ability of PCa-MSCs compared with
BMMSCs. To achieve this aim, we used cell proliferation and
MSC-RM-1 cell culture. The growth curve indicated that the growth
ability of PCa-MSCs was markedly higher than that of BMMSCs. In
addition, the activity of PCa-MSCs, which could stimulate the cell
proliferation of RM-1, was significantly higher when compared with
that of BMMSCs. Our results indicate that it may be mediated at
least in part by growth factors/chemokines. This observation is
consistent with the hypothesis that MSCs locate to the tumor
environment since tumors mimic tissue injury (10,11,27).
Conversely, MSCs are precursors of stromal cells, which generate
the extracellular matrix supporting hematopoiesis within the bone
marrow microenvironment (28).
Stromal components derived from MSCs may therefore play a role in
tumor growth within the tumor microenvironment.
Taking these findings together, it is unlikely that
the localization of PCa-MSCs within prostate tumors grown in mice
was merely the result of a species-specific tropism. Instead, MSCs
appear to have an intrinsic, cell-specific capacity to localize to
PCa. Detailed characterization of the properties of MSCs following
tumor grafting will be addressed in future studies.
Acknowledgements
This study was sponsored by a grant
from the National Natural Sciences Foundation of China (NSFC)
(grant no. 81001145).
References
|
1
|
Richman EL, Kenfield SA, Stampfer MJ,
Paciorek A, Carroll PR and Chan JM: Physical activity after
diagnosis and risk of prostate cancer progression: data from the
cancer of the prostate strategic urologic research endeavor. Cancer
Res. 71:3889–3895. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pittenger MF and Marshak DR: Mesenchymal
stem cells of human adult bone marrow. Stem Cell Biology. Marshak
DR, Gardner RL and Gottlieb D: Cold Spring Harbor Laboratory Press;
Cold Spring Harbor: pp. 349–373. 2001
|
|
3
|
Orkin SH: Hematopoietic stem cells:
molecular diversification and developmental interrelationships.
Stem Cell Biology. Marshak DR, Gardner RL and Gottlieb D: Cold
Spring Harbor Laboratory Press; Cold Spring Harbor: pp. 289–306.
2001
|
|
4
|
Keller G: The hemangioblast. Stem Cell
Biology. Marshak DR, Gardner RL and Gottlieb D: Cold Spring Harbor
Laboratory Press; Cold Spring Harbor: pp. 329–348. 2001
|
|
5
|
Kopen GC, Prockop DJ and Phinney DG:
Marrow stromal cells migrate throughout forebrain and cerebellum,
and they differentiate into astrocytes after injection into
neonatal mouse brains. Proc Natl Acad Sci USA. 96:10711–10716.
1999. View Article : Google Scholar
|
|
6
|
Woodbury DJ, Schwarz EJ, Prockop DJ and
Black IB: Adult rat and human bone marrow stromal cells
differentiate into neurons. J Neurosci. 61:364–370. 2000.PubMed/NCBI
|
|
7
|
Noël D, Djouad F and Jorgensen C:
Regenerative medicine through mesenchymal stem cells for bone and
cartilage repair. Curr Opin Investig Drugs. 3:1000–1004.
2002.PubMed/NCBI
|
|
8
|
Nakamura K, Ito Y, Kawano Y, Kurozumi K,
Kobune M, Tsuda H, Bizen A, Honmou O, Niitsu Y and Hamada H:
Anti-tumor effect of genetically engineered mesenchymal stem cells
in a rat glioma model. Gene Ther. 11:1155–1164. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Djouad F, Plence P, Bony C, Tropel P,
Apparailly F, Sany J, Noel D and Jorgensen C: Immunosuppressive
effect of mesenchymal stem cells favors tumor growth in allogeneic
animals. Blood. 102:3837–3844. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bissell MJ and Radisky D: Putting tumors
in context. Nat Rev Cancer. 1:46–54. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ben-Baruch A: Host microenvironment in
breast cancer development: inflammatory cells, cytokines and
chemokines in breast cancer progression: reciprocal
tumor-microenvironment interactions. Breast Cancer Res. 5:31–36.
2003. View
Article : Google Scholar
|
|
12
|
Studeny M, Marini FC, Champlin RE,
Zompetta C, Fidler IJ and Andreeff M: Bone marrow-derived
mesenchymal stem cells as vehicles for interferon-beta delivery
into tumors. Cancer Res. 62:3603–3608. 2002.PubMed/NCBI
|
|
13
|
Peister A, Mellad J, Larson B, Hall B,
Gobson L and Prockop D: Adult stem cells from bone marrow (MSCs)
isolated from different strains of in bred mice vary in surface
epitopes, rates of proliferation, and differential potential.
Blood. 103:1662–1668. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Igarashi M, Yogiashi Y, Mihara M, Takada
I, Kitagawa H and Kato S: Vitamin K induces osteoblast
differentiation through pregnane X receptor-mediated
transcriptional control of the Msx2 gene. Mol Cell Biol.
27:7947–7954. 2007. View Article : Google Scholar
|
|
15
|
Meirelles Lda S and Nardi NB: Murine
marrow-derived mesenchymal stem cell: isolation, in vitro
expansion, and characterization. Br J Haematol. 123:702–711.
2003.PubMed/NCBI
|
|
16
|
Phinney DG, Kopen G, Isaacson RL and
Prockop DJ: Plastic adherent stromal cells from the bone marrow of
commonly used strains of inbred mice: variations in yield, growth,
and differentiation. J Cell Biochem. 72:570–585. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pittenger MF, Mackay AM, Beck SC, Jaiswal
RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S and
Marshak DR: Multilineage potential of adult human mesenchymal stem
cells. Science. 284:143–147. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Huss R, Lange C, Weissinger EM, Kolb HJ
and Thalmeier K: Evidence of peripheral blood-derived,
plastic-adherent CD34(-/low) hematopoietic stem cell clones with
mesenchymal stem cell characteristics. Stem Cells. 18:252–260.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Majumdar MK, Banks V, Peluso DP and Morris
EA: Isolation, characterization, and chondrogenic potential of
human bone marrow-derived multipotential stromal cells. J Cell
Physiol. 185:98–106. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
De Ugarte DA, Alfonso Z, Zuk PA, Elbarbary
A, Zhu M, Ashjian P, Benhaim P, Hedrick MH and Fraser JK:
Differential expression of stem cell mobilization-associated
molecules on multi-lineage cells from adipose tissue and bone
marrow. Immunol Lett. 89:267–270. 2003.PubMed/NCBI
|
|
21
|
Niyibizi C, Wang S, Mi Z and Robbins PD:
The fate of mesenchymal stem cells transplanted into
immunocompetent neonatal mice: implications for skeletal gene
therapy via stem cells. Mol Ther. 9:955–963. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Majumdar MK, Thiede MA, Mosca JD, Moorman
M and Gerson SL: Phenotypic and functional comparison of cultures
of marrow-derived mesenchymal stem cells (MSCs) and stromal cells.
J Cell Physiol. 176:57–66. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Phinney DG: Building a consensus regarding
the nature and origin of mesenchymal stem cells. J Cell Biochem
Suppl. 38:7–12. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Conget PA and Minguell JJ: Phenotypical
and functional properties of human bone marrow mesenchymal
progenitor cells. J Cell Physiol. 181:67–73. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Minguell JJ, Erices A and Conget P:
Mesenchymal stem cells. Exp Biol Med. 226:507–520. 2001.PubMed/NCBI
|
|
26
|
Huttmann A, Li CL and Duhrsen U: Bone
marrow-derived stem cells and ‘plasticity’. Ann Hematol.
82:599–604. 2003.
|
|
27
|
Coussens LM and Werb Z: Inflammation and
cancer. Nature. 420:860–867. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Prockop DJ: Marrow stromal cells as stem
cells for nonhematopoietic tissues. Science. 276:71–74. 1997.
View Article : Google Scholar : PubMed/NCBI
|