Introduction
The failure of any component of joints participating
in load transmission, particularly bone and articular cartilage,
may lead to sport-induced joint injuries. The skeletal challenges
appear to vary considerably among different sports. In soccer, the
incidence of knee- and ankle-joint injuries is conceivably higher
than in any other sports, possibly since soccer athletes expose the
lower extremity joints to excessive and repetitive axial loading.
Such skeletal injuries are predicted, not only to prevent players
from participating in practice and competition, but also to
increase the risk of osteoarthritis (OA). Results of various
clinical studies have indicated that soccer is one of the sports
most likely to cause knee OA (1–4).
Gelber et al (5) reported
that young adults with knee injuries have a considerably increased
risk of developing OA.
It is possible that soccer- and certain other
sports-related skeletal injuries, as well as acute or chronic
physical loadings, are expected to affect the turnover rate of the
afflicted parts of the skeleton, particularly the joint tissues,
and are detected using systemic biomarker assays. Various molecular
markers have been reported as indicators of bone turnover and
cartilage metabolism in patients with bone and joint disorders
(6,7). Among the biomarkers that have been
extensively used are the C-terminal crosslinked telopeptides of
type II collagen (CTX-II) and C-terminal propeptides of type II
collagen (CPII) as the cartilage-specific type II collagen (CII)
degradation and synthesis markers, respectively. N-terminal
telopeptides of bone-specific type I collagen (NTx) have been used
as the marker of bone resorption (8). Previously, we revealed that CTX-II,
CPII and NTx are significantly or substantially elevated in the
urine or sera of young athletes compared with non-athlete controls
(9).
A commercially available product of dietary
supplement containing a chicken comb extract (CCE), which is rich
in hyaluronan, (Kojun®) has been shown relieve joint
pain and other symptoms, as well as to potentially improve the
balance of CII degradation/synthesis in patients with knee OA
(10,11). Based on these observations, we
hypothesized that the CCE-containing supplement product (test
product) has an effect on the three biomarkers in athletes.
Therefore, in this study, we evaluated the effect of a
CCE-containing supplement on cartilage and bone metabolism in
athletes.
Materials and methods
Subjects
The study was approved by the Human Experimentation
Ethics Committee of Juntendo University (Japan) and informed
consent was obtained from the participants. A total of 66
collegiate athletes belonging to three different intercollegiate
soccer teams were recruited to participate in the study. It was
expected that the rate of skeletal injuries and the levels of
soccer-related activities or physical loadings are influenced by
the position of players within the team and/or on the pitch. These
levels were predicted to be higher in the midfielder position
compared to any other player positions, including the forward,
defender and goal keeper, since midfielders cover a longer distance
than the players of other positions (12). For this reason, only midfielders
were selectively analyzed in this study.
Intervention and subject group
assignment
The test product was a 300-mg capsule preparation
consisting of 157.5 mg of CCE, of which approximately 4.5 mg was
hyaluronan, 20 mg calcium lactate, 10 mg propolis extract, 4.9 mg
chitosan oligosaccharide, 5.0 mg each of vitamins B1 and
B6, 2.5 mg vitamin E, 2.0 mg ferric pyrophosphate, 0.1 mg vitamin
B12 and 192.5 mg of vehicle (a mixture of crystalline
cellulose, dextrin and fatty acid sugar esters) (11). Fourteen and 15 subjects were
randomly assigned to receive 16 capsules/day of the test product
(test group) and those of dummy placebo containing only vehicle
(placebo group), respectively. The subjects in the two groups were
instructed to take allocated capsules at a dose of 8 capsules twice
daily for 12 weeks. The study was executed in accordance with the
principles of the amended Declaration of Helsinki and the Ethical
Guidelines for Epidemiological Research (established by the
Japanese Government in 2004) during the 2010 summer-fall soccer
competition season.
Procedures
The urine and blood/serum samples were collected at
baseline (before the intervention) and at 4, 8 and 12 weeks after
the intervention, and stored at less than −40°C before assay.
Urinal CTX-II and NTx were measured using CartiLaps EIA®
(Immunodiagnostic Systems, Inc., Tyne & Wear, UK) and
Osteomark® (Inverness Medical Japan Co., Ltd., Tokyo,
Japan), respectively, and the values were normalized with
creatinine (Cr). Serum CPII was measured using Procollagen II C
Propeptide ELISA® (Ibex Pharmaceuticals, Inc.,
Mont-Royal, QC, Canada).
Statistical analysis
Unpaired and paired Student’s t-tests were used for
the between-group comparison and within-group comparison,
respectively. Values were presented as the means ± SD. P<0.05
was considered to indicate a statistically significant result.
Results
Data from 29 subjects (14 and 15 in the test and
placebo groups, respectively) who completed the study were
evaluated. Demographic characteristics [age, height, weight, body
mass index (BMI), blood pressures and pulse rate] as well as
biomarker profiles (CTX-II, CPII, CTX-II/CPII ratio and NTx) were
not significantly different between the test and placebo groups
(Table I).
 | Table IBaseline data of subjects in the test
and placebo groups who completed the study. |
Table I
Baseline data of subjects in the test
and placebo groups who completed the study.
| Variables | Test group (n=14)
(mean ± SD) | Placebo group (n=15)
(mean ± SD) | P-value |
|---|
| Age (years) | 20.0±1.0 | 20.0±1.2 | 1.000 |
| Height (cm) | 170.2±4.3 | 172.6±4.9 | 0.159 |
| Weight (kg) | 63.0±4.4 | 64.7±5.2 | 0.359 |
| Body mass index
(kg/m2) | 21.8±1.2 | 21.7±0.6 | 0.808 |
| Systolic blood
pressure (mmHg) | 116.3±11.0 | 116.1±8.7 | 0.953 |
| Diastolic blood
pressure (mmHg) | 63.6±6.4 | 63.0±7.0 | 0.821 |
| Pulse rate
(beats/min) | 60.4±8.7 | 56.5±8.8 | 0.251 |
| Urinary CTX-II
(ng/mmol Cr) | 1,710±883 | 1,265±1,096 | 0.242 |
| Serum CPII
(ng/ml) | 1,544±458 | 1,498±438 | 0.784 |
| Urinary CTX-II/serum
CPII ratio | 1.25±0.81 | 0.88±0.69 | 0.195 |
| Urinary NTx (nmol
BCE/mmol Cr) | 66.8±19.4 | 60.0±30.1 | 0.479 |
Table II shows the
changes in the biomarker profiles during the intervention. CTX-II
levels were clearly reduced from the baseline in the test group
with a statistical significance at week 12 (P<0.01), while no
significant reduction was observed in the placebo group at any
timepoint during the intervention. Unlike CTX-II, CPII levels were
elevated significantly from the baseline at all timepoints (weeks
4, 8 and 12; P<0.01 or <0.05). As a result, the ratios of
CTX-II to CPII were decreased to a significant level (P<0.01 or
<0.05) at weeks 4, 8 and 12 in the test group and at weeks 8 and
12 in the placebo group. Notably, NTx levels were reduced only in
the test group, reaching a significant level at week 12
(P<0.01).
 | Table IIChanges in the levels of urinary
CTX-II, serum CPII, the CTX-II/CPII ratio and urinary NTx during
the 12-week intervention period in the test (n=14) and placebo
groups (n=15). |
Table II
Changes in the levels of urinary
CTX-II, serum CPII, the CTX-II/CPII ratio and urinary NTx during
the 12-week intervention period in the test (n=14) and placebo
groups (n=15).
| Groups | Baselinea | Week 4a | Week 8a | Week 12a |
|---|
| CTX-II (ng/mmol
Cr) | Test | 1,710±883 | 1,596±852 (−7) | 1,388±719 (−19) | 1,301±706
(−24)c |
| Placebo | 1,265±1,096 | 1,424±1,239 (13) | 1,182±884 (−7) | 1,056±693 (−17) |
| CPII (ng/ml) | Test | 1,544±458 | 1,823±393
(18)b | 1,993±396
(29)c | 1,947±544
(26)c |
| Placebo | 1,498±438 | 1,764±399
(18)b | 1,919±384
(28)c | 1,964±515
(31)c |
| CTX-II/CPII
ratio | Test | 1.25±0.811 | 0.93±0.54
(−26)b | 0.74±0.45
(−41)c | 0.71±0.38
(−43)c |
| Placebo | 0.88±0.69 | 0.85±0.75 (−3) | 0.65±0.47
(−26)b | 0.56±0.33
(−36)b |
| NTx (nmol BCE/mmol
Cr) | Test | 66.8±19.4 | 58.8±18.5 (−12) | 58.1±21.0 (−13) | 56.7±17.0
(−15)c |
| Placebo | 60.0±30.1 | 65.0±32.4 (8) | 58.0±18.9 (−3) | 62.1±23.0 (4) |
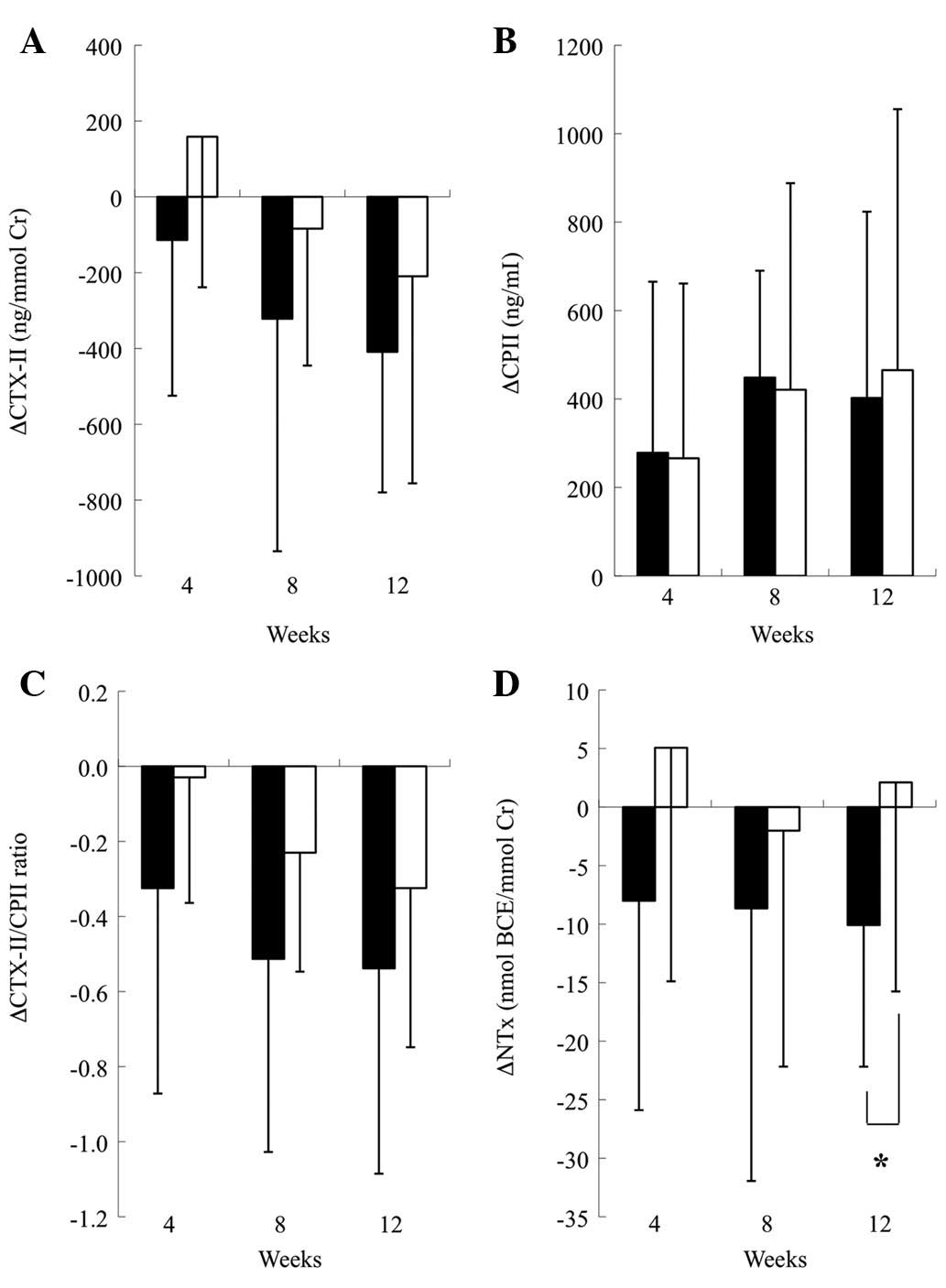
Fig. 1 shows the
changed values from the baseline in all biomarkers used in this
study during the intervention. The changes in the CPII levels were
not different between the two groups at any timepoints. By
contrast, the changes from the baseline levels of CTX-II, the
CTX-II/CPII ratio and NTx were greater in the test group than in
the placebo group, and the between-group difference in the NTx was
statistically significant at week 12 (P<0.05).
Discussion
Joint injury and OA are similarly characterized by
the remodeling and degradation of cartilage, bone and other joint
tissues. Lohmander et al (13) reported the release of CTX-II into
synovial fluid in human OA and joint injury, which provides the
evidence that the integrity of the CII network of cartilage is
compromised shortly after joint injury and in arthritis. With
regard to OA, accumulating evidence indicates that serum or urinary
levels of CTX-II and/or certain other CII degradation markers,
including C1, 2C and C2C, increase with the progression of OA
(10,11). The presence of NTx in the urine has
been evaluated by Bettica et al (8) as a risk marker in progressive knee OA
and was found to be elevated in patients with the radiographic
progression of knee OA. This finding suggests that the progression
of OA reflects the changes of both cartilage metabolism and bone
turnover, and that the subchondral bone alterations play a role in
the pathogenesis of OA. Based on these findings with regard to OA
and the similarity of the change in the skeletal marker profiles
between OA and joint injury, we have hypothesized that, similar to
OA, joint injury is also characterized by cartilage degradation and
subchondral bone abnormalities in athletes.
In the present study, we have shown that the daily
oral intake of the CCE-containing test product clearly decreases
the urinary levels of both CTX-II and NTx at 12 weeks after the
initiation of the intervention. The results suggest that the test
product is effective in inhibiting cartilage degradation and bone
remodeling. In this context, hyaluronan, a putative active
component of the CCE-containing supplement, has been reported to
stimulate bovine chondrocytes, being effective in maintaining the
cell phenotype with increased matrix deposition of
glycosaminoglycan and CII (14,15).
Although the actual mechanism of this beneficial action on joint
injury remains unclear, the CCE-containing supplement may be useful
for the management of joint health in athletes.
In conclusion, the oral administration of the
CCE-containing supplement decreases the levels of CTX-II and NTx in
the urine of young athletes, suggesting a potential use for
relieving the joint injury-associated cartilage degradation and
bone remodeling.
References
|
1
|
Roos H, Lindberg H, Gärdsell P, Lohmander
LS and Wingstrand H: The prevalence of gonarthrosis and its
relation to meniscectomy in former soccer players. Am J Sports Med.
22:219–222. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kujala UM, Kettunen J, Paananen H, Aalto
T, Battié MC, Impivaara O, Videman T and Sarna S: Knee
osteoarthritis in former runners, soccer players, weight lifters,
and shooters. Arthritis Rheum. 38:539–546. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rannou F, Poiraudeau S and Revel M:
Cartilage: from biomechanics to physical therapy. Ann Readapt Med
Phys. 44:259–267. 2001.(In French).
|
|
4
|
Lequesne MG, Dang N and Lane NE: Sport
practice and osteoarthritis of the limbs. Osteoarthritis Cartilage.
5:75–86. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gelber AC, Hochberg MC, Mead LA, Wang NY,
Wigley FM and Klag MJ: Joint injury in young adults and risk for
subsequent knee and hip osteoarthritis. Ann Intern Med.
133:321–328. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Poole AR, Ionescu M, Fitzcharles MA and
Billinghurst RC: The assessment of cartilage degradation in vivo:
development of an immunoassay for the measurement in body fluids of
type II collagen cleaved by collagenases. J Immunol Methods.
294:145–153. 2004. View Article : Google Scholar
|
|
7
|
Rousseau JC and Delmas PD: Biological
markers in osteoarthritis. Nat Clin Pract Rheumatol. 3:346–356.
2007. View Article : Google Scholar
|
|
8
|
Bettica P, Cline G, Hart DJ, Meyer J and
Spector TD: Evidence for increased bone resorption in patients with
progressive knee osteoarthritis: longitudinal results from the
Chingford study. Arthritis Rheum. 46:3178–3184. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yoshimura M, Sakamoto K, Tsuruta A,
Yamamoto T, Ishida K, Yamaguchi H and Nagaoka I: Evaluation of the
effect of glucosamine administration on biomarkers for cartilage
and bone metabolism in soccer players. Int J Mol Med. 24:487–494.
2009.PubMed/NCBI
|
|
10
|
Hatayama T, Nagano M, Yamaguchi N, Kumagai
S and Ohnuki K: The effect of a supplement on knee pain and
discomfort evaluated by visual analog scale (VAS): a randomized,
double-blind, placebo-controlled study. Kenko-shien. 10:13–17.
2008.(In Japanese).
|
|
11
|
Nagaoka I, Nabeshima K, Murakami S,
Yamamoto T, Watanabe K, Tomonaga A and Yamaguchi H: Evaluation of
the effects of a supplementary diet containing chicken comb extract
on symptoms and cartilage metabolism in patients with knee
osteoarthritis. Exp Ther Med. 1:817–827. 2010.PubMed/NCBI
|
|
12
|
Bangsbo J, Nørregaard L and Thorsø F:
Activity profile of competition soccer. Can J Sport Sci.
16:110–116. 1991.PubMed/NCBI
|
|
13
|
Lohmander LS, Atley LM, Pietka TA and Eyre
DR: The release of crosslinked peptides from type II collagen into
human synovial fluid is increased soon after joint injury and in
osteoarthritis. Arthritis Rheum. 48:3130–3139. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Patti AM, Gabriele A, Vulcano A, Ramieri
MT and Della Rocca C: Effect of hyaluronic acid on human
chondrocytes cell lines from articular cartilage. Tissue Cell.
33:294–300. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Akmal M, Singh A, Anand A, Kesani A, Aslam
N, Goodship A and Bentley G: The effects of hyaluronic acid on
articular chondrocytes. J Bone Joint Surg Br. 87:1143–1149. 2005.
View Article : Google Scholar : PubMed/NCBI
|