Introduction
Nuclear factor-κB (NF-κB) was first identified as a
regulator of the expression of the κ light-chain gene in murine B
lymphocytes in 1986 (1), and a
number of groups are currently researching the mechanism and effect
of NF-κB. As an inducible nuclear transcription factor, NF-κB plays
a key role in physiological or pathological conditions, and NF-κB
and various signaling pathways regulate the expression of many
genes involved in growth, differentiation, embryonic development,
innate and adaptive immune responses, inflammation and apoptosis
(2,3).
The occurrence and development of cancer is an
extremely complex process which is affected by a variety of
cytokines, signals and genetic changes. Cancer therapy has been
ungoing for a long period of time, yet the effect of anticancer
drugs remains inefficient. The primary cause may be
chemotherapeutic resistance caused by either the deletion of a
pro-apoptotic gene or the overexpression of an anti-apoptotic gene
(4).
More recently, it has become clear that NF-κB
signaling also plays a critical role in cancer development and
progression. The activation of NF-κB results in the resistance of
tumor cells to radiochemotherapy-induced cytotoxicity (3,5,6).
NF-κB may also regulate tumor angiogenesis and invasiveness, and
the signaling pathways that mediate its activation provide
candidate targets for new chemopreventive and chemotherapeutic
approaches (7,8). In the present study, we used the
colon cancer cell line HT-29 to observe the effect of the NF-κB
signaling pathway on apoptosis induced by chemotherapy drugs.
Materials and methods
Materials
The colon cancer cell line HT-29 was purchased from
the Chinese Academy of Sciences (Shanghai, China). The arsenic acid
sodium (As2O3) injection was purchased from
Harbin Yida Pharmaceutical (Haerbin, Heilongjiang, China),
fluorouracil (5FU) injection from the Tianjin Kingyork Group
(Hedong, Tianjin, China), paclitaxel injection from Beijing Shiqiao
Biological Pharmaceutical (Beijing, China) and oxaliplatin
injection from Jiangsu Hengrui Medicine (Lianyungang, Jiangsu,
China). The NF-κB inhibitors, bortezomib from Xian-Janssen
Pharmaceutical (Beijing, China), SN50 from Alexis Biochemicals (San
Diego, CA, USA) and ammonium pyrrolidine dithiocarbamate (PDTC)
from Sigma (St. Louis, MO, USA) were used in this study. DMEM (high
glucose) and fetal bovine serum were from HyClone (Logan, UT, USA),
the Annexin V-PI apoptosis detection kit was from BD Biosciences,
the SDA-PAGE gel configuration kit, RIPA cell lysates (strong),
PMSF and BCA protein concentration determination kit were purchased
from Beyotime Institute of Biotechnology (Haimen, Jiangsu, China).
Protein liquid sample buffer 4X was purchased from Beijing Solarbio
Science and Technology (Beijing, China). Prestained protein ladder
was from Fermentas. The mouse monoclonal antibody to the NF-κB p65
subunit was obtained from Santa Cruz Biotechnology Inc. (Santa
Cruz, CA, USA). Rabbit monoclonal to IKBα and rabbit polyclonal to
survivin was from Abcam (Cambridge, MA, USA). Nuclear protein
extraction kit and electrophoretic mobility shift assay (EMSA) kit
were from Viagene Biotech (Los Angeles, CA, USA).
Cell culture
Colon cancer HT-29 cells were grown in DMEM
supplemented with 10% fetal bovine serum and 0.1%
penicillin/streptomycin and maintained at 37°C in an atmosphere of
5% CO2. Cells were passaged to the next generation every
two to three days and digested by 0.25% trypsin. Logarithmic
growing cells were prepared.
Cell viability assay
The cells were dispersed and plated at
6×104 cells/well in 96-well microplates to determine the
concentration and time-course of the response of HT-29 cells to
As2O3, 5FU, oxaliplatin or paclitaxel, or
combined with PDTC, bortezomib or SN50. Cell viability was assessed
using an MTT assay following drug treatment at various
concentrations or days in culture. The absorbance value (A) at 490
nm was read using a microplate reader (Thermo, Rockford, IL, USA).
The inhibition rate was calculated as follows: Cell inhibition rate
(%) = (1 - A of experiment well/A of positive control well) x 100%.
Cell viability was assessed three times.
Apoptosis assays and flow cytometry
(FCM)
Following drug treatment for various hours, the
HT-29 cell suspension was prepared using 0.125% trypsin and was
rinsed and centrifuged with ice-cold PBS at 1,000 rpm for 5 min.
The collected cells were treated with Annexin V-FITC or PI
according to the manufacturer’s instructions (tube 1, unstained
cells; tube 2, stained with PI; tube 3, stained with Annexin
V-FITC; tube 4, stained with both Annexin V-FITC and PI) for 20 min
away from the light, and Annexin fuorescence intensity was detected
using FCM.
NF-κB assays and western blotting
HT-29 cells were treated with a chemotherapy drug (5
mg/l As2O3, 300 mg/l 5FU, 20 mg/l oxaliplatin
or 2.5 mg/l paclitaxel alone for 0, 3, 6, 12, 24 and 48 h, NF-κB
inhibitor (50 μmol/l PDTC, 100 nmol/l bortezomib, 12.5 mg/l SN50)
alone for 24 h, or combined with a chemotherapy drug for 24 h. The
cell lysates were then prepared using standard methods. The protein
concentration of each sample was measured using a BCA kit. Proteins
from each sample were subjected to electrophoresis by SDS-PAGE,
transferred onto polyvinylidene difluoride membranes (PVDF) with a
transfer system (Bio-Rad, Hercules, CA, USA), and then blocked with
a buffer containing 5% skimmed milk and 0.1% Tween-20 in
Tris-buffered saline (TBST) at room temperature for 1 h. All
antibodies were diluted in TBST. The membranes were incubated
overnight with a primary antibody at 4°C, washed with TBST (3x10
min), followed by incubation with horseradish peroxidase
(HRP)-conjugated secondary antibody for 1 h at room temperature,
and then washed (3×10 min). Detection of chemiluminescence was
performed with a DAB kit.
EMSA
Nuclear proteins were extracted with a nuclear
protein extraction kit in accordance with the manufacturer’s
instructions. The protein concentration was determined using a BCA
kit. The NF-κB probe was 5′-AGTTGAGGGGACTTTCCC AGGC-3′. Binding
reactions were performed according to the non-radioactive EMSA kit.
The specificity of the DNA and protein complex was confirmed by
cold competition with a 50-fold excess of unlabeled NF-κB
oligonucleotides. Binding reaction, gel electrophoresis, membrane
transfer and immobilization, DNA binding, chemiluminescent reaction
and imaging were performed sequentially.
Statistical analysis
All data are expressed as means ± SD. Statistical
analyses were performed using the Student’s t-test or ANOVA by SPSS
11.0. P<0.05 was considered to indicate a statistically
significant result.
Results
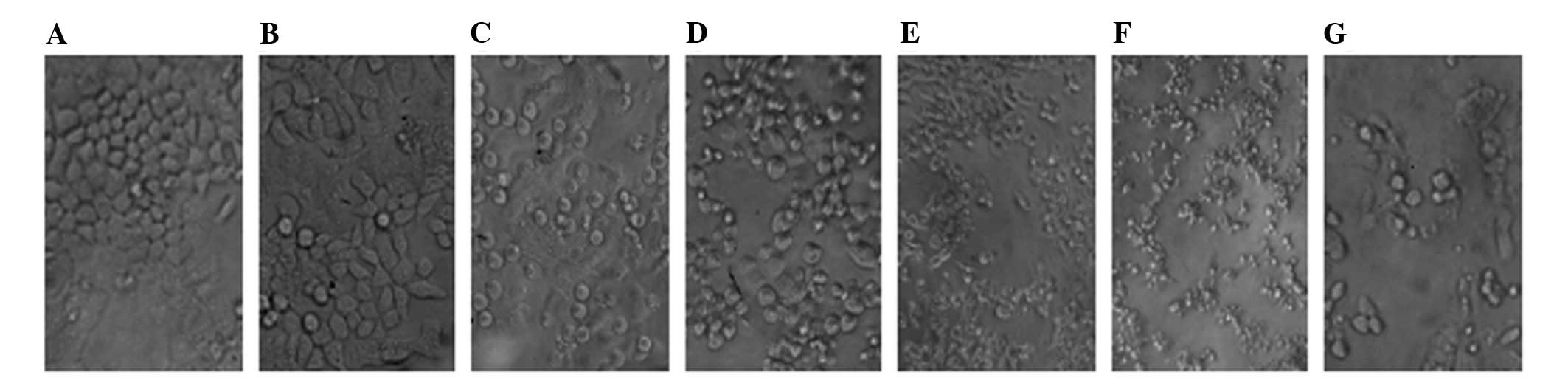
Cell morphology
Cell morphologic changes were clearly observed using
an inverted microscope. With an increase in time or increase in
concentration of the chemotherapy drug, the HT-29 cell membranes
gradually became blurred, rounding was reduced; cells were shrunken
and rounded to malapposition and even death, while changes were
more marked when the chemotherapy drug was combined with an
inhibitor (Fig. 1).
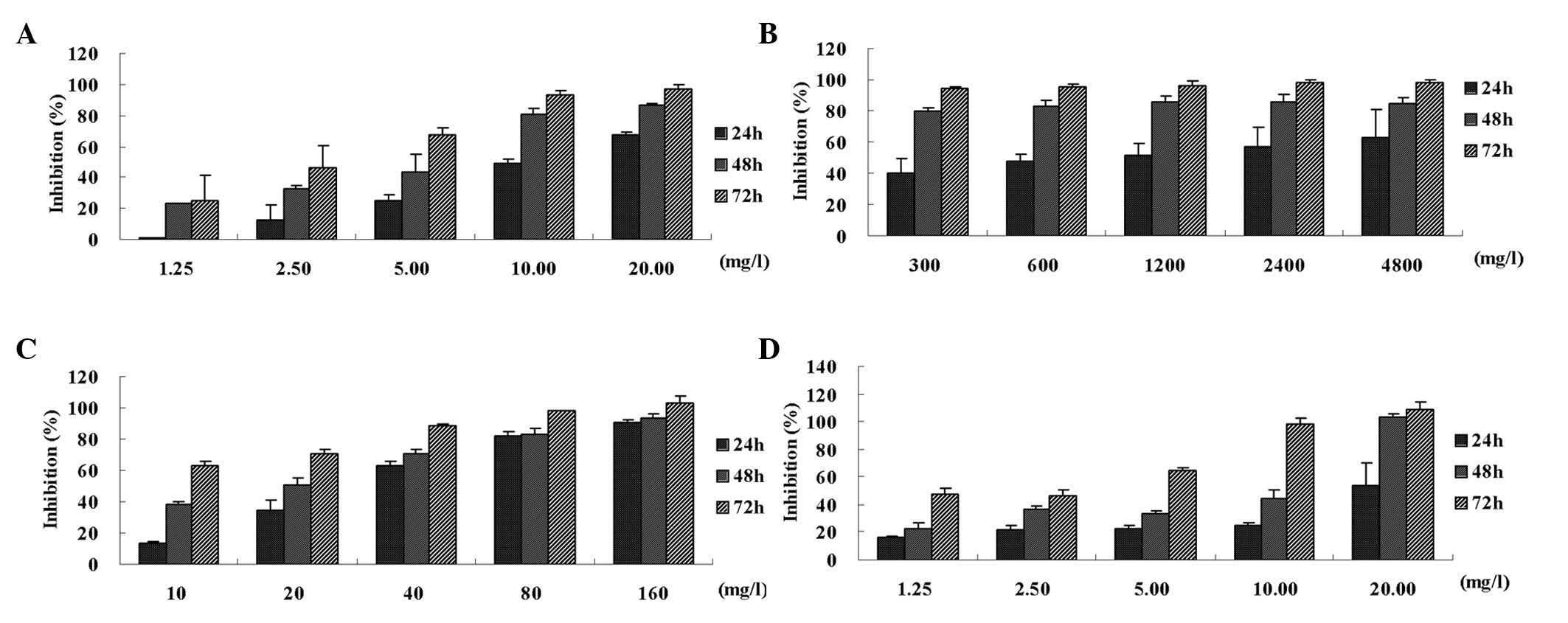
Chemotherapy-induced growth inhibition
and apoptosis in the HT-29 cells
Chemotherapy drugs are able to inhibit cell
proliferation and promote apoptosis and NF-κB inhibitors are
capable of enhancing chemotherapy-induced growth inhibition and
apoptosis of HT-29 cells. As2O3, oxaliplatin
and paclitaxel inhibited cell proliferation in a time- and
concentration-dependent manner (P<0.05), while 5FU inhibited
cell proliferation in a time-dependent manner (P<0.05; Fig. 2). The chemotherapy drugs promoted
apoptosis in a time-dependent manner. NF-κB inhibitors enhanced
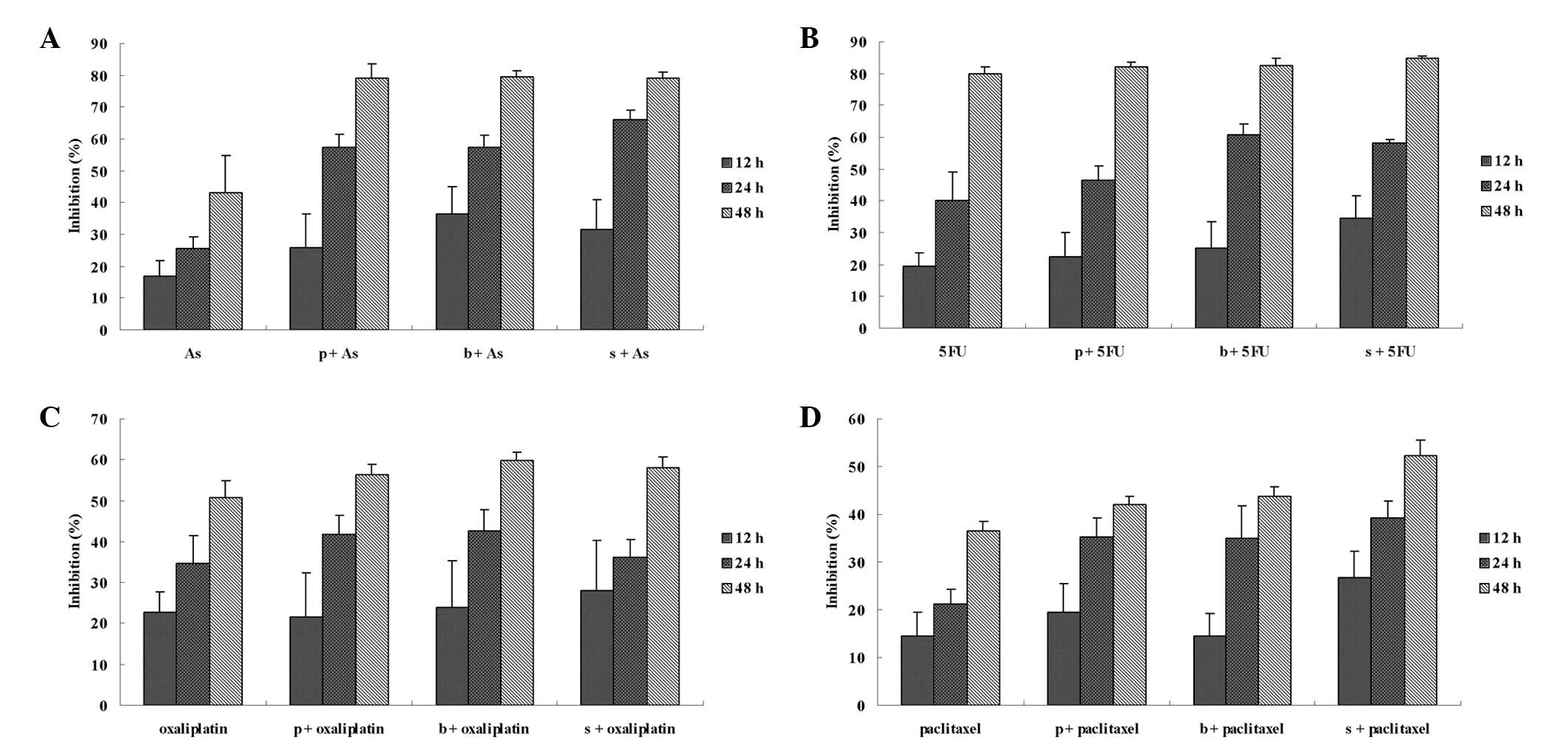
chemotherapy drug-mediated growth inhibition (Fig. 3) and apoptosis induction (Fig. 4).
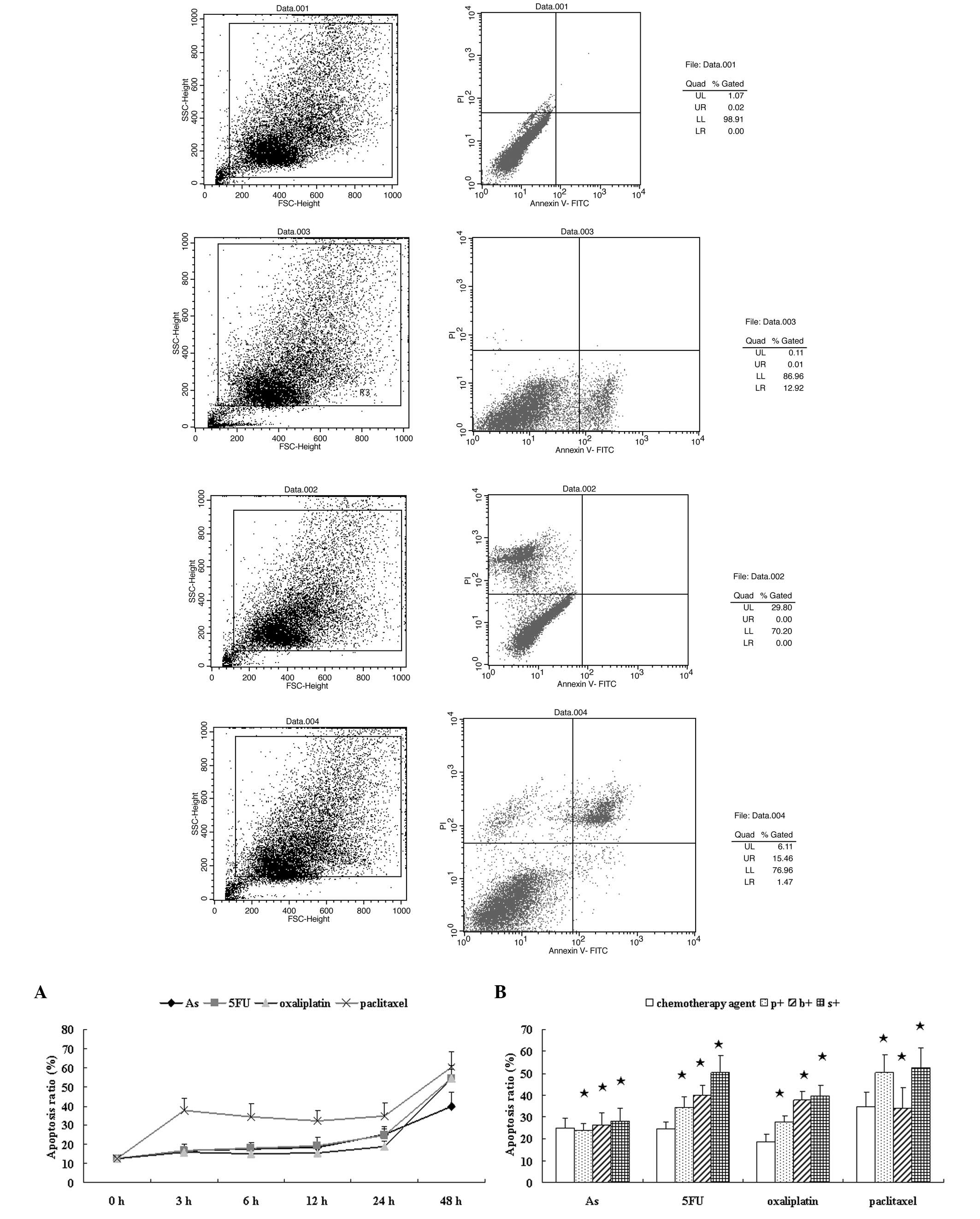 | Figure 4Effects of a chemotherapy drug alone
or in combination with an NF-κB inhibitor on the apoptosis of HT-29
cells. (A) HT-29 cells were incubated with 5 mg/l
As2O3, 300 mg/l 5FU, 20 mg/l oxaliplatin, 2.5
mg/l paclitaxel alone for 3, 6, 12, 24 and 48 h. (B) Cells were
incubated with a chemotherapy drug alone for 24 h or combined with
an NF-κB inhibitor: 50 μmol/l PDTC (p+), 100 nmol/l bortezomib (b+)
or 12.5 mg/l SN50 (s+) for 24 h, and then Annexin fluorescence
intensity was determined by FCM. As2O3, 5FU,
oxaliplatin and paclitaxel induced cell apoptosis from the start of
treatment. At 3, 6, 12 and 24 h the apoptosis rate continued; at 48
h the apoptosis rate increased suddenly, but late apoptosis was
noted. NF-κB inhibitors enhanced chemotherapy-induced apoptosis
when compared with the chemotherapy drug alone at 24 h;
*P>0.05. NF-κB, nuclear factor-κB; As,
As2O3; 5FU, fluorouracil; PDTC, pyrrolidine
dithiocarbamate; p+, chemotherapy drug combined with PDTC; b+,
chemotherapy drug combined with bortezomib; s+, chemotherapy drug
combined with SN50; FCM, flow cytometry. |
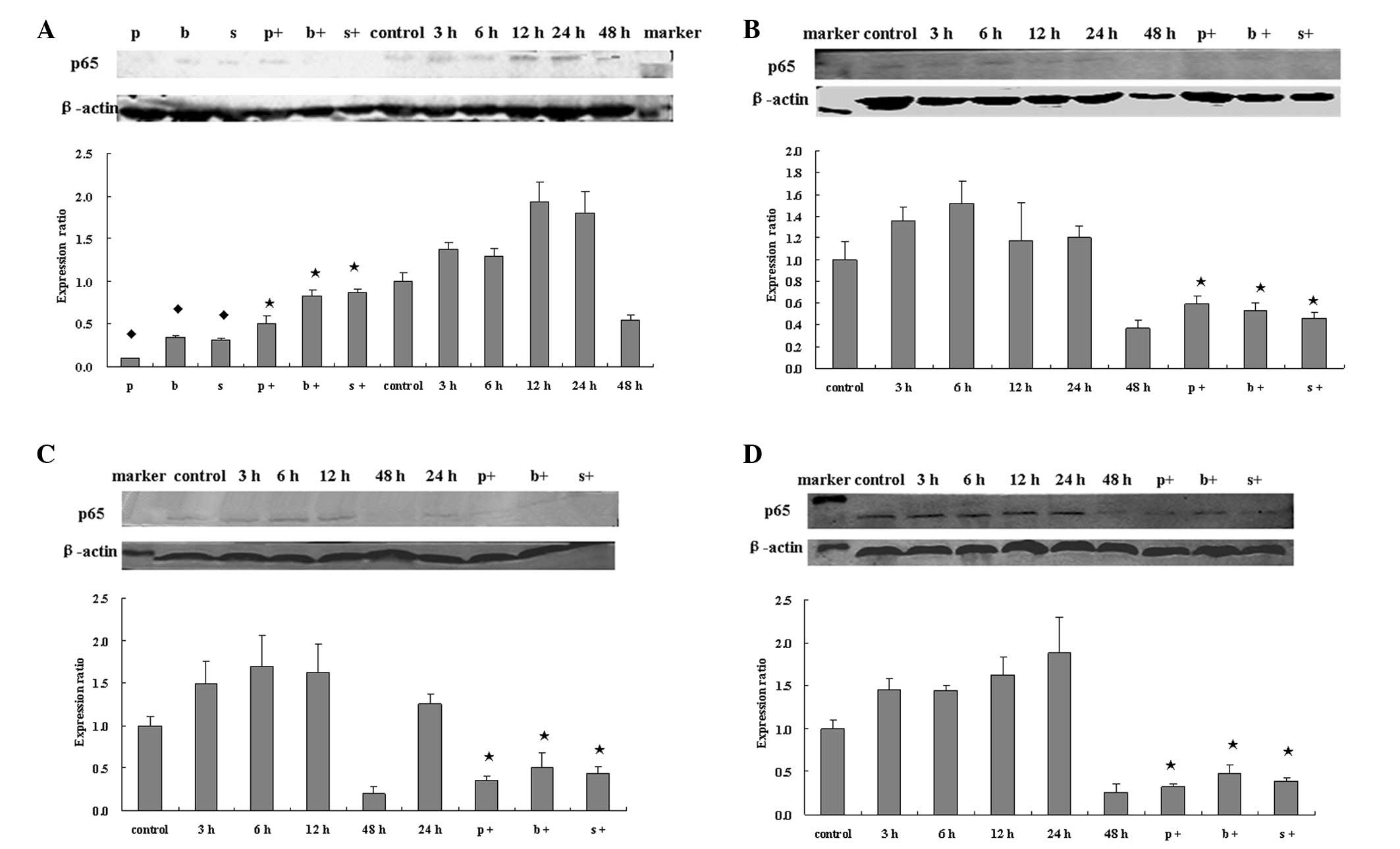
NF-κB activity
NF-κB activity was activated by chemotherapy drugs
and was reduced by NF-κB inhibitors. The expression of total NF-κB
p65 was detected by western blotting and that of nuclear
translocated activated p65 by EMSA (Figs. 5 and 6). Both levels increased following
treatment with a chemotherapy drug for 3, 6, 12 and 24 h, but were
weakened at 48 h. p65 expression was significantly inhibited
compared with chemotherapy drug treatment alone.
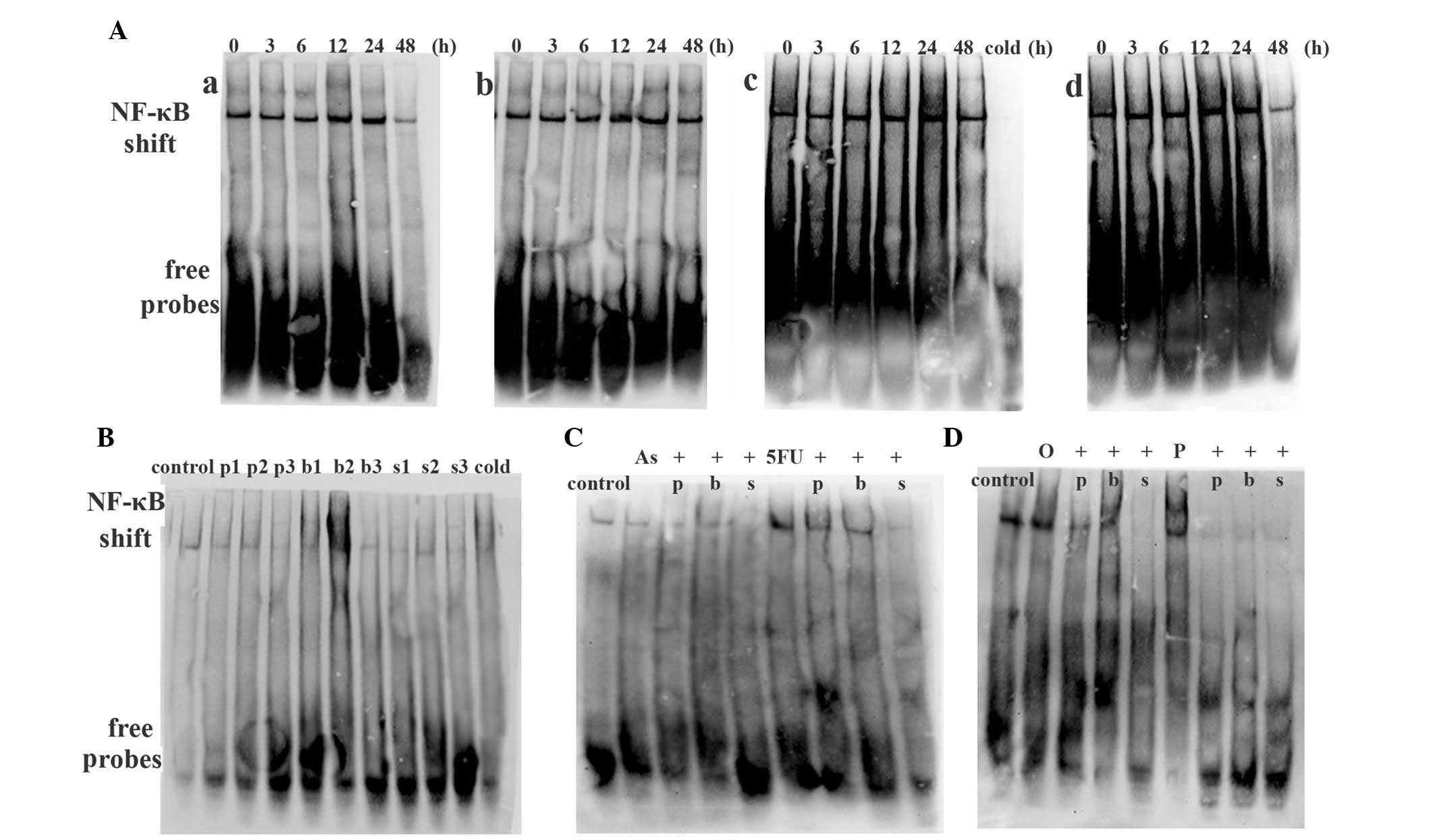 | Figure 6Expression of NF-κB in HT-29 cells as
detected by EMSA. (A) Chemotherapy drug activated NF-κB activity:
(a) 5 mg/l As2O3 alone for 3, 6, 12, 24 and
48 h; (b) 300 mg/l 5FU alone for 3, 6, 12, 24 and 48 h; (c) 20 mg/l
oxaliplatin alone for 3, 6, 12, 24 and 48 h and a cold competition
control; (d) 2.5 mg/l paclitaxel alone for 3, 6, 12, 24 and 48 h.
(B) NF-κB inhibitors [50 μmol/l PDTC (p), 100 nmol/l bortezomib (b)
or 12.5 mg/l SN50 (s)] decreased NF-κB activity. ‘1, 2, 3’ denote
6, 12 and 24 h respectively. (C) The effect of NF-κB inhibitors on
As2O3- or 5FU-treated cells. (D) The effect
of NF-κB inhibitors on oxaliplatin- or paclitaxel-treated cells.
The untreated HT-29 cells or induced by a chemotherapy drug
obtained p65 activity, and NF-κB inhibitors inhibited the NF-κB
activity induced by the chemotherapy drug. As,
As2O3; 5FU, fluorouracil; O, oxaliplatin; P,
paclitaxel. EMSA, electrophoretic mobility shift assay; NF-κB,
nuclear factor-κB. |
Discussion
Colorectal cancer is a common malignancy and is
strongly associated with a Western lifestyle. In the past several
decades, much has been learned about the dietary, lifestyle and
medical risk factors for this malignancy (9,10).
It is the third leading cause of cancer-related death in
individuals of each gender and the second overall in men and women
combined in the US (11). In
China, with the improvement of living standards and changes in
diet, the incidence of colorectal cancer has gradually increased,
but half of colorectal cancer treatment fails. Even in regions with
improved living conditions the overall 5-year survival rate is
approximately 30%.
NF-κB is an important nuclear transcription factor,
comprised of a complex system. It exists in a variety of cells, and
plays a great role in the gene regulation of inflammation, immune
response, cell proliferation and apoptosis (12). In resting cells, NF-κB is
sequestered in the cytoplasm in association with inhibitory
proteins IκB which cover the nuclear localization signal (NLS) of
NF-κB. When cells are subjected to a variety of stimuli, including
bacteria or virus infection, inflammatory cytokines, TNF, LPS,
ultraviolet ray and ionizing radiation, IκB is phosphorylated,
ubiquitinated and is quickly degraded by proteasomes, NF-κB is
released and activated, then translocates into the nucleus and
binds to the promoter region of target genes to regulate a series
of gene expression patterns involved in the control of different
cellular responses (13,14).
The anti-apoptotic ability of NF-κB was identified
by chance. Beg et al (15)
studied the function of NF-κB using gene knockout mice, RelA was
knocked out from embryonic stem cells to study the influence on
mouse survival, and the embryos died on the 15th or 16th day;
autopsies and pathological inspection revealed a large quantity of
liver cell apoptosis. The identification of NF-κB involvement in
cell apoptosis has aroused great interest. Numerous groups are
currently researching NF-κB and have found that NF-κB plays a key
role in cancer anti-apoptotic mechanisms (3,16,17);
Wu et al (18) found that
adenosine arrested hepatocellular carcinoma cells in the G0–G1
phase of the cell cycle, enhanced the activity of caspase-3 and
upregulated p53, but at the same time upregulated NF-κB p65
expression and downregulated Bcl-2 expression. NF-κB inhibition of
PDTC decreased p65 expression, enhanced cell apoptosis ratio and
increased caspase-3 activity. NF-κB may play an anti-apoptotic role
in adenosine-induced HepG2 cytotoxicity; Furuta et al
(19) applied NBD peptide which
disrupted the association of NF-κB essential modulator (NEMO) with
IκB kinases on oral squamous cell carcinoma, and the conclusion was
that NBD peptide treatment inhibited TNFα-induced, and
constitutive, NF-κB activation, increased apoptosis and suppressed
proliferation. Zhu et al (20) investigated the antitumor effects of
the NF-κB inhibitor SN50 in gastric carcinoma SGC-7901 cells and
revealed that NF-κB inhibition triggers an impairment of cell
proliferation and the induction of apoptosis of cancer cells.
Blocking NF-κB may increase the expression of p53 and induce
pro-apoptotic and autophagic proteins.
Many different sites may be exploited to block NF-κB
activation in the NF-κB pathway. PDTC is a type of metal chelating
agent and antioxidant. It inhibits the release of the IκB subunit
from the cytoplasm and prevents the separation between IκB and
NF-κB to inhibit the activation of NF-κB (21). Proteasome inhibitor bortezomib
inhibits IκB degradation following phosphorylation and
ubiquitination (22,23) and SN50 inhibits coupling between
NF-κB and the effective DNA (24).
The effect site of each of the inhibitors is closer, sequentially,
to the terminal of the NF-κB pathway and the specificity increases
accordingly.
Different chemotherapy drugs have their own
mechanisms. The mechanism of As2O3 is
unclear, but it induces apoptosis and inhibits telomerase activity
to inhibit cell division; 5FU is classified as an antimetabolite
which is a cell-cycle-specific chemotherapy drug and attacks cells
at specific phases in the cycle. 5FU and its metabolites are
similar to normal substances within the cell. When they are
incorporated into cells, they inhibit essential biosynthetic
processes, or are incorporated into the macromolecular DNA and RNA
to inhibit their normal fuction. Oxaliplatin is an alkylating agent
which is cell-cycle non-specific and is most active in the resting
phase of the cell. It forms a coordination metal salt complex and
inhibits DNA synthesis in cancer cells. Paclitaxel is a taxane
plant alkaloid and an antimicrotubule agent which is cell-cycle
specific and attacks cells during various phases of division. It
stabilizes the microtubule structures and inhibits spindle
formation, which are part of the cell division and replication
apparatus, resulting in cell death.
In our study, we applied
As2O3, 5FU, oxaliplatin, paclitaxel alone or
combined with PDTC, bortezomib or SN50 to the colon cancer cell
line HT-29. We confirmed that As2O3,
oxaliplatin and paclitaxel inhibited cell proliferation in a time-
and concentration-dependent manner, while 5FU only inhibited cell
proliferation in a time-dependent manner (Fig. 2). NF-κB inhibitors had enhanced
chemotherapy-mediated growth inhibition (Fig. 3). The cell apoptosis rate was also
higher when the chemotherapy drug was combined with an NF-κB
inhibitor. The inhibitors, 50 μmol/l PDTC, 100 nmol/l bortezomib
and 12.5 mg/l SN50, suppressed the NF-κB expression of the tumor
cells themselves, which was stimulated by chemotherapy (P<0.05).
The result of NF-κB nuclear transfer tested by EMSA was consistent
with the total protein expression tested by western blotting.
Therefore, we come to the conclusion that the NF-κB inhibitors,
PDTC, bortezomib and SN50, inhibit NF-κB activation and improve the
cell inhibition rate and apoptosis ratio to influence the effect of
chemotherapy on HT-29 cells. The NF-κB protein expression was
inhibited by NF-κB inhibitors significantly compared with the
chemotherapy drugs (P<0.05), while the cell inhibition rate and
apoptosis ratio were improved (P>0.05).
When 5 mg/l As2O3, 300 mg/l
5FU, 20 mg/l oxaliplatin or 2.5 mg/l paclitaxel was applied to
HT-29 cells alone, the total protein expression of NF-κB was
increased, and the highest increase was 1.93±0.23, 1.51±0.21,
1.70±0.37 and 1.88±0.41 times, respectively. The inhibitors, 50
μmol/l PDTC, 100 nmol/l bortezomib, 12.5 mg/l SN50, suppressed the
NF-κB expression which was stimulated by the tumor cells
themselves; the degrees of inhibtion were 0.11±0.00, 0.35±0.01 and
0.31±0.03 times, respectively (P<0.05). NF-κB inhibitors were
able to inhibit the NF-κB expression which was stimulated by
chemotherapy (P<0.05). The result of NF-κB nuclear transfer
tested by EMSA was consistent with the total protein expression
tested by western blotting.
We conclude that the NF-κB inhibitors, PDTC,
bortezomib and SN50, inhibit NF-κB activation, improve the cell
inhibition rate and apoptosis ratio to influence the effect of
chemotherapy on HT-29 cells. The NF-κB protein expression was
inhibited by NF-κB inhibitors significantly compared with the
chemotherapy drugs (P<0.05), while the cell inhibition rate and
apoptosis ratio were improved (P>0.05). This differs from other
research, maybe due to the effect time, dose or the two combined.
Therefore, the best concentration and incubation time of the NF-κB
inhibitor, and whether the effect would be improved when cells had
acquired chemotherapy drug resistance, require further
investigation.
Acknowledgements
We would like to thank the National
Natural Science Foundation of China, no. 30871152 for supporting
our study.
References
|
1
|
Sen R and Baltimore D: Multiple nuclear
factors interact with the immunoglobulin enhancer sequences. Cell.
46:705–716. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zandi E and Karin M: Bridging the gap:
composition, regulation, and physiological function of the IkappaB
kinase complex. Mol Cell Biol. 19:4547–4551. 1999.PubMed/NCBI
|
|
3
|
Baldwin AS: Control of oncogenesis and
cancer therapy resistance by the transcription factor NF-kappaB. J
Clin Invest. 3:241–246. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gottesman MM, Fojo T and Bates SE:
Multidrug resistance in cancer: role of ATP-dependent transporters.
Nat Rev Cancer. 2:48–58. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang CY, Guttridge DC, Mayo MW and Baldwin
AS Jr: NF-kappaB induces expression of the Bcl-2 homologue A1/Bfl-1
to preferentially suppress chemotherapy-induced apoptosis. Mol Cell
Biol. 19:5923–5929. 1999.PubMed/NCBI
|
|
6
|
Wang CY, Mayo MW, Korneluk RG, Goeddel DV
and Baldwin AS Jr: NF-kappaB antiapoptosis: induction of TRAF1 and
TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation.
Science. 281:1680–1683. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Karin M: Nuclear factor-kappaB in cancer
development and progression. Nature. 441:431–436. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mitsiades N, Mitsiades CS, Poulaki V,
Anderson KC and Treon SP: Intracellular regulation of tumor
necrosis factor-related apoptosis-inducing ligand-induced apoptosis
in human multiple myeloma cells. Blood. 99:2162–2171. 2002.
View Article : Google Scholar
|
|
9
|
Poynter JN, Haile RW, Siegmund KD,
Campbell PT, Figueiredo JC, Limburg P, Young J, Le Marchand L,
Potter JD, Cotterchio M, Casey G, Hopper JL, Jenkins MA, Thibodeau
SN, Newcomb PA and Baron JA; Colon Cancer Family Registry:
Associations between smoking, alcohol consumption, and colorectal
cancer, overall and by tumor microsatellite instability status.
Cancer Epidemiol Biomarkers Prev. 18:2745–2750. 2009. View Article : Google Scholar
|
|
10
|
Bostick RM, Potter JD, Kushi LH, Sellers
TA, Steinmetz KA, McKenzie DR, Gapstur SM and Folsom AR: Sugar,
meat and fat intake, and non-dietary risk factors for colon cancer
incidence in Iowa women. Cancer Causes Control. 5:38–52. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chan AT and Giovannucci EL: Primary
prevention of colorectal cancer. Gastroenterology. 138:2029–2043.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim HJ, Hawke N and Baldwin AS: NF-kappaB
and IKK as therapeutic targets in cancer. Cell Death Differ.
13:738–747. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Legrand-Poels S, Schoonbroodt S, Matroule
JY and Piette J: NF-kappaB: an important transcription factor in
photobiology. Photochem Photobiol B. 45:1–8. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Romano MF, Lamberti A, Bisogni R, Tassone
P, Pagnini D, Storti G, Del Vecchio L, Turco MC and Venuta S:
Enhancement of cytosine arabinoside induced apoptosis in human
myeloblastic leukemia cells by NF kappaB/Rel-specific decoy
oligodeoxy-nucleotides. Gene Ther. 7:1234–1237. 2000. View Article : Google Scholar
|
|
15
|
Beg AA, Sha WC, Bronson RT, Ghosh S and
Baltimore D: Embryonic lethality and liver degeneration in mice
lacking the RelA component of NF-kappaB. Nature. 376:167–170. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Holmes-McNary M and Baldwin AS Jr:
Chemopreventive properties of trans-resveratrol are associated with
inhibition of activation of IkappaB kinase. Cancer Res.
60:3477–3483. 2000.PubMed/NCBI
|
|
17
|
Yemelyanov A, Gasparian A, Lindholm P,
Dang L, Pierce JW, Kisseljov F, Karseladze A and Budunova I:
Effects of IKK inhibitor PS1145 on NF-kappaB function,
proliferation, apoptosis and invasion activity in prostate
carcinoma cells. Oncogene. 25:387–398. 2006.PubMed/NCBI
|
|
18
|
Wu LF, Li GP, Su JD, Pu ZJ, Feng JL, Ye YQ
and Wei BL: Involvement of NF-kappaB activation in the apoptosis
induced by extracellular adenosine in human hepatocellular
carcinoma HepG2 cells. Biochem Cell Biol. 88:705–714.
2010.PubMed/NCBI
|
|
19
|
Furuta H, Osawa K, Shin M, Ishikawa A,
Matsuo K, Khan M, Aoki K, Ohya K, Okamoto M, Tominaga K, et al:
Selective inhibition of NF-κB suppresses bone invasion by oral
squamous cell carcinoma in vivo. Int J Cancer. Jan 19–2012.(Epub
ahead of print).
|
|
20
|
Zhu BS, Xing CG, Lin F, Fan XQ, Zhao K and
Qin ZH: Blocking NF-κB nuclear translocation leads to p53-related
autophagy activation and cell apoptosis. World J Gastroenterol.
17:478–487. 2011.
|
|
21
|
Si X, McManus BM, Zhang JC, Yuan J, Cheung
C, Esfandiarei M, Suarez A, Morgan A and Luo HL: Pyrrolidine
dithiocarbamate reduces coxsackievirus B3 replication through
inhibition of the ubiquitin-proteasome pathway. J Virol.
79:8014–8023. 2005. View Article : Google Scholar
|
|
22
|
Caravita T, de Fabritiis P, Palumbo A,
Amadori S and Boccadoro M: Bortezomib: efficacy comparisons in
solid tumors and hematologic malignancies. Nat Clin Pract Oncol.
3:374–387. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nencioni A, Grünebach F, Patrone F,
Ballestrero A and Brossart P: Proteasome inhibitors: antitumor
effects and beyond. Leukemia. 21:30–36. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Orange JS and May MJ: Cell penetrating
peptide inhibitors of nuclear factor-kappaB. Cell Mol Life Sci.
65:3564–3591. 2008. View Article : Google Scholar : PubMed/NCBI
|