Introduction
Colorectal cancer is the world’s third most common
malignant tumor, after lung and breast cancer and is associated
with a poor prognosis (1). The
incidence of colorectal cancer is rising (2) and patients often present in the later
stages. Tumor invasion and metastasis are the leading cause of
death in colorectal cancer, therefore identification of those
factors that regulate colorectal cancer metastasis, prognosis and
optimal treatment are of great significance. The activation of
oncogenes and the inactivation or loss of function of
tumor-suppressor genes are important steps in colorectal
carcinogenesis; however, to date, no sensitive and specific
biological indicators of malignancy and prognosis exist for
colorectal cancer.
Tumor development, invasion and metastasis are
dependent on signaling between cells and lymphangiogenesis. In
recent years, the effect of deletion of the chromosome 10
phosphatase and tensin homolog gene (PTEN), and expression of
signal transducer and activator of transcription-3 (STAT3) and
vascular endothelial growth factor-C (VEGF-C) have been
investigated in malignant tumors (3,4).
PTEN exerts protein phosphatase activity to inhibit Ras-mediated
MAPK signaling cascades, thereby reducing STAT3 transcriptional
activity and inhibiting cell migration and invasion (3). Klatte et al proposed that PTEN
inhibits the expression of VEGF-C in renal cell carcinoma (4), while Lee et al demonstrated
that VEGF-C is a downstream target gene under the regulation of
STAT3 (5).
Despite these advances, the role of PTEN, STAT3 and
VEGF-C in colorectal cancer has yet to be reported. In the present
study, we examined PTEN, STAT3 and VEGF-C protein expression in
colorectal cancer and analyzed their prognostic ability.
Materials and methods
Colorectal tumor samples
We selected tissue samples from 68 cases of
colorectal adenocarcinoma undergoing radical surgery at the First
Affiliated Hospital of Henan University of Science and Technology
between June 2004 and October 2005. Written informed consent was
obtained from each patient and Ethics Committee approval from our
institution was obtained. All specimens were confirmed by
pathology, and complete clinical data and follow-up records were
available to the follow-up deadline of December 2010. For all
patients, surgery was the first treatment for cancer. In total,
there were 40 males and 28 females, aged between 32 and 78 years;
median age was 57 years. A total of 39 patients received a FOLFOX4
chemotherapy scheme after surgery and completed the chemotherapy,
12 patients did not have chemotherapy and 17 only took it
irregularly. Normal control colorectal tissues were obtained from
20 individuals during surgery for other diseases.
Reagents
The Universal SP immunohistochemistry kit, DAB kit
color, mouse anti-human PTEN monoclonal antibodies, rabbit
anti-STAT3 monoclonal antibodies and rabbit anti-human VEGF-C
polyclonal antibody were purchased from Sigma, St. Louis, MO,
USA.
Immunohistochemistry
Immunohistochemistry was performed using the
Universal SP immunohistochemistry kit following the manufacturer’s
instructions, followed by moderate hematoxylin staining,
dehydration in a graded ethanol series and xylene, after which
sections were mounted with neutral gum and observed by light
microscopy. The negative control was performed with PBS instead of
the primary antibody. The number of positive cells and color
intensity was scored using a semi-quantitative method from 5
high-power fields of view (6).
Clear brown particles appearing in the cytoplasm or nucleus were
recorded as positive cells, and the percentage of positive cells
was scored as follows: 0, <10% of cells; 1, 10–49% of cells; 2,
50–74% of cells; 3, >75% of cells. Staining intensity was scored
as follows: 0, colorless; 1, light yellow; 2, brown; 3, tan. The
scores for the number of stained cells and staining intensity were
added together: Scores of 0–2 were considered negative and scores
>2 were considered positive.
Prognostic analysis
Follow-up data from the 68 colorectal cancer
patients were analyzed and summarized. Time from surgery to patient
mortality was recorded as the survival time. Kaplan-Meier survival
curves were used to calculate 3-and 5-year survival rates, and
univariate analysis was used to analyze the independent prognostic
ability of PTEN, STAT3 and VEGF-C.
Statistical analysis
SPSS 17.0 was used for statistical analysis.
Numerical data were analyzed by the χ2 test and pairwise
comparisons of multiple samples were analyzed by the segmentation
method. One-way ANOVA was used to analyze the measurement data.
Spearman’s rank correlation was used to analyze the correlation
between PTEN, STAT3 and VEGF-C expression. The log-rank test was
used to determine significant differences between survival rates.
P-values of <0.05 were considered indicative of statistical
significance.
Results
Relationship between PTEN, STAT3 and
VEGF-C expression and clinicopathological features of colorectal
carcinoma
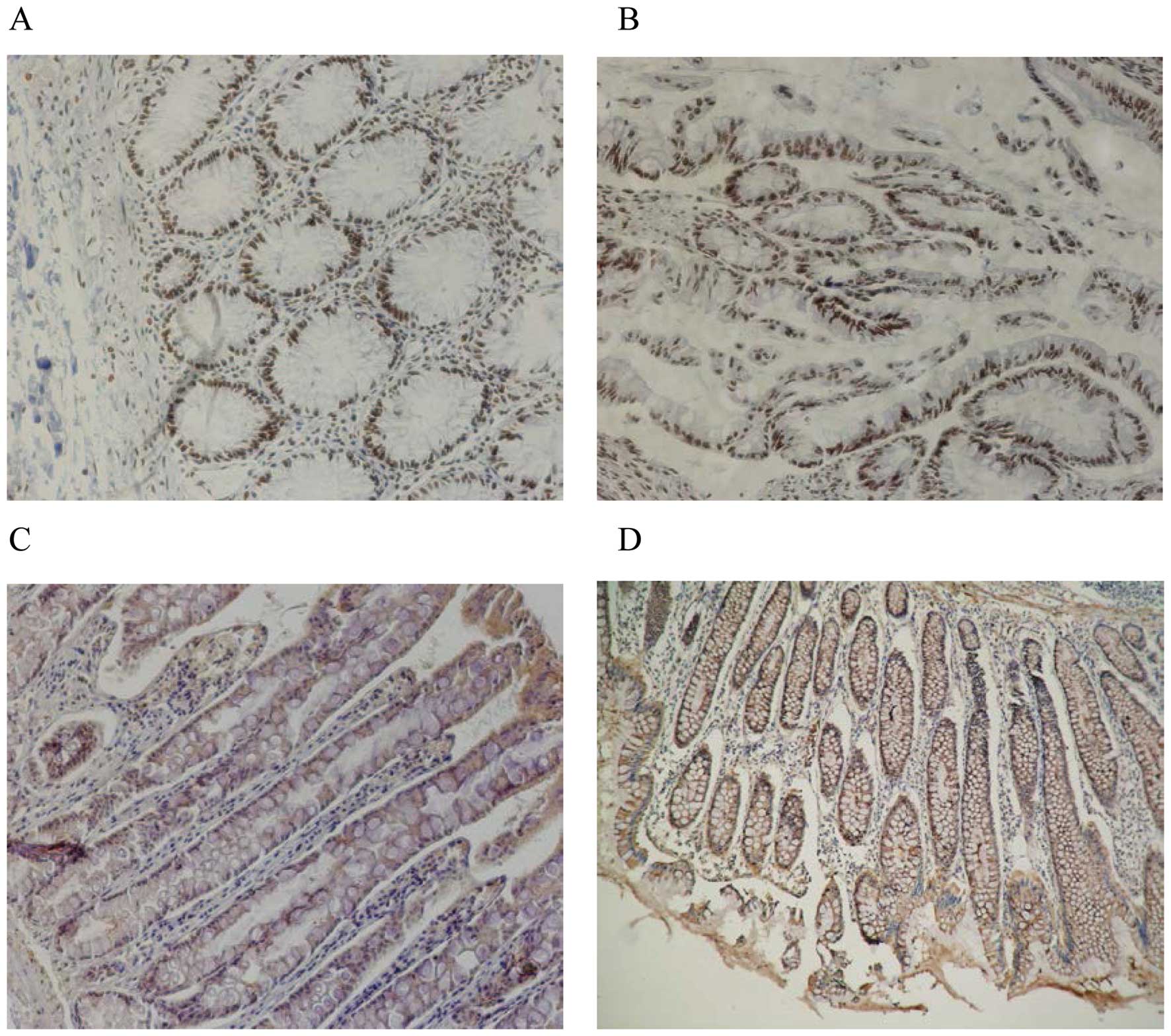
PTEN protein expression was located in the nucleus,
and STAT3 and VEGF-C protein expression was located in the
cytoplasm, as yellow or brown granules (Fig. 1). The expression rates of PTEN,
STAT3 and VEGF-C were 32.4, 60.3 and 63.2% in colorectal cancer
samples, and 90.0, 0 and 0% in normal tissues, respectively; these
differences were significant (p<0.05). Expression of PTEN and
STAT3 protein was significantly related to the pathological grade
of colorectal cancer (p=0.011 and 0.001, respectively) with no
relationship between PTEN and STAT3 expression and tumor size,
lymph node metastasis or clinical (TNM) stage. Expression of VEGF-C
protein was significantly related to lymph node metastasis in
colorectal cancer (p=0.002); with no relationship between VEGF-C
and tumor size, pathological grade or clinical stage (Table I).
 | Table IRelationship between
clinicopathological features and expression of PTEN, STAT3 and
VEGF-C in colorectal carcinoma. |
Table I
Relationship between
clinicopathological features and expression of PTEN, STAT3 and
VEGF-C in colorectal carcinoma.
| | PTEN expression
| STAT3 expression
| VEGF-C expression
|
|---|
| Characteristics | n | n, rate (%) | P-value | n, rate (%) | P-value | n, rate (%) | P-value |
|---|
| Tumor size (cm) | | | | | | | |
| ≤4 | 30 | 10 (33.3) | 0.878 | 17 (56.7) | 0.587 | 18 (60.0) | 0.632 |
| >4 | 38 | 12 (40.0) | | 24 (63.1) | | 25 (65.8) | |
| Grade | | | | | | | |
| Middle/low | 48 | 20 (41.6) | 0.011 | 23 (47.9) | 0.001 | 29 (60.4) | 0.662 |
| High | 20 | 2 (10.0) | | 18 (90.0) | | 14 (70.0) | |
| Node metastasis | | | | | | | |
| Positive | 38 | 10 (26.3) | 0.231 | 26 (68.4) | 0.123 | 30 (78.9) | 0.002 |
| Negative | 30 | 12 (40.0) | | 15 (50.0) | | 13 (43.3) | |
| Clinical stage | | | | | | | |
| I–II | 32 | 12 (37.5) | 0.392 | 18 (56.3) | 0.520 | 19 (59.4) | 0.534 |
| III–IV | 36 | 10 (27.8) | | 23 (63.8) | | 24 (66.7) | |
Correlation between PTEN, STAT3 and
VEGF-C expression in colorectal carcinoma
In colorectal carcinoma, PTEN and STAT3 protein
expression was significantly negatively correlated (r=−0.402,
p=0.001) as was PTEN and VEGF-C protein expression (r=−0.320,
p=0.008). STAT3 and VEGF-C protein expression was positively
correlated (r=0.254, p=0.036).
Relationship of PTEN protein expression
with colorectal cancer prognosis
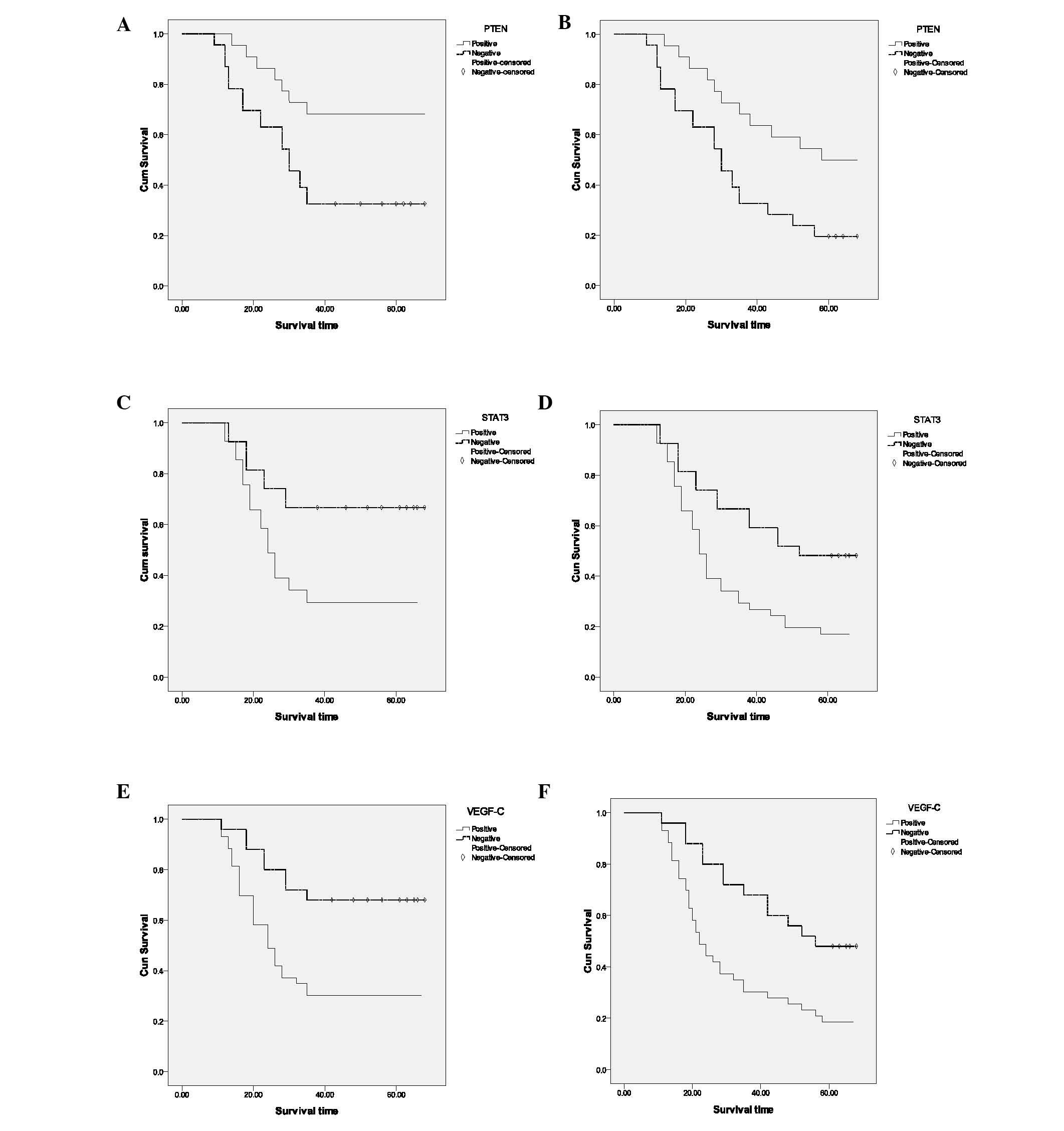
The number of deaths and survival rate in PTEN
protein-positive and -negative patients at 3 and 5 years are
indicated in Table II. Median
survival times for PTEN protein positive and negative patients were
38 and 23 months, respectively. Kaplan-Meier single factor survival
analysis and the log-rank test indicated that the 3- and 5-year
survival rates of PTEN protein-positive colorectal cancer patients
were significantly higher than PTEN protein-negative patients
(p=0.008, Fig. 2A and B).
 | Table IIRelationship of PTEN, STAT3 and VEGF-C
protein expression with colorectal cancer prognosis. |
Table II
Relationship of PTEN, STAT3 and VEGF-C
protein expression with colorectal cancer prognosis.
| | 3-year
| 5-year
| |
|---|
| Index | n | Mortality | Survival | % | Mortality | Survival | % | Median (months) |
|---|
| PTEN | | | | | | | | |
| Positive | 22 | 7 | 15 | 68.1 | 11 | 11 | 50.0 | 38 |
| Negative | 46 | 31 | 15 | 32.6 | 37 | 9 | 19.6 | 23 |
| STAT3 | | | | | | | | |
| Positive | 41 | 29 | 12 | 29.3 | 34 | 7 | 17.1 | 21 |
| Negative | 27 | 9 | 18 | 66.7 | 14 | 13 | 48.1 | 29 |
| VEGF-C | | | | | | | | |
| Positive | 43 | 30 | 13 | 30.2 | 35 | 8 | 18.6 | 20 |
| Negative | 25 | 8 | 17 | 68.0 | 13 | 12 | 48.0 | 28 |
Relationship of STAT3 protein expression
with colorectal cancer prognosis
The number of deaths and survival in STAT3
protein-positive and -negative patients at 3 and 5 years are
indicated in Table II. Median
survival times for STAT3 protein-positive and -negative patients
were 21 and 29 months, respectively. Kaplan-Meier single factor
survival analysis and the log-rank test indicated that the 3- and
5-year survival rates of STAT3 protein-positive colorectal cancer
patients were significantly lower than STAT3 protein-negative
patients (p=0.005, Fig. 2C and
D).
Relationship of VEGF-C protein expression
with colorectal cancer prognosis
The number of deaths and survival in VEGF-C
protein-positive and -negative patients at 3 and 5 years are shown
in Table II. The median survival
times for VEGF-C protein-positive and -negative patients were 20
and 28 months, respectively. Kaplan-Meier single factor survival
analysis and the log-rank test indicated that the 3- and 5-year
survival rates of VEGF-C protein-positive colorectal cancer
patients were significantly lower than those of VEGF-C
protein-negative patients (p=0.003, p=0.004, respectively; Fig. 2E and F).
Prognostic factors in colorectal
cancer
The 3- and 5-year survival rates and related
prognostic factors were analyzed using Kaplan-Meier univariate
survival analysis and the log-rank test. Pathological grade,
clinical stage, and the presence of visible residual tumor and
completion of regular chemotherapy after surgery were prognostic
factors in colorectal carcinoma (Table
III). To further determine the effects of independent
prognostic factors in colorectal cancer, pathological grade,
clinical stage, PTEN, STAT3 and VEGF-C protein expression,
post-operative chemotherapy and residual tumor after surgery were
analyzed using multivariate survival analysis. Clinical stage,
postoperative chemotherapy, VEGF-C protein expression and
histological grade were independent prognostic factors in
colorectal cancer, while presence of residual tumor after surgery
and PTEN and STAT3 protein expression were not independent
prognostic factors (Table IV).
 | Table IIISurvival rates of 68 patients with
colorectal cancer stratified according to various factors. |
Table III
Survival rates of 68 patients with
colorectal cancer stratified according to various factors.
| Stratification | n | 3-year (%) | 5-year (%) |
|---|
| Grade | | | |
| High | 20 | 30.0 | 10.0 |
| Middle/low | 48 | 50.0 | 37.5 |
| Clinical stage | | | |
| I | 13 | 100 | 76.9 |
| II | 19 | 68.4 | 52.6 |
| III | 24 | 45.8 | 29.1 |
| IV | 12 | 16.7 | 8.3 |
| Residual tumor | | | |
| 0 cm | 26 | 73.0 | 65.4 |
| <2 cm | 30 | 40.0 | 16.7 |
| ≥2 cm | 12 | 19.0 | 9.8 |
| Chemotherapy | | | |
| No | 12 | 16.7 | 8.3 |
| Regular | 39 | 56.4 | 48.7 |
| Irregular | 17 | 35.3 | 11.8 |
 | Table IVIndependent risk coefficients of
independent prognostic factors in colorectal carcinoma determined
using multivariate survival analysis. |
Table IV
Independent risk coefficients of
independent prognostic factors in colorectal carcinoma determined
using multivariate survival analysis.
| Factor | Coefficient |
|---|
| Stage | 2.128 |
| Grade | 1.765 |
| Postoperative
chemotherapy | 0.610 |
| VEGF-C
expression | 0.512 |
Discussion
Numerous studies have investigated the prognostic
factors in colorectal cancer; however, many studies have
concentrated on a limited number of clinical stages, pathological
grades or traditional prognostic factors, and have failed to
correlate clinical stage with prognosis or make accurate prognostic
judgments. Identification of new molecular biological markers to
predict metastasis and recurrence is of major interest in cancer
research. This study was designed to identify the correlation
between PTEN, STAT3 and VEGF-C expression and prognostic ability in
colorectal cancer.
The results of our study indicated that PTEN protein
is expressed in colorectal cancer at significantly lower levels
than in normal tissues and is indicative of poor prognosis,
consistent with previous reports (7–9). The
3- and 5-year survival rates of patients with PTEN protein-positive
colorectal cancer were higher than those of PTEN protein-negative
patients. PTEN is a tumor-suppressor gene with lipid phosphatase
activity, which negatively regulates the PI3K/AKT signaling
pathway. Activation of PI3K/AKT signaling is linked to cell
immortalization, promotes angiogenesis, and is involved in cell
growth and differentiation (10).
Hameed et al demonstrated that PTEN significantly inhibits
the growth of esophageal cancer cells in vitro by inducing
expression of the pro-apoptotic gene Bcl-2, and PTEN inhibits tumor
growth in vivo (11).
Absence of PTEN in colorectal cancer may affect the cell cycle and
signal transduction pathways, thus promoting cell proliferation and
tumorigenesis and leading to poorer prognosis. Clinical detection
of PTEN protein expression may help to accurately predict prognosis
in colorectal cancer and guide reasonable and effective clinical
treatment.
Our findings demonstrate that STAT3 protein
expression is significantly higher in colorectal cancer than in
normal tissue and is associated with tumor grade. The 3- and 5-year
survival rates of STAT3 protein-positive colorectal cancer patients
were lower than the rates in STAT3 protein-negative patients,
indicating that STAT3 may play a role in tumorigenesis, is related
to the degree of malignancy, and influences the prognosis of
colorectal cancer. STAT3 is an important member of the STAT family.
Normally, activation of STAT3 signaling is subject to strict
regulation, and STAT3 activation is related to malignant
transformation (12). Zhang et
al found that inhibitors of the JAK-2/STAT3 signaling pathway
inhibit glioma proliferation, thereby preventing metastasis and
invasion (13). STAT3 is
abnormally activated and is related to tumor stage and prognosis in
gastrointestinal tumors (14–16).
STAT3 activation is linked to tumor progression, mainly through
promotion of angiogenesis, anti-apoptotic effects and immune
escape, and poor prognosis (17,18).
Therefore, the detection of STAT3 expression in colorectal tumors
may be a useful predictor of the degree of malignancy and
prognosis, and may provide a novel target for cancer treatment.
VEGF-C was the first lymphatic growth stimulating
factor to be reported. It regulates proliferation and
differentiation of lymphatic endothelial cells and promotes tumor
lymph node metastasis (19). In
esophageal cancer, expression of VEGF-C is related to poor
prognosis, and was suggested as an effective indicator for
prediction of lymph node metastasis (20). In many tumors expression of VEGF-C
is closely related to lymphatic vessel invasion, sentinel lymph
node metastasis and distant metastasis and is indicative of poor
prognosis (21). In colorectal
cancer, we observed that VEGF-C expression was significantly
increased compared to normal tissues and was related to lymph node
metastasis.
The 3- and 5-year survival rates of VEGF-C
protein-positive colorectal cancer patients were significantly
lower than those of VEGF-C protein-negative patients, indicating
VEGF-C has prognostic value in colorectal cancer. VEGF-C was also
an independent prognostic factor of lymphatic system metastasis in
bladder cancer and gastric cancer (22,23),
and multivariate analysis in our study indicated that VEGF-C was
also an independent prognostic factor in colorectal cancer. VEGF-C
induces intracellular signal transduction to stimulate lymphatic
endothelial cell proliferation via VEGF-R3 (19). VEGF-C may also directly activate
VEGF-R3 in tumor cells to promote tumor growth and increase tumor
invasiveness (24,25). By increasing formation of lymphatic
vessels, VEGF-C increases the tumor cell contact with lymphatic
vessels to promote metastasis. Additionally, VEGF-C increases
lymphatic vessel permeability and tumor interstitial pressure,
enhancing the risk of tumor cells entering lymphatic vessels and
veins (26). Saharinen et
al demonstrated that VEGF-C alters lymphatic endothelial cell
adhesion properties, increases secretion of chemokines and
cytokines and promotes tumor cell proliferation (27). Further investigation is required to
determine the potential of VEGF-C as a potential target to reduce
lymph node metastases and improve prognosis in colorectal
cancer.
Numerous studies have shown that expression of PTEN,
STAT3 and VEGF-C, which can regulate tumor cell growth, is closely
related during tumor development. PTEN inhibits HIF-lα
transcription factor activity, via PI3K/Akt/mTOR, reducing VEGF
expression (28). PTEN and VEGF-C
expression was negatively correlated in colorectal cancer, similar
to the observations of Klatte et al in gastric cancer
(4). VEGF is also a target gene of
STAT3 (29), indicating that PTEN
and STAT3 have a common target. In our study STAT3 and VEGF-C
expression was also positively correlated. Evidence from the
literature and our findings indicates that the expression of PTEN
STAT3 and VEGF-C may be interdependent, and that each has their own
role and prognostic value in colorectal cancer; downregulation of
PTEN tumor suppressor activity and increased STAT3 and VEGF-C
expression promote tumor growth, leading to increased invasion and
metastasis in colorectal cancer.
In conclusion, PTEN, STAT3 and VEGF-C are prognostic
factors in colorectal cancer and VEGF-C can be used as an
independent prognostic factor. Combined detection of PTEN, STAT3
and VEGF-C expression may provide an index with which to determine
the degree of malignancy, metastasis and prognosis in colorectal
cancer and guide clinical treatment. Future research should focus
on the methods by which to alter expression of PTEN, STAT3 and
VEGF-C in order to provide new targets for clinical treatment, and
to improve survival and quality of life of colorectal cancer
patients.
Acknowledgements
The authors thank the Oncology
Department of the First Affiliated Hospital of Henan University of
Science and Technology for their assistance.
References
|
1
|
Higa R: Colorectal cancer: epidemiology
and primary profilaxis. Acta Gastroenterol Latinoam. 41:70–73.
2011.PubMed/NCBI
|
|
2
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar
|
|
3
|
Yoon YK, Kim HP, Han SW, Oh do Y, Im SA,
Bang YJ and Kim TY: KRAS mutant lung cancer cells are
differentially responsive to MEK inhibitor due to AKT or STAT3
activation: implication for combinatorial approach. Mol Carcinog.
49:353–362. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Klatte T, Seligson DB, LaRochelle J, Shuch
B, Said JW, Riggs SB, Zomorodian N, Kabbinavar FF, Pantuck AJ and
Belldegrun AS: Molecular signatures of localized clear cell renal
cell carcinoma to predict disease-free survival after nephrectomy.
Cancer Epidemiol Biomarkers Prev. 18:894–900. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lee J, Kang WK, Park JO, Park SH, Park YS,
Lim HY, Kim J, Kong J, Choi MG, Sohn TS, et al: Expression of
activated signal transducer and activator of transcription 3
predicts poor clinical outcome in gastric adenocarcinoma. APMIS.
117:598–606. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yokoyama Y, Sato S, Futagami M, Fukushi Y,
Sakamoto T, Umemoto M and Saito Y: Prognostic significance of
vascular endothelial growth factor and its receptors in endometrial
carcinoma. Gynecol Oncol. 77:413–418. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bedolla R, Prihoda TJ, Kreisberg JI, Malik
SN, Krishnegowda NK, Troyer DA and Ghosh PM: Determining risk of
biochemical recurrence in prostate cancer by immunohistochemical
detection of PTEN expression and Akt activation. Clin Cancer Res.
13:3860–3867. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sano T, Lin H, Chen X, Langford LA, Koul
D, Bondy ML, Hess KR, Myers JN, Hong YK, Yung WK and Steck PA:
Differential expression of MMAC/PTEN in glioblastoma multiforme:
relationship to localization and prognosis. Cancer Res.
59:1820–1824. 1999.PubMed/NCBI
|
|
9
|
Lee HS, Lee HK, Kim HS, Yang HK and Kim
WH: Tumour suppressor gene expression correlates with gastric
cancer prognosis. J Pathol. 200:39–46. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang S and Yu D: PI(3)king apart PTEN’s
role in cancer. Clin Cancer Res. 16:4325–4330. 2010.PubMed/NCBI
|
|
11
|
Hameed M, Lange KH, Andersen JL,
Schjerling P, Kjaer M, Harridge SD and Goldspink G: The effect of
recombinant human growth hormone and resistance training on IGF-I
mRNA expression in the muscles of elderly men. J Physiol.
555:231–240. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Couffinhal T, Dufourcq P and Duplàa C:
Beta-catenin nuclear activation: common pathway between Wnt and
growth factor signaling in vascular smooth muscle cell
proliferation? Circ Res. 99:1287–1289. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang HY, Zhang Q, Zhang X, Yu C, Huo X,
Cheng E, Wang DH, Spechler SJ and Souza RF: Cancer-related
inflammation and Barrett’s carcinogenesis: interleukin-6 and STAT3
mediate apoptotic resistance in transformed Barrett’s cells. Am J
Physiol Gastrointest Liver Physiol. 300:G454–G460. 2011.
|
|
14
|
Kusaba T, Nakayama T, Yamazumi K, Yakata
Y, Yoshizaki A, Nagayasu T and Sekine I: Expression of p-STAT3 in
human colorectal adenocarcinoma and adenoma; correlation with
clinicopathological factors. J Clin Pathol. 58:833–838. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu JR, Wang Y, Zuo LF, Li FL, Wang Y and
Liu JL: Expression and clinical significance of COX-2, p-Stat3, and
p-Stat5 in esophageal carcinoma. Ai Zheng. 26:458–462.
2007.PubMed/NCBI
|
|
16
|
Kusaba T, Nakayama T, Yamazumi K, Yakata
Y, Yoshizaki A, Inoue K, Nagayasu T and Sekine I: Activation of
STAT3 is a marker of poor prognosis in human colorectal cancer.
Oncol Rep. 15:1445–1451. 2006.PubMed/NCBI
|
|
17
|
Senft C, Priester M, Polacin M, Schröder
K, Seifert V, Kögel D and Weissenberger J: Inhibition of the
JAK-2/STAT3 signaling pathway impedes the migratory and invasive
potential of human glioblastoma cells. J Neurooncol. 101:393–403.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kortylewski M, Kujawski M, Wang T, Wei S,
Zhang S, Pilon-Thomas S, Niu G, Kay H, Mulé J, Kerr WG, et al:
Inhibiting Stat3 signaling in the hematopoietic system elicits
multicomponent antitumor immunity. Nat Med. 11:1314–1321. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Joukov V, Pajusola K, Kaipainen A, Chilov
D, Lahtinen I, Kukk E, Saksela O, Kalkkinen N and Alitalo K: A
novel vascular endothelial growth factor, VEGF-C, is a ligand for
the Flt4 (VEGFR-3) and KDR (VEGFR-2) receptor tyrosine kinases.
EMBO J. 15:17511996.
|
|
20
|
Tanaka T, Ishiguro H, Kuwabara Y, Kimura
M, Mitsui A, Katada T, Shiozaki M, Naganawa Y, Fujii Y and Takeyama
H: Vascular endothelial growth factor C (VEGF-C) in esophageal
cancer correlates with lymph node metastasis and poor patient
prognosis. J Exp Clin Cancer Res. 29:832010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rinderknecht M and Detmar M: Tumor
lymphangiogenesis and melanoma metastasis. J Cell Physiol.
216:347–354. 2008. View Article : Google Scholar
|
|
22
|
Han FH, Li HM, Zheng DH, He YL and Zhan
WH: The effect of the expression of vascular endothelial growth
factor (VEGF)-C and VEGF receptor-3 on the clinical outcome in
patients with gastric carcinoma. Eur J Surg Oncol. 36:1172–1179.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang H, Kim C, Kim MJ, Schwendener RA,
Alitalo K, Heston W, Kim I, Kim WJ and Koh GY: Soluble vascular
endothelial growth factor receptor-3 suppresses lymphangiogenesis
and lymphatic metastasis in bladder cancer. Mol Cancer. 10:362011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Matsuura M, Onimaru M, Yonemitsu Y, Suzuki
H, Nakano T, Ishibashi H, Shirasuna K and Sueishi K: Autocrine loop
between vascular endothelial growth factor (VEGF)-C and VEGF
receptor-3 positively regulates tumor-associated lymphangiogenesis
in oral squamoid cancer cells. Am J Pathol. 175:1709–1721. 2009.
View Article : Google Scholar
|
|
25
|
Kodama M, Kitadai Y, Tanaka M, Kuwai T,
Tanaka S, Oue N, Yasui W and Chayama K: Vascular endothelial growth
factor C stimulates progression of human gastric cancer via both
autocrine and paracrine mechanisms. Clin Cancer Res. 14:7205–7214.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bednarek W, Wertel I and Kotarski J:
Lymphangiogenesis in cancerous tumours. Ginekol Pol. 79:625–629.
2008.(In Polish).
|
|
27
|
Saharinen P, Tammela T, Karkkainen MJ and
Alitalo K: Lymphatic vasculature: development, molecular regulation
and role in tumor metastasis and inflammation. Trends Immunol.
25:387–395. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Park GS, Joo YE, Kim HS, Choi SK, Rew JS,
Park CS and Kim SJ: Expression of PTEN and its correlation with
angiogenesis in gastric carcinoma. Korean J Gastroenterol.
46:196–203. 2005.PubMed/NCBI
|
|
29
|
Zhao M, Liu F, Wang JY, Zhang WY, Gao FH
and Jiang B: Effects of JAK2/STAT3 signaling pathway on
angiogenesis in non-small cell lung cancer. Zhonghua Yi Xue Za Zhi.
91:375–381. 2011.(In Chinese).
|