Introduction
Hepatocarcinoma (liver cancer) is the third-leading
cause of cancer-related mortality and the fifth most common
malignancy worldwide (1). In China
presently, hepatocarcinoma is the second major cause of
cancer-related mortalities, with a mortality rate of 26.26 per
100,000 individuals (males, 37.55; and females, 14.45 per 100,000),
accounting for 19.33% of all types of cancers. Accordingly, the
estimated annual incidence of cases and mortalities from
hepatocarcinoma are 360,000 and 350,000, respectively (2,3).
Type VIII collagen, a non-fibrillar short-chain collagen, is a
structural component of numerous extracellular matrices (4,5). It
is highly expressed by vascular smooth muscle cells and is
considered to be a key component in vascular remodeling (6,7).
Type VIII collagen exists as a heterodimer composed of two distinct
α-chains (COL8A1 and COL8A2), each with a molecular weight of
approximately 60 kDa (8). The high
expression level of COL8A1 in vascular endothelial cells and tumor
cells has gained extensive attention (9,10).
High expression of COL8A1 in tumor cells may be associated with
tumor cell proliferation. The present study focused on the role of
COL8A1 in the tumor metastasis of liver cancer, which may provide
experimental basis for research and development of antitumor
drugs.
Materials and methods
Cell culture
The mouse hepatocarcinoma cell line Hepa1-6, which
lacks metastatic potential in the lymph nodes (provided by the Cell
Center of Peking Union Medical College, China) was cultured in DMEM
medium (HyClone, Logan, UT, USA), supplemented with 10% FBS
(HyClone), streptomycin 100 U/ml, penicillin 100 U/ml. The cells
were incubated in a humidified atmosphere containing 5%
CO2 at 37°C for 24 h. The cells were in logarithmic
growth phase.
Cell transfection
COL8A1-1-pEGFP-N2 and pEGFP-N2 were transfected,
into the experimental and control group cells, respectively. The
specific steps were as follows. i) Hepa1-6 cells were cultured in
DMEM culture medium for 24 h, up to 40–80% confluency in cell
fusion. ii) Carrier (6 μg) was dissolved in DMEM culture medium
(without FBS and antibiotics) and when the volume reached 300 μl,
50 μl liposome reagent was added, mixed well and then kept at room
temperature for 5 min, and ultimately reaction complexes were
formed. iii) Hepa1-6 cells were prepared by adding 7 ml DMEM
medium. Inclusion of such a complex containing the reaction of the
test tube and 1 ml DMEM was mixed well and immediately transferred
onto a dish. Cells were incubated in a humidified atmosphere
containing 5% CO2 at 37°C for 8 h by replacing the fluid
for normal cell growth and continuing for an additional 48 h. The
experimental group cells were cultured in G418 (800 μg/ml)
selective medium, and the control group of untransfected Hepa1-6
cells were cultured in G418 medium. Cells were cultured in the G418
(400 μg/ml) selective medium until the majority of the
untransfected cells were dead in the control group and the
transfected cells in the experimental group were cloned. The
selective medium was replaced every 3 days for 20 days. A single
clonal cell was picked for screening into 24-well plates using the
cloning ring method together with cultured cells in G418 (400
μg/ml) selective medium.
RT-PCR analysis
For RT-PCR analysis of COL8A1 mRNA levels, total RNA
was isolated from cells using TRIzol (Invitrogen, Carlsbad, CA,
USA), and cDNA was synthesized using an RT-PCR kit (Takara, Shiga,
Japan) according to the manufacturer’s instructions. The primer
sequences were as follows; 5′-GCTGCTGGGAATACTGTTCA-3′(F) and
5′-GGGAGGTATGGGTACTCTTT-3′(R) for COL8A1;
5′-GGCCGTGAAGTCGTCAGAAC-3′(F) and 5′-GCCACGATGCCCAGGAA-3′(R) for
GAPDH. PCR analysis was performed under the following conditions:
denaturation at 94°C for 1 min, and 30 cycles of denaturation for
20 sec at 97°C, annealing for 20 sec at 64°C and extension for 20
sec at 72°C. The amplified products were analyzed by agarose gel
electrophoresis using 1.0% gel, followed by ethidium bromide
staining.
Western blot analysis
Western blot analysis was performed to evaluate
COL8A1 protein levels. The protein was harvested from cells using a
2X concentrated electrophoresis sample buffer (125 mmol/l Tris-HCl,
pH 6.8, 2% sodium dodecyl sulfate, 5% glycerol and 1%
β-mercaptoethanol). Equal amounts of denatured proteins (35 μg)
were resolved by 10% SDS-PAGE and then blotted onto PVDF membranes
(Pall Corporation). After blocking for 2 h with phosphate-buffered
saline containing 0.1% Tween 20 and 5% powdered skimmed milk, the
blots were incubated with rabbit anti-mouse COL8A1 polyclonal
antibodies (Abcam, Cambridge, UK; ab58776, 1:200 dilution)
overnight in 5% powdered skimmed milk buffer, washed thrice with
phosphate-buffered saline with 0.1% Tween 20 and then incubated
with secondary antibody anti-rabbit-HRP (Santa Cruz Biotechnology
Inc., Santa Cruz, CA, USA; 1:3000 dilution). GAPDH antibody (Santa
Cruz Biotechnology, Inc.; 1:200 dilution) was used as a control.
All bands were detected using ECL western blot kit (Amersham
Biosciences, Buckinghamshire, UK).
MTT assay
Hepa1-6, Hepa1-6/COL8A1 and Hepa1-6/mock cells
(1×106) in 200 μl RPMI-1640 were seeded in duplicate
into each well of 96-well culture plates, and 100 μl MTT (5 mg/ml,
Sigma, St. Louis, MO, USA) was added at 24, 48, 72, 96 and 120 h.
After a 4-h incubation at 37°C in 5% CO2, 100 μl DMSO
(Gibco, Carlsbad, CA, USA) was pipetted to solubilize the formazan
product for 30 min at room temperature. The absorbancy of A570 was
measured using a microplate reader (Bio-Rad, Hercules, CA, USA).
Growth rate (%) = A570(Hepa1-6/COL8A1) ÷ A570(Hepa1-6) × 100%.
In vitro ECM invasion assay
Cell invasion in vitro was demonstrated using
24-well Transwell units (Corning, NY, USA) with 8-μm pore size
polycarbonate filter coated with ECMatrix gel (Chemicon, Temecula,
CA, USA) to form a continuous thin layer. Hepa1-6, Hepa1-6/COL8A1
and Hepa1-6/mock cells (3×105) were harvested in
RPMI-1640 containing 1% FBS and added to the upper chamber. The
lower chamber was filled with 500 μl RPMI-1640 containing 10% FBS.
The cells were incubated for 24 h at 37°C with 5% CO2.
At the end of incubation, the cells on the upper surface of the
filter were completely removed by wiping with a cotton swab. The
filters were then fixed in methanol and were stained with
Wright-Giemsa. The number of cells that had invaded the Matrigel
and reached the lower surface of the filter were counted under a
light microscope at a magnification of x200. Triplicate samples
were acquired, and the data were expressed as the average cell
number of 5 fields. Invasive cells were calculated and analyzed
with the Image-Pro Plus 4.5 software (Media Cybernetics).
In vivo tumorigenicity analysis
A total of 60 C57L mice (provided by Dalian Medical
University, Dalian, China) were randomly divided into three groups,
each group having 20 mice. The logarithmic phase Hepa1-6,
Hepa1-6/COL8A1 and Hepa1-6/mock cells were injected subcutaneously
in mice. The mice were sacrificed three weeks later. Swollen
axillary lymph nodes, fixed with 4% formaldehyde from the 3 groups
were generally compared for tumor weight and metastatic rates.
Paraffin sections, hematoxylin and eosin staining and observation
of tumor cells were performed under a microscope.
Drug sensitivity assay
To assess chemosensitivity to D-limonene (Sigma);
Hepa1-6, Hepa1-6/COL8A1 and Hepa1-6/mock cells (3×105)
cultured for 24 h, were incubated with various concentrations of
D-limonene (0, 0.2, 0.4, 0.8 and 1.6 g/ml) for another 48 h. Then
cells were treated with MTT as described previously and each group
contained three wells. Cell survival rate (%) =
A570(D-limonene+) ÷ A570(D-limonene−) ×
100.
Statistical analysis
SPSS 14.0 software (SPSS, Chicago, IL, USA) was
used. Each assay was performed at least three times. The data are
expressed as mean ± SD and the Student’s t-test was used to
determine the significance of differences in multiple comparisons.
P<0.05 was considered to indicate a statistically significant
result.
Results
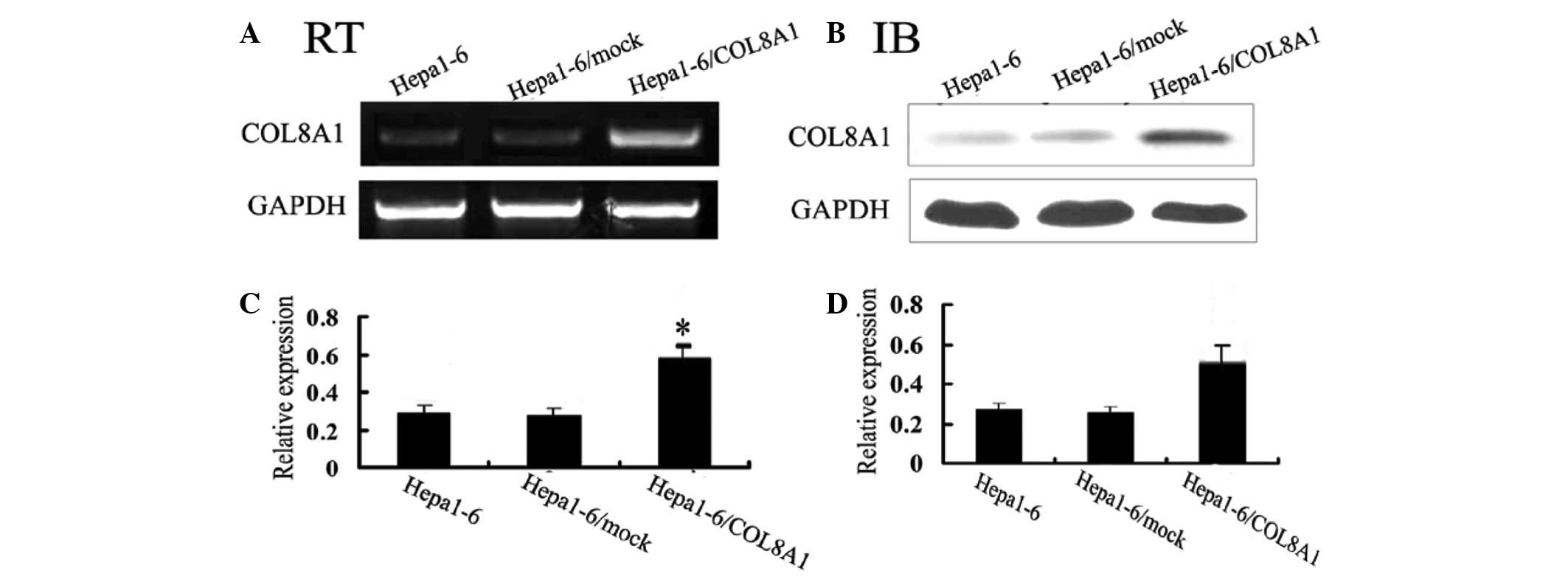
cDNA transfection increases the
expression of COL8A1
In order to verify whether stable expression of
COL8A1 affects hepatocarcinoma tumors in mice, we transfected
Hepa1-6 cells that expressed COL8A1 at a low level with the
constructed plasmid COL8A1-pEGFP-N2. After 3 weeks, we analyzed the
stable transfected Hepa1-6/COL8A1 cells by RT-PCR and western blot
analysis. We set two negative control groups; i) nontransfected
Hepa1-6 cells; ii) empty plasmid-transfected Hepa1-6/mock cells,
through which transfection of Hepa1-6 cells was observed. Following
stable transfection, Hepa1-6/COL8A1 cells highly expressed COL8A1
and the other two control groups expressed COL8A1 at lower levels
(Fig. 1). These results confirmed
that it was feasible, reliable and effective to apply this method
of stable transfection.
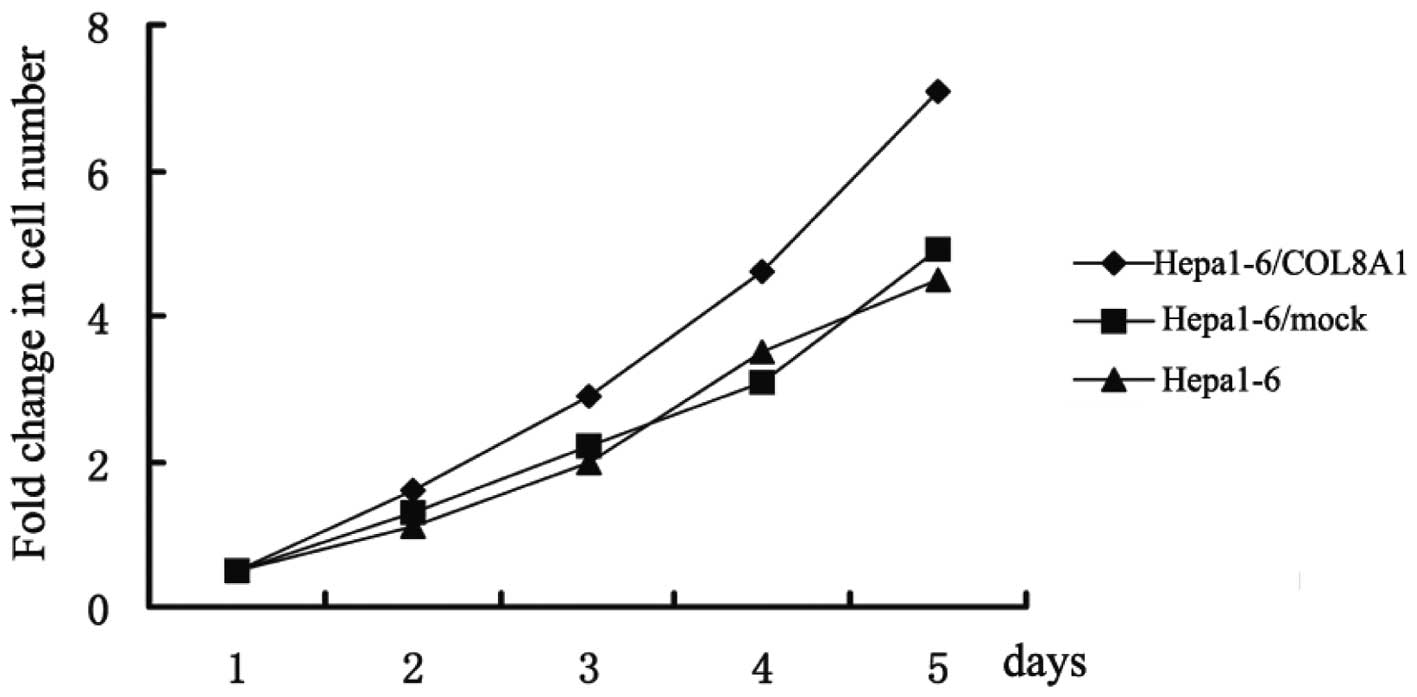
Enhanced COL8A1 expression increases
Hepa1-6 cell proliferation in vitro
After enhancing the expression of the COL8A1 gene in
Hepa1-6 cells, we determined the cell proliferation using MTT at
24, 48, 72, 96 and 120 h, and also set a control group of
untransfected Hepa1-6 cells and another of empty
plasmid-transfected Hepa1-6/mock cells. The results demonstrated
that the growth rate curve of Hepa1-6/COL8A1 cells increased, while
the growth rate curves of the control group exhibited a flat trend.
Therefore, enhancing COL8A1 expression increased the proliferative
ability of Hepa1-6 cells in vitro (Fig. 2).
Enhancing COL8A1 expression increases
Hepa1-6/COL8A1 cell invasion in vitro
In order to verify whether the stable expression of
COL8A1 plays a key role in Hepa1-6/COL8A1 cell invasion in
vitro we compared the cell proliferation of Hepa1-6/COL8A1
cells of the experimental group with that of the two control
groups: the untransfected cells of the Hepa1-6 control group and
the empty plasmid-transfected cells of the Hepa1-6/mock control
group (Fig. 3A). The number of
cells that crossed the membrane was higher in the experimental
group (21±3) (P<0.05; Fig. 3B).
These results indicate that stable expression of COL8A1 is able to
promote the in vitro cell invasion of Hepa1-6/COL8A1 cells
and plays a key role in cancer metastasis.
Enhanced COL8A1 expression promotes the
tumorigenicity of Hepa1-6 cells
The tumor formation experiment in vivo
(Fig. 4A) showed that, compared
with the control groups of untransfected Hepa1-6 cells and empty
plasmid-transfected Hepa1-6 cells, the experimental group
Hepa1-6/COL8A1 cells clearly exhibited tumor formation. These
results indicate that the stable expression of COL8A1 promotes the
tumorigenicity of Hepa1-6/COL8A1 cells.
Increased COL8A1 expression reduces the
sensitivity of Hepa1-6 cells to D-limonene treatment
Compared with the control group of untransfected
Hepa1-6 cells and empty plasmid-transfected Hepa1-6/mock cells,
increased COL8A1 expression inhibited the sensitivity of
Hepa1-6/COL8A1 cells to D-limonene treatment.
Discussion
One of the main purposes of cancer research is to
identify the genetic factors that contribute to tumor formation and
to elucidate the mechanism of tumor development. Therefore, certain
specific gene proteins may be investigated due to their potential
role as markers of tumor progression or as a treatment goal. It has
been reported that at initial diagnosis, the cancer in over 60% of
patients has progressed. Metastasis is a characteristic of
malignant tumors and is the main cause of mortality in patients
with malignant tumors. Elucidation of the mechanisms involved in
tumor metastasis has great theoretical significance and practical
value. The formation and development of tumors involves cell
proliferation, differentiation disorders, apoptosis, an abnormal
angiogenesis pathway and numerous other factors. It is a complex
process involving multiple genetic changes.
Currently, increasing attention has been paid to
COL8A1 gene due to its expression in a number of rapidly
proliferating cell types (10,11).
COL8A1 which is located around the cell may play a sustaining role
in the cell proliferative stage. COL8A1 has also been known to take
on the role of chemokine stromal cells, including smooth muscle
cells, and provides a substrate to enhance cell transfer (12–14).
COL8A1 gene expression profiles divide gastrointestinal stromal
tumors into different tumor groups (9). D-limonene is an available monomer
composition which is extracted from traditional Chinese medicinal
orange peel and belongs to the natural monoterpene set of
compounds. Due to its citrus aroma, D-limonene is widely used as an
additive in perfumes, soap, food, chewing gum and drinks (15). A large number of experiments have
proven that D-limonene exhibits anticancer activity and plays a
preventive and therapeutical role in rodent gastric, breast, liver,
colon, pancreatic and skin cancer, and other tumors which are
chemically induced. Its mechanisms include direct cytotoxicity to
tumor cells, inducing tumor cell death and inhibition of the enzyme
activity and levels of matrix protein in tumor cells (16–20).
D-limonene also inhibits the formation of tumor microvasculature
and lymphatic tubes, resulting in low microvascular density. Thus
tumor metastasis was found to be inhibited (21–23).
In the present study, we transfected a constructed
plasmid of COL8A1-pEGFP-N2 to low COL8A1-expressing Hepa1-6 cells
and analyzed the stably transfected Hepa1-6/COL8A1 cells using
RT-PCR and western blot analysis. Following stable transfection,
Hepa1-6/COL8A1 cells expressed COL8A1 at high levels, and the other
two control groups expressed COL8A1 at low levels. These results
sufficiently demonstrated that it was feasible, reliable and
effective for us to perform this method of stable transfection.
After using the cDNA transfection technique to
upregulate COL8A1 expression, the cell proliferation and invasion
of Hepa1-6/COL8A1 cells increased. The tumorigenicity in
vivo trials demonstrated that the tumorigenicity of
Hepa1-6/COL8A1 cells was clear (P<0.05). The stable expression
of COL8A1 promoted the tumorigenicity of Hepa1-6/COL8A1 cells,
which indicated that enhanced expression of COL8A1 affected
Hepa1-6/COL8A1 cell in vivo tumorigenicity.
In conclusion, our results suggest that COL8A1
expression is closely related to tumor cell proliferation, invasion
and tumorigenicity in vivo. COL8A1 overexpression inhibits
drug sensitivity as determined by the effects of D-limonene on
Hepa1-6/COL8A1 cells. COL8A1 expression may be one of the key
mechanisms in the regulation of cytokines in tumor metastasis.
Increased COL8A1 expression enhances tumorigenicity of the cells in
the body, which provides new targets for the diagnosis and
treatment of over 60% of the cancer patients lymph node metastasis.
It also provides valuable experimental evidence and clues for the
design of anticancer drugs.
Abbreviations:
|
COL8A1
|
type VIII collagen one;
|
|
FBS
|
phosphate buffered saline;
|
|
SDS-PAGE
|
sodium dodecyl sulfate polyacrylamide
gel electrophoresis;
|
|
RT-PCR
|
reverse transcription-polymerase chain
reaction;
|
|
MTT
|
3-(4,5)-dimethylthiazol (-2-yl)-2,5
diphenyltetrazolium bromide
|
Acknowledgements
This work was supported by the Science
Foundation of Liaoning Province (no. 20092164), the Creating Team
Item of Liaoning Province (no. 2008T033), China.
References
|
1
|
Andrisani OM, Studach L and Merle P: Gene
signatures in hepatocellular carcinoma (HCC). Semin Cancer Biol.
21:4–9. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen JG, Zhang SW and Chen WQ: Analysis of
liver cancer mortality in the national retrospective sampling
survey of death causes in China, 2004–2005. Zhonghua Yu Fang Yi Xue
Za Zhi. 44:383–389. 2010.(In Chinese).
|
|
3
|
Wang Y, Zhang Y, Peitgen HO, Schenk A,
Yuan L, Wei G and Sun Y: Precise local resection for hepatocellular
carcinoma based on tumor-surrounding vascular anatomy revealed by
3D analysis. Dig Surg. 29:99–106. 2012. View Article : Google Scholar
|
|
4
|
Muragaki Y, Shiota C, Inoue M, Ooshima A,
Olsen BR and Ninomiya Y: alpha 1(VIII)-collagen gene transcripts
encode a short-chain collagen polypeptide and are expressed by
various epithelial, endothelial and mesenchymal cells in newborn
mouse tissues. Eur J Biochem. 207:895–902. 1992. View Article : Google Scholar
|
|
5
|
Kittelberger R, Davis PF, Flynn DW and
Greenhill NS: Distribution of type VIII collagen in tissues: an
immunohistochemical study. Connect Tissue Res. 24:303–318. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hou G, Mulholland D, Gronska MA and
Bendeck MP: Type VIII collagen stimulates smooth muscle cell
migration and matrix metalloproteinase synthesis after arterial
injury. Am J Pathol. 156:467–476. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Adiguzel E, Hou G, Mulholland D, Hopfer U,
Fukai N, Olsen B and Bendeck M: Migration and growth are attenuated
in vascular smooth muscle cells with type VIII collagen-null
alleles. Arterioscler Thromb Vasc Biol. 26:56–61. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhao Y, Jia L, Mao X, Xu H, Wang B and Liu
Y: siRNA-targeted COL8A1 inhibits proliferation, reduces invasion
and enhances sensitivity to D-limonene treatment in hepatocarcinoma
cells. IUBMB Life. 61:74–79. 2009. View
Article : Google Scholar
|
|
9
|
Kapoor R, Sakai LY, Funk S, Roux E,
Bornstein P and Sage EH: Type VIII collagen has a restricted
distribution in specialized extracellular matrices. J Cell Biol.
107:721–730. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Koon N, Schneider-Stock R, Sarlomo-Rikala
M, Lasota J, Smolkin M, Petroni G, Zaika A, Boltze C, Meyer F,
Andersson L, Knuutila S, Miettinen M and El-Rifai W: Molecular
targets for tumour progression in gastrointestinal stromal tumours.
Gut. 53:235–240. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Illidge C, Kielty CM and Shuttleworth CA:
Stability of type VIII collagen homotrimers: comparison with
alpha1(X). Biochem Soc Trans. 26:S181998.PubMed/NCBI
|
|
12
|
Yasuda O, Zhang SH, Miyamoto Y and Maeda
N: Differential expression of the alpha1 type VIII collagen gene by
smooth muscle cells from atherosclerotic plaques of
apolipoprotein-E-deficient mice. J Vasc Res. 37:158–169. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Plenz GM, Deng MC, Robenk H and Völker W:
Vascular collagens: spotlight on the role of type VIII collagen in
atherogenesis. Atherosclerosis. 166:1–11. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Alitalo K, Bornstein P, Vaheri A and Sage
H: Biosynthesis of an unusual collagen type by human astrocytoma
cells in vitro. J Biol Chem. 258:2653–2661. 1983.PubMed/NCBI
|
|
15
|
Crowell PL: Prevention and therapy of
cancer by dietary monoterpenes. J Nutr. 129:775S–778S.
1999.PubMed/NCBI
|
|
16
|
Satomi Y, Ohara K, Yazaki K, Ito M, Honda
G and Nishino H: Production of the monoterpene limonene and
modulation of apoptosis-related proteins in embryonic-mouse NIH 3T3
fibroblast cells by introduction of the limonene synthase gene
isolated from Japanese catnip (Schizonepeta tenuifolia).
Biotechnol Appl Bioch. 52:185–190. 2009. View Article : Google Scholar
|
|
17
|
Tsuda H, Ohshima Y, Nomoto H, Fujita K,
Matsuda E, Iigo M, Takasuka N and Moore MA: Cancer prevention by
natural compounds. Drug Metab Pharmacokinet. 19:245–263. 2004.
View Article : Google Scholar
|
|
18
|
Mo H and Elson CE: Studies of the
isoprenoid-mediated inhibition of mevalonate synthesis applied to
cancer chemotherapy and chemoprevention. Exp Biol Med (Maywood).
229:567–585. 2004.PubMed/NCBI
|
|
19
|
Van der Logt EM, Roelofs HM, van Lieshout
EM, Nagengast FM and Peters WH: Effects of dietary anticarcinogens
and nonsteroidal anti-inflammatory drugs on rat gastrointestinal
UDP-glucuronosyltransferases. Anticancer Res. 24:843–849.
2004.PubMed/NCBI
|
|
20
|
Del Toro-Arreola S, Flores-Torales E,
Torres-Lozano C, Del Toro-Arreola A, Tostado-Pelayo K, Guadalupe
Ramirez-Dueñas M and Daneri-Navarro A: Effect of D-limonene on
immune response in BALB/c mice with lymphoma. Int Immunopharmacol.
5:829–838. 2005.PubMed/NCBI
|
|
21
|
Ji J, Zhang L, Wu YY, Zhu XY, Lv SQ and
Sun XZ: Induction of apoptosis by d-limonene is mediated by a
caspase-dependent mitochondrial death pathway in human leukemia
cells. Leuk Lymphoma. 47:2617–2624. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lu XG, Zhan LB, Feng BA, Qu MY, Yu LH and
Xie JH: Inhibition of growth and metastasis of human gastric cancer
implanted in nude mice by d-limonene. World J Gastroenterol.
10:2140–2144. 2004.PubMed/NCBI
|
|
23
|
Zheng HH, Li YL, Yang HK, Lv YH and Gong
CL: The empirical study of the mice-transplanted tumor of S-180
mediated by D-Limonene. Anatomy Research. 1:25–28. 2008.
|