Introduction
Estrogen has been reported to produce a variety of
pleiotropic effects in target tissues as diverse as bone, brain,
breast, blood vessel and the male and female gonads (1). It is widely accepted that estrogen
plays a significant role in the regulation of bone remodeling and
the development and maintenance of the skeleton (2). Estrogen replacement therapy has long
been an important therapeutic modality for the prevention and
treatment of post-menopausal osteoporosis (3,4).
Several types of naturally occurring estrogen have been reported in
the literature (5). It has been
reported that the most potent naturally occurring estrogen in
humans is 17β-estradiol, followed by estrone and estriol (6).
The inhibition of bone resorption by 17β-estradiol
is relatively well established (7), but studies investigating the effect
of estrogen on osteoblast proliferation and differentiation have
produced inconsistent results (8).
There is a limited number of studies available with regard to the
effects of estrone on the differentiation and mineralization of
osteoblasts (3,8). However, the effects of low doses of
estrone on bone cells and the underlying mechanisms have not yet
been fully investigated.
The present study aimed to examine the effects of
various dosages of estrone (0.01 to 10 nM) on the cellular
proliferation, differentiation and mineralization of
preosteoblasts. Cell viability was evaluated using the
3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT)
assay, and the alkaline phosphatase (ALP) activity test and
alizarin red S staining were used to assess the differentiation and
mineralization of treated cells, respectively. The expression of
proteins associated with bone formation, including estrogen
receptor-α (ER-α), estrogen receptor-β (ER-β) and osteopontin (OPN)
was evaluated using western blot analysis. To the best of the
author’s knowledge, this is the first study to demonstrate the
effects of low doses of estrone on the expression of OPN in
osteoprecursor cells.
Materials and methods
Cell culture
Murine osteoprecursor cells (MC3T3-E1 cells) were
grown in α-minimum essential medium (αMEM; Invitrogen, Carlsbad,
CA, USA) supplemented with 10% fetal bovine serum (Invitrogen),
antibiotics (100 U/ml of penicillin and streptomycin, 100
µg/ml; Invitrogen). The culture medium was changed to
osteogenic differentiation medium [(αMEM supplemented with 50
µg/ml ascorbic acid (Sigma, St. Louis, MO, USA) and 10 mM
β-glycerophosphate (Sigma)] to induce osteogenic differentiation.
The cultures were kept in a humidified atmosphere containing 5%
CO2 and 95% air at 37°C. Estrone was dissolved in
dimethyl sulfoxide (DMSO; Sigma) and filter-sterilized. In order to
minimize any differences in cellular growth and differentiation
between the controls and treated cultures, an equal amount of DMSO
was administered to the controls and treated cultures in each
experiment.
Cellular proliferation
Cells were plated at a density of 1.0×104
cells, 1 ml/well in 12-well plates and the cultures were stimulated
with estrone at a range of final concentrations between 0.01 nM and
10 nM. The effects of estrone on the cellular proliferation of the
osteoprecursor cells were assessed on day 4. At the end of the
incubation time, the MTT reagents were added at a final
concentration of 0.5 mg/ml. The cells were incubated for 1 h at
37°C then washed with phosphate-buffered saline (PBS), pH 7.4,
followed by the addition of DMSO. Complete dissolution was achieved
after gentle agitation. Aliquots of the resulting solutions were
transferred into 96-well plates, and absorbance was recorded at 560
and 670 nm using a microplate spectrophotometer system.
ALP activity assays
Cells were lysed into a buffer which contained 10 mM
Tris-HCl, pH 7.4, and 0.2% Triton X-100 and then sonicated for 20
sec at 4°C. Samples were then added to a glycine buffer (100 mM, pH
10.5) containing 10 mM p-nitrophenylphosphate and 1 mM
MgCl2 and incubated at 37°C in a water bath. Total
protein content was determined by comparison with a bovine serum
albumin series as an internal standard. The optical density of
p-nitrophenol at 405 nm was determined spectrophotometrically and
ALP activities were normalized with respect to total protein
content.
Mineralization assay
Cell cultures obtained at day 14 were washed twice
with PBS, fixed for 1 h in ice-cold 70% ethanol and then rinsed
twice with deionized water. The cultures were stained with 40 mM
alizarin red S for 30 min under gentle agitation. To remove
non-specifically bound stain, cultures were washed three times with
deionized water and once with PBS for 15 min at ambient
temperature. To quantify the bound dye, the stain was solubilized
by agitation with 10% cetylpyridinium chloride. The absorbance of
the solubilized stain was measured at 562 nm.
Western blot analysis
Osteoprecusor cells were washed twice with ice-cold
PBS and solubilized in lysis buffer containing 10 mM Tris-HCl, pH
7.4, and 0.2% Triton X-100. The lysates were centrifuged at 14,000
rpm for 20 min at 4°C to remove the nuclear pellet. The
supernatants were boiled in a sodium dodecyl sulfate sample buffer
containing β-mercaptoethanol. Equal quantities of the cell extracts
were separated using sodium dodecyl sulfate-polyacrylamide gel
electrophoresis and transferred onto polyvinylidene fluoride
microporous membranes (Immobilon-P membranes; Millipore
Corporation, Billerica, MA, USA). Membranes were then blocked for
at least 1 h in 0.1% (v/v) Tween-20 in PBS containing 5% (w/v)
powdered milk. The membrane was immunoblotted with the desired
antibodies which were diluted in the same buffer at the recommended
concentrations. The membrane was incubated with horseradish
peroxidase-conjugated secondary antibody. The washed blot was
developed using enhanced chemiluminescence detection kits.
Mouse antibodies against ER-α, ER-β, OPN and β-actin
and the secondary antibodies conjugated to horseradish peroxidase
were purchased from Cell Signaling Technology, Inc. (Danvers, MA,
USA), Abcam (Cambridge, MA, USA) and Santa Cruz Biotechnology, Inc.
(Santa Cruz, CA, USA).
Statistical analysis
Results are presented as mean ± SD of the
experiments and a one-way analysis of variance (ANOVA) was
performed to determine the differences between groups using a
commercially available program (PASW Statistics 18; SPSS Inc.,
Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Cellular proliferation
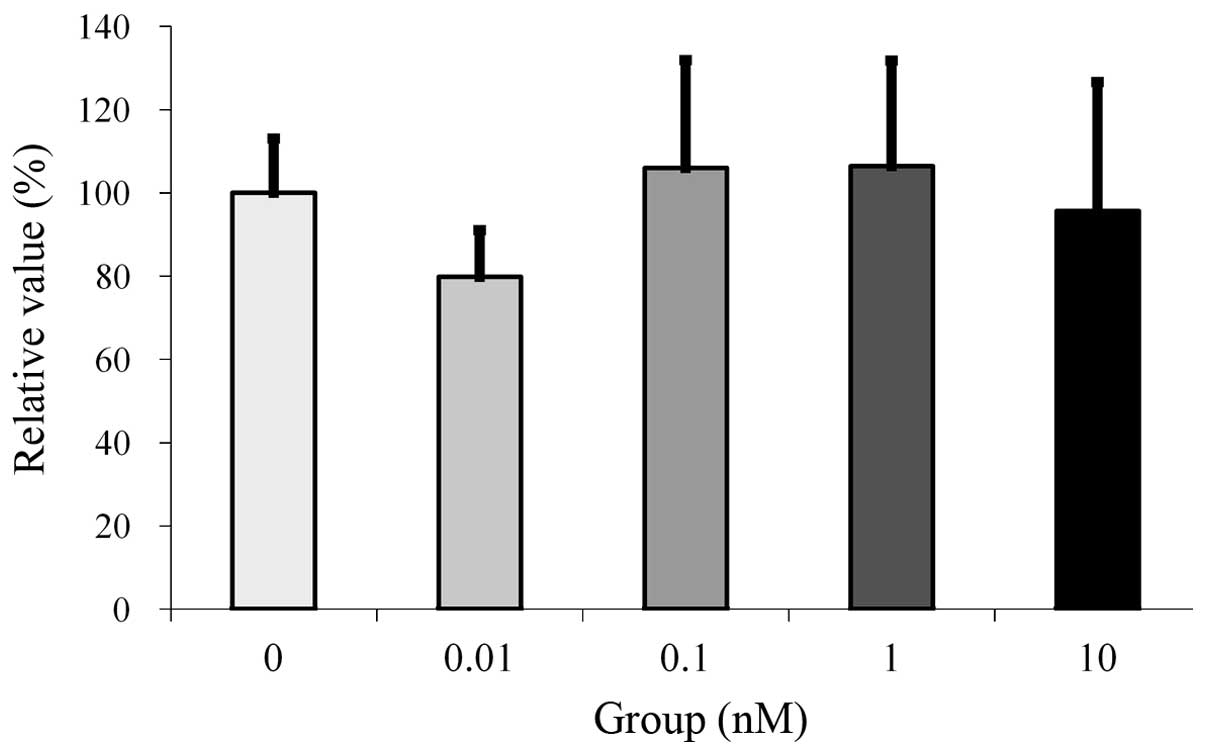
Cultures grown in the presence of estrone at the 0.1
and 1 nM concentrations demonstrated an increase in relative values
in the MTT assays. However, no significant differences were
observed between the groups (Fig.
1).
ALP assays
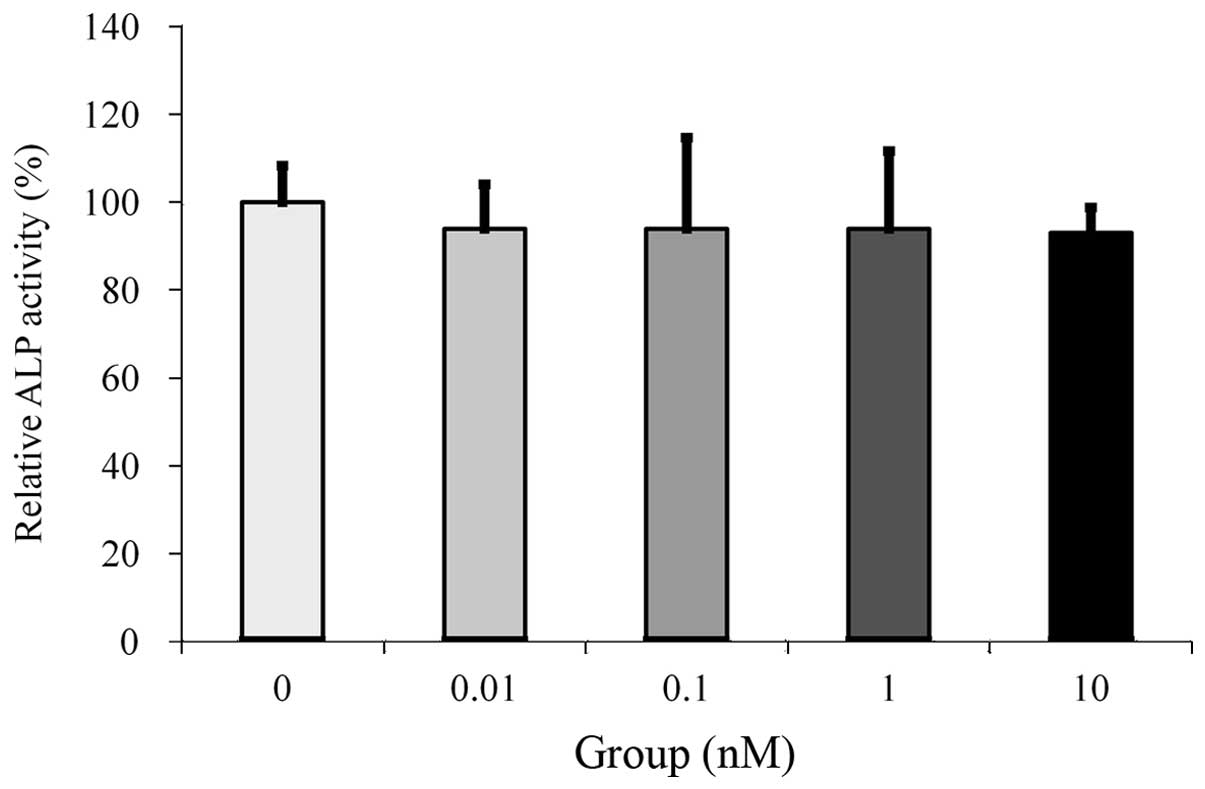
Cultures grown in the absence of estrone presented
the highest value for the ALP activity, but no significant
differences were observed between the groups (Fig. 2).
Mineralization/calcium deposition
assay
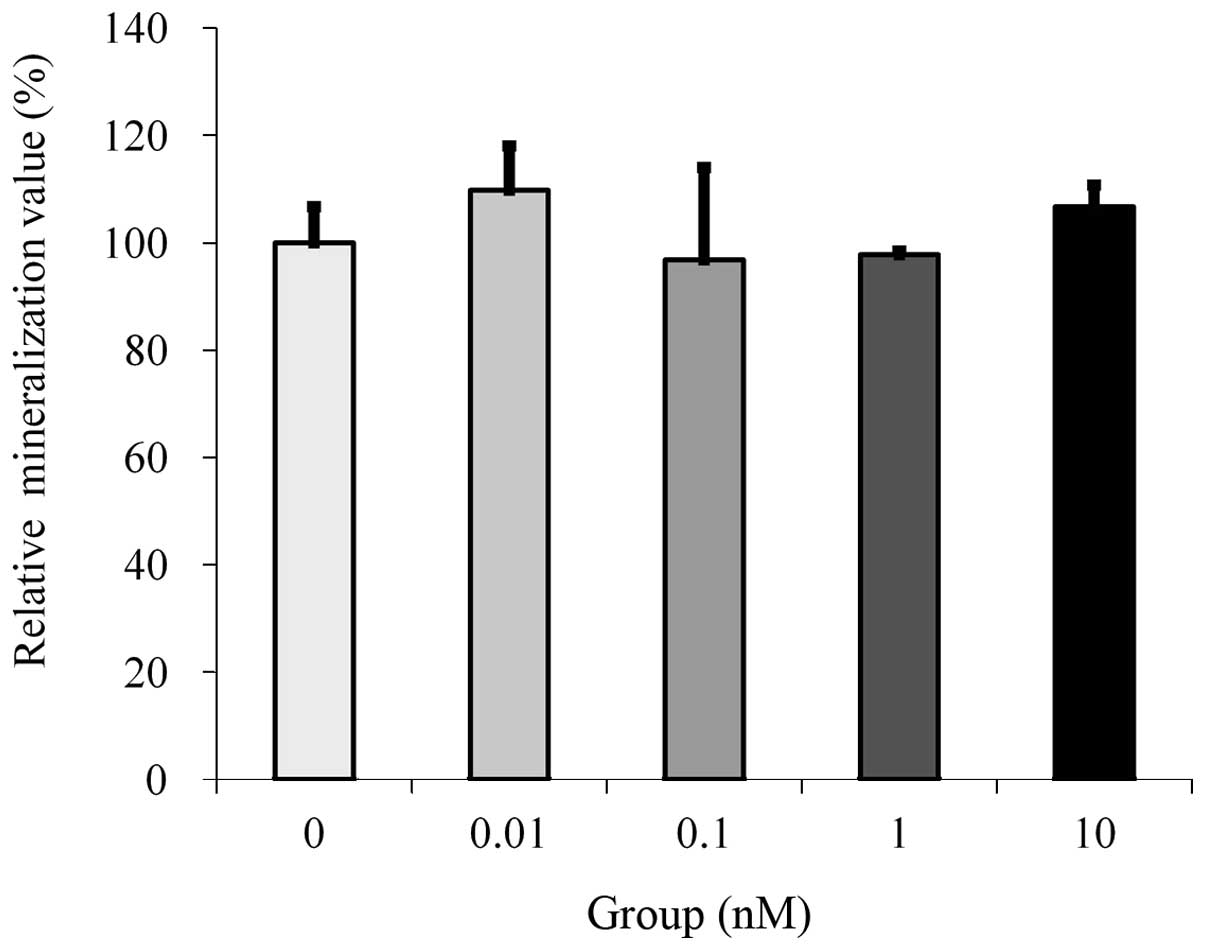
Cultures grown in the presence of estrone at the 10
nM concentration demonstrated an increase in mineralization.
However, statistically significant differences were not observed
between the tested groups (Fig.
3).
Western blot analysis
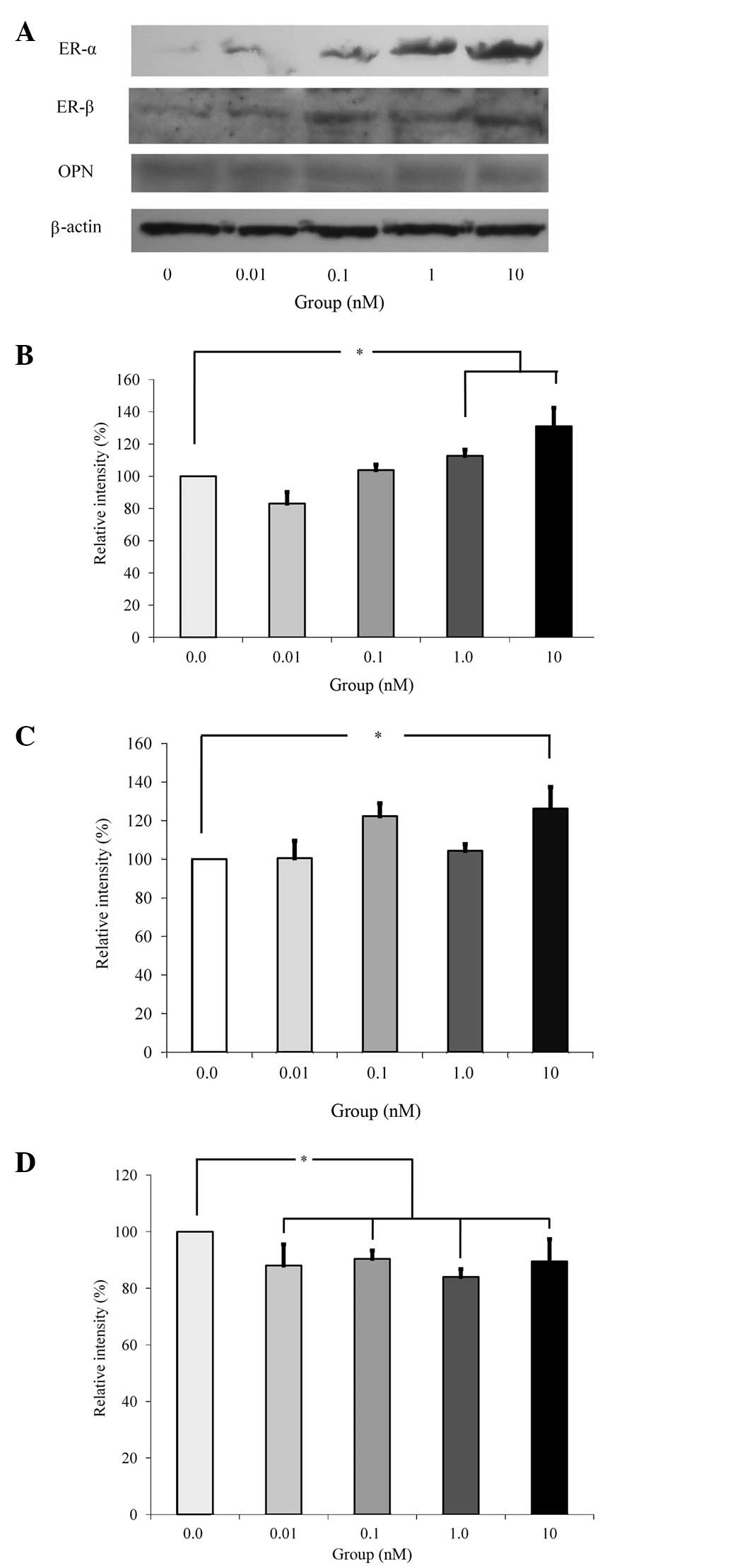
Western blot analysis was performed to detect
protein expression following treatment with estrone (Fig. 4A). The results demonstrated that
the addition of estrone increased the expression of ER-α and ER-β
following normalization with β-actin expression (Fig. 4B and C). Normalization of protein
expression revealed that the group treated with 10 nM estrone
yielded 131.0±11.5 and 126.3±11.1% of the ER-α and ER-β expression
levels compared with the control, respectively. The increase of
ER-α and ER-β expression at the 10 nM estrone group was
statistically significant (P<0.05). However, estrone appeared to
reduce the expression of OPN (Fig.
4D).
Discussion
In the present study, we examined the effects of low
doses of estrone on cell viability and the differentiation and
mineralization of osteoblast progenitor cells at predetermined
concentrations (0.01 to 10 nM). Additionally, experiments were
performed to identify through which pathway the effects of estrone
occur.
MTT assays were used in the present study to
evaluate cellular proliferation since this assay allows
mitochondrial dehydrogenases to oxidize MTT to an insoluble blue
formazan product (9,10). The results indicated that although
there were increases in the relative values, they were not
significant increases, suggesting that cellular proliferation was
not affected by the treatment. Previous studies have demonstrated
that estrone at a concentration of 10 nM markedly stimulated the
proliferation of human breast epithelial HBL-100 cells and that
estrone at concentrations of 10 and 100 nM significantly promoted
the proliferation and survival of human osteoblastic MG-63 cells
(8). This difference in results
may be due to the expression of large amounts of ER-α and ER-β,
which are responsive to estrogen, in MG-63 cells (11).
Osteoblast differentiation was assessed by ALP
activity, which has been reported to be an early marker of
osteoblastic cell differentiation (12,13).
The results of the present study revealed that estrone demonstrated
no significant effects within the range of administered doses. In
MG-63 cells, estrone at 100 nM concentration has been demonstrated
to markedly increase ALP activity (11). The presence of calcium deposits was
evaluated using alizarin red S staining with the aid of
cetylpyridinium chloride for quantification (14). Although treatment with estrone at
higher concentrations demonstrated a tendency to increase the
calcium levels of cellular deposits, this did not reach a
statistically significant level. The difference in differentiation
and mineralization behavior with regard to the estrone dosage may
be attributed to the type of cells, system model, the culturing
period or different affinity for ERs (8,15).
Western blot analysis was performed to detect the
expression levels of ER-α, ER-β and OPN when cells were treated
with estrone. Estrogens and ER modulators bind to ER-α and/or ER-β
to form discrete molecular complexes that exert pleiotropic
tissue-specific effects by modulating the expression of their
target genes (16). The present
study demonstrated that estrone influences ER-α, ER-β and OPN
expression. OPN is also an important mediator of bone remodeling,
and it has been reported to be a negative regulator of
calcification (17). Previous
studies have revealed that increasing OPN levels through the
overexpression of OPN mRNA caused a significant decrease in
BMP-2-inducible ALP activity and mineral deposition (17). The western blotting data in the
present study may demonstrate the same mechanism.
Based on these findings, it was hypothesized that a
low dose of estrone may produce positive effects on the
mineralization of osteoprecursor cells. Moreover, these results
also suggest that higher doses of estrone may be required to
significantly enhance the differentiation and mineralization of
these osteoprecursor cells.
References
|
1
|
Dechering K, Boersma C and Mosselman S:
Estrogen receptors alpha and beta: two receptors of a kind? Curr
Med Chem. 7:561–576. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Syed F and Khosla S: Mechanisms of sex
steroid effects on bone. Biochem Biophys Res Commun. 328:688–696.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang Y, Li LZ, Zhang YL, Zhu YQ, Wu J and
Sun WJ: LC, a novel estrone-rhein hybrid compound, concurrently
stimulates osteoprotegerin and inhibits receptor activator of
NF-kappaB ligand (RANKL) and interleukin-6 production by human
osteoblastic cells. Mol Cell Endocrinol. 337:43–51. 2011.
View Article : Google Scholar
|
|
4
|
López-Marcos JF, García-Valle S and
García-Iglesias AA: Periodontal aspects in menopausal women
undergoing hormone replacement therapy. Med Oral Patol Oral Cir
Bucal. 10:132–141. 2005.(In English and Spanish).
|
|
5
|
Tarakji B, Nassani MZ and Sloan P:
Immunohistochemical expression of estrogens and progesterone
receptors in carcinoma ex pleomorphic adenoma-undifferentiated and
adenocarcinoma types. Med Oral Patol Oral Cir Bucal. 15:e432–e436.
2010. View Article : Google Scholar
|
|
6
|
Coelingh Bennink HJ: Are all estrogens the
same? Maturitas. 47:269–275. 2004.PubMed/NCBI
|
|
7
|
Qu Q, Perälä-Heape M, Kapanen A, et al:
Estrogen enhances differentiation of osteoblasts in mouse bone
marrow culture. Bone. 22:201–209. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang Y, Li LZ, Zhang YL, et al: LC, a
novel estrone-rhein hybrid compound, promotes proliferation and
differentiation and protects against cell death in human
osteoblastic MG-63 cells. Mol Cell Endocrinol. 344:59–68. 2011.
View Article : Google Scholar
|
|
9
|
Park JB: Effects of fibroblast growth
factor 2 on osteoblastic proliferation and differentiation by
regulating bone morphogenetic protein receptor expression. J
Craniofac Surg. 22:1880–1882. 2011. View Article : Google Scholar
|
|
10
|
Odabaş ME, Ertürk M, Çinar Ç, Tuzüner T
and Tulunoğlu Ö: Cytotoxicity of a new hemostatic agent on human
pulp fibroblasts in vitro. Med Oral Patol Oral Cir Bucal.
16:e584–e587. 2011.PubMed/NCBI
|
|
11
|
Luo XH and Liao EY: Effects of estriol on
the proliferation and differentiation of human osteoblastic MG-63
cells. Endocr Res. 29:343–351. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Park JB: The effects of dexamethasone,
ascorbic acid, and beta-glycerophosphate on osteoblastic
differentiation by regulating estrogen receptor and osteopontin
expression. J Surg Res. 173:99–104. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Todorovic T, Dozic I, Vicente-Barrero M,
et al: Salivary enzymes and periodontal disease. Med Oral Patol
Oral Cir Bucal. 11:E115–E119. 2006.(In Spanish and English).
|
|
14
|
Fernández-Tresguerres-Hernández-Gil I,
Alobera-Gracia MA, del-Canto-Pingarrón M and Blanco-Jerez L:
Physiological bases of bone regeneration. I. Histology and
physiology of bone tissue. Med Oral Patol Oral Cir Bucal.
11:E47–E51. 2006.(In English and Spanish).
|
|
15
|
Park JB: Effects of doxycycline,
minocycline, and tetracycline on cell proliferation,
differentiation, and protein expression in osteoprecursor cells. J
Craniofac Surg. 22:1839–1842. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pinzone JJ, Stevenson H, Strobl JS and
Berg PE: Molecular and cellular determinants of estrogen receptor
alpha expression. Mol Cell Biol. 24:4605–4612. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Huang W, Carlsen B, Rudkin G, et al:
Osteopontin is a negative regulator of proliferation and
differentiation in MC3T3-E1 preosteoblastic cells. Bone.
34:799–808. 2004. View Article : Google Scholar : PubMed/NCBI
|