Introduction
Bladder cancer is the fourth most common cancer
among men in the United States. In 2010, it was estimated that
69,250 new cases of bladder cancer would be diagnosed and 14,990
deaths of this cancer would occur among men in the country
(1). To date, the only firmly
established risk factors for this disease appear to be age, gender,
smoking history and occupational exposure to certain chemicals
(2). However, these well-defined
factors cannot fully explain the observed differences in incidence
and mortality from bladder cancer amongst countries. Therefore,
other potential risk factors need to be identified.
Dietary factors have been thought to account for
about 30% of cancers in Western countries, making diet second only
to tobacco consumption as a preventable cause of cancer (3). The role of dietary factors on the
risk of bladder cancer is clearly plausible, as most substances or
metabolites are excreted through the urinary tract and are
consequently in direct contact with the mucosa of the bladder. Eggs
are an important source of protein and fat and are widely consumed
worldwide. A high intake of eggs has been associated with increased
risk of several cancers (4–6),
although other studies reported no association (7,8) or
even decreased risk (9).
Associations between bladder cancer incidence and intake of eggs
have also been investigated, yielding inconclusive results.
The purpose of the present study was to investigate
the association between bladder cancer and eggs intake. We
conducted a meta-analysis of all published studies with greater
statistical power to provide summary risk estimates for bladder
cancer in relation to egg consumption.
Materials and methods
Database search
Eligible studies were identified by searching the
PubMed database for relevant epidemiological studies of egg
consumption in relation to the risk of bladder cancer dating from
between January 1, 1980 and December 31, 2011. Additional
publications identified by hand-searching of reference lists were
also included. For computer searches, we used the following terms
in any field: ‘eggs’ or ‘egg’ or ‘meat’ or ‘meats’ or ‘animal
products’ combined with ‘bladder cancer’ or ‘urothelial cancer’.
Studies were included in the meta-analyses if they presented risk
estimates with corresponding 95% confidence intervals (CIs) from a
cohort or case-control study in English language on the association
between egg intake and incidence of bladder cancer. We also
included articles evaluating the risk of urinary tract cancer with
egg consumption, as the majority of cancers in the urinary tract
are urothelial cancers, and bladder cancer comprises almost 90%
cases of cancer in the lower urinary tract.
Study analysis
The following pieces of information were extracted
from the included studies: the name of the first author, the year
of publication, the country in which the study was conducted, study
design, year of follow-up (cohort studies) or data collection
(case-control studies), sample size, anatomical site of the
neoplasm, risk estimates with corresponding 95% CIs for highest vs.
lowest level of egg consumption, exposure assessment and range of
exposure, and adjusted covariates. We used risk estimates (REs) as
the measure of the association in cohort and case-control studies.
Crude (unadjusted) and adjusted REs were used for meta-analysis.
Adjusted REs were extracted directly from the original reports. For
the crude RE analysis, we extracted the number of cases and
controls for the case-control studies and the number of cases and
the person-years in the cohort studies, and calculated them from a
two by two table.
Statistical analysis
We estimated a pooled RE with 95% CI based on fixed-
and random-effects models depending on the heterogeneity of the
analysis. Statistical heterogeneity among studies included in the
meta-analysis was assessed using the Q (10) and I2 statistics
(11). Statistically significant
heterogeneity was defined as p<0.05. Publication bias was
assessed by the funnel plot and Egger’s test (12). Statistically significant
publication bias was defined as p<0.05. Statistical analyses
were performed using Stata 11.0 (Stata Corp, College Station, TX,
USA).
Results
Study characteristics
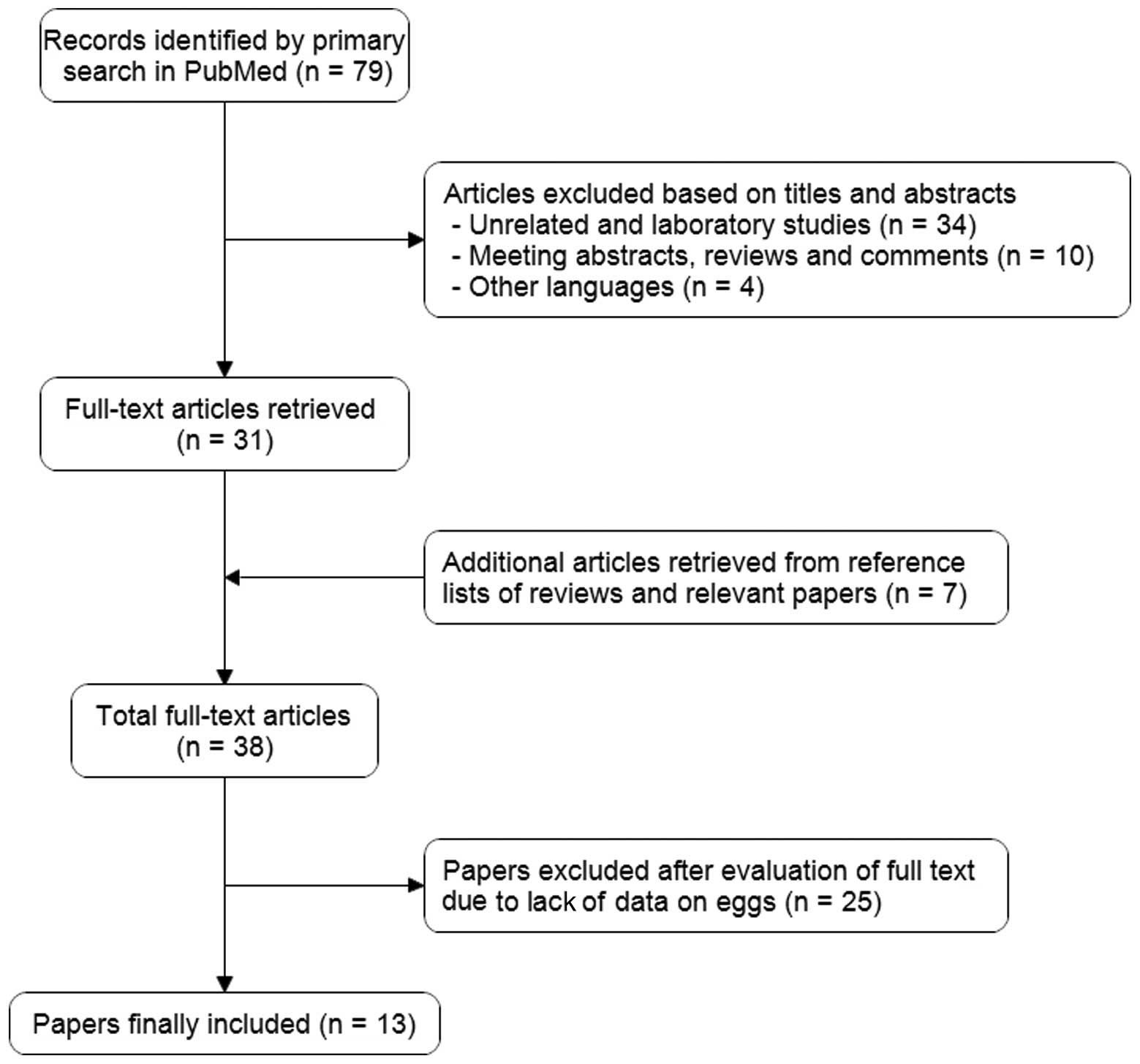
Fig. 1 shows the
process of identifying and selecting studies. We finally included
13 articles that examined the risk of bladder cancer with egg
intake published between January 1980 and December 2011, including
four cohort, seven hospital-based and two population-based
case-control studies (13–25). Six of these studies were conducted
in Europe (13–15,22,23,25),
one in the US (16), two in
Uruguay (19,24), and four in Japan (17,18,20,21).
Five studies included neoplasms of the urinary tract as cases
(13,15,16,20,21).
Table I presents the basic
characteristics of each study included in our meta-analysis.
 | Table IStudy characteristics of the published
cohort and case-control studies on egg intake and bladder
cancer. |
Table I
Study characteristics of the published
cohort and case-control studies on egg intake and bladder
cancer.
| Authors, year
(ref.) | Study design | Country | Study period | Cases/subjects | Location of
neoplasm | Egg consumption | RR (95% CI) | Variables of
adjustment | Assessment |
|---|
| Steineck et
al, 1988 (13) | Cohort | Sweden | 1968–1982 | 80/16,477 | Urothelial | Ever vs. never | 1.0 (0.6–1.6) | Age, gender and
smoking | Questionnaire |
| La Vecchia et
al, 1989 (14) | HCC | Italy | 1985–1987 | 163/344 | Bladder | The highest vs. the
first tertile | Unadjusted: 0.82
(0.44–1.54) | None | Interview |
| Steineck et
al, 1990 (15) | PCC | Sweden | 1985–1987 | 326/719 | Urothelial | Weekly vs. less
frequently | Boiled: 1.1
(0.7–1.8)
Fried: 1.8 (1.0–3.1) | Age, gender and
smoking | Questionnaire |
| Chyou et al,
1993 (16) | Cohort | USA | 1965–1991 | 96/7,090 | Lower urinary
tract | 5 times/week vs. ≤
once/week | Unadjusted: 0.85
(0.5–1.52)
Males: 0.82 (0.48–1.38) | Age, smoking | Both methods |
| Nagano et al,
2000 (17) | Cohort | Japan | 1979–1993 | 114/38,540 | Bladder | ≥ 5 times/week vs. ≤
once/week | 0.83 (0.5–1.36) | Age, gender,
radiation dose, smoking status, education level, body mass index
and calendar time | Questionnaire |
| Wakai et al,
2000 (18) | HCC | Japan | 1996–1999 | 297/592 | Bladder | The highest vs. the
first quartile | 1.0
(0.61–1.64)
Men: 0.89 (0.52–1.52) | Age, gender, smoking
and occupational history as a cook | Interview |
| Balbi et al,
2001 (19) | HCC | Uruguay | 1998–1999 | 144/720 | Bladder | The highest vs. the
first tertile | 1.82
(1.15–2.86)
Boiled:1.54 (0.96–2.46)
Fried: 2.24 (1.37–3.66) | Age, gender,
residence, urban/rural status, education, body mass index, tobacco
smoking, mate drinking, and total calories | Interview |
| Wakai et al,
2004 (20) | HCC | Japan | 1994–2000 | 124/744 | Urothelial | ≥ 5 times/week vs.
≤1–3 times/wk | Unadjusted: 0.47
(0.24–0.92)
Adjusted: 0.5 (0.27–0.9) | Age, gender, year of
first visit and cumulative consumption of cigarettes | Questionnaire |
| Sakauchi et
al, 2005 (21) | Cohort | Japan | 1988–1997 | 115/65,184 | Urothelial | ≥3–4 times/week vs.
1–2 times/month | Unadjusted: 0.8
(0.41–1.73)
Adjusted: 0.74 (0.38–1.44) | Age, gender and
smoking | Questionnaire |
| Radosavljević et
al, 2005 (22) | HCC | Serbia | 1997–1999 | 130/260 | Bladder | The highest vs. the
first tertile | Adjusted: 3.12
(1.1–8.8) | All variables that
independently contributed to risk for bladder cancer | Interview |
| Baena et al,
2006 (23) | HCC | Spain | Not mentioned | 74/163 | Bladder | ≥ 4 times/week vs.
never | 0.57
(0.28–1.14) | None | Interview |
| Anue et al,
2009 (24) | HCC | Uruguay | 1996–2004 | 254/2,371 | Bladder | ≥ 4 times/week vs.
never | 2.23
(1.3–3.83) | Age, gender,
residence, education, income, interviewer, smoking status, age at
starting smoking, cigarettes per day, years since quitting smoking,
duration of smoking, alcohol intake, intake of fruits and
vegetables, grains, dairy foods, total meat, other fatty foods,
mate drinking status, energy intake, BMI, men/women | Interview |
| Brinkman et
al, 2011 (25) | PCC | Belgium | 1999–2004 | 200/486 | Bladder | The highest vs. the
first tertile | 1.02
(0.62–1.67) | Gender, age,
smoking status, number of cigarettes smoked per day, number of
years smoking, occupational exposure to PAHs or aromatic amines and
energy intake | Questionnaire |
Meta-analysis
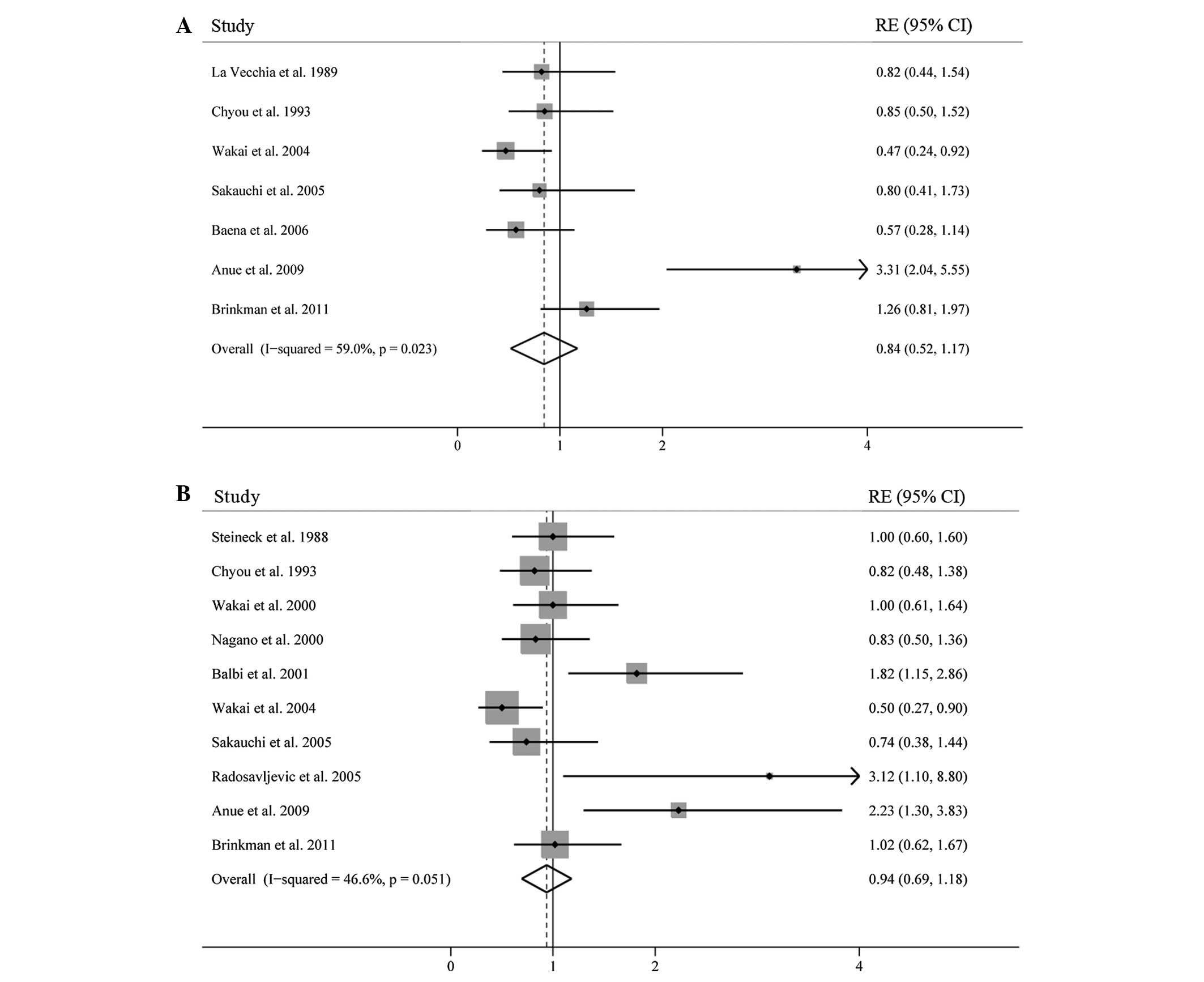
We first calculated the summary RE for the highest
vs. lowest category of egg consumption using the crude data; the
pooled RE was 0.84 (95% CI, 0.52–1.12) in a random effects analysis
(Fig. 2A). There was a
statistically significant heterogeneity across studies
(I2=66.6%; p=0.023). After excluding one study by Anue
et al, which reported the highest point estimates, the
p-value for heterogeneity among these studies was no longer
statistically significant (I2=20.5%, p=0.279), and a
significant inverse association was observed between egg
consumption and bladder cancer risk (RE=0.73; 95% CI,
0.51–0.95).
However, when using the adjusted data, high egg
consumption was not associated with a reduction in risk of bladder
cancer (RE=0.94; 95% CI, 0.69–1.18) (Fig. 2B). There was a weak heterogeneity
among all studies combined (I2=46.6%; p=0.051). To
evaluate the stability of the results, we also performed a
sensitivity analysis, which removed one study at a time. This
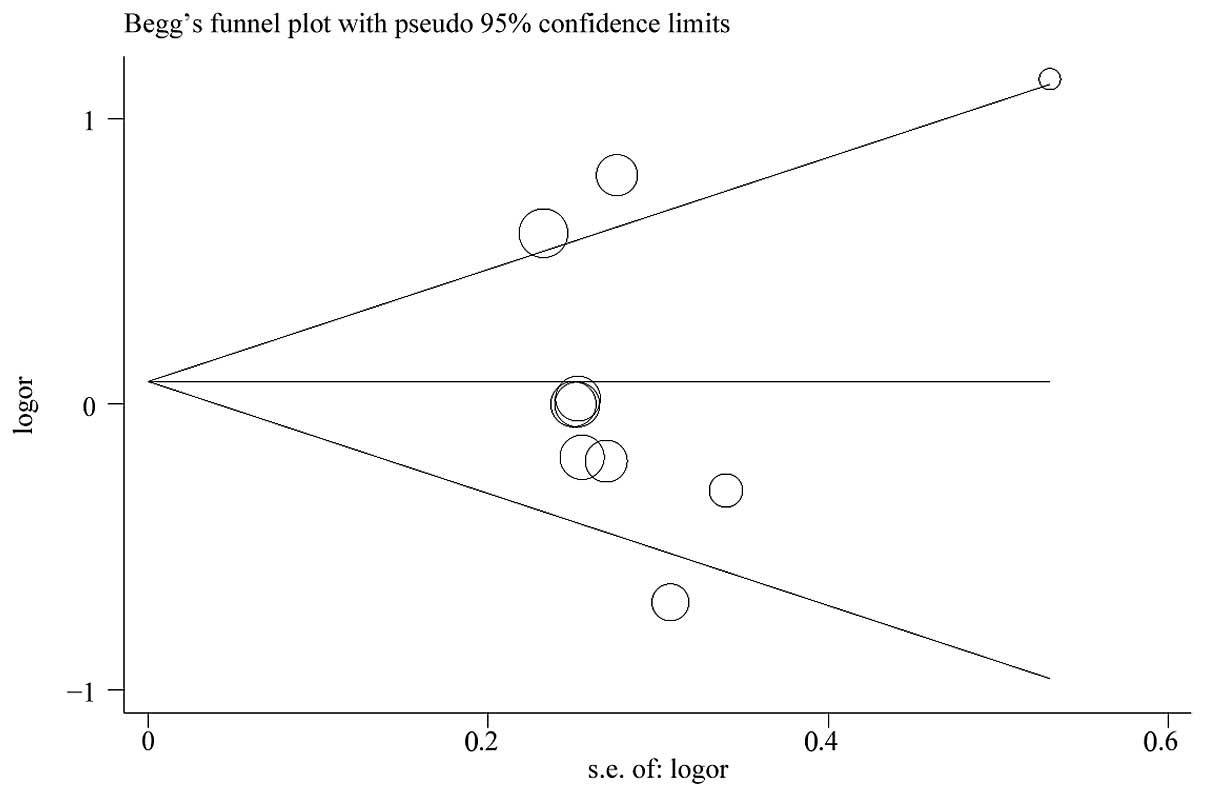
analysis confirmed the stability of our results. No indication of
publication bias was detected from either visualization of funnel
plot (Fig. 3) or Egger’s test
(p=0.883).
Results of the subgroup analyses by study
design, study population, egg cooking method and assessment are
shown in Table II
In the subgroup analyses by study design, neither
cohort studies (RE=0.85; 95% CI, 0.61–1.08) nor case-control
studies (RE=1.17; 95% CI, 0.66–1.68) showed that egg intake was
related to decreased bladder cancer risk. There was no evidence of
heterogeneity among cohort studies, but some evidence among
case-control studies. When subgroup analysis was conducted by study
population, we found a statistically significant protective effect
of egg consumption on bladder cancer for Japanese [including one
study of Japanese in Hawaii (16);
RR=0.72, 95% CI, 0.53–0.91], whereas a significantly increased risk
was observed Uruguayans (RR=1.95, 95% CI, 1.24–2.66), and no
association was found in Western countries (RR=1.19; 95% CI,
0.77–1.60). A subgroup analysis was also performed according to the
assessment method of egg consumption. A statistically significant
association was observed among studies using self-administered
questionnaire techniques (RR=0.76; 95% CI, 0.55–0.97) but not among
studies using interviews (RR=1.30; 95% CI, 0.77–1.84). In addition,
it was noted that bladder cancer appeared to have a stronger
positive association with fried egg intake (RR=2.00; 95% CI,
1.23–2.77), but not with boiled eggs.
Discussion
Eggs are one of nature’s most nutritious foods, with
low levels of saturated fat and high levels of protein. Although
various health concerns are associated with egg consumption, it
remains a popular ingredient in cooking worldwide. We
systematically reviewed published epidemiological studies on the
association between egg intake and the risk of bladder cancer. To
our knowledge, this is the first meta-analysis to evaluate the
relationship between them.
Egg consumption has not been studied as thoroughly
as the consumption of meat and dairy products in relation to cancer
risk. The most convincing evidence points to egg consumption as
increasing risk for colorectal cancer (24,26,27).
Several studies also found a positive association between egg
intake and cancers of the oral cavity and pharynx (4,28,29).
Recently, a large cohort study calculated that men who consumed 2.5
or more eggs per week had an 81% increased risk of lethal prostate
cancer compared with men who consumed fewer than 0.5 eggs per week
(30). The biological mechanism by
which eggs have a detrimental effect on cancer risk possibly
involves the high cholesterol content in egg yolk. Hu et al
(31) found that high cholesterol
intake is linked to increased risk of various cancers. Cholesterol
is a precursor of steroid hormones, and accumulation of cholesterol
in cells may affect prostate cancer risk through the formation of
androgen. Alterations in cholesterol level could also contribute to
cellular inflammation (32), which
is a critical component of tumor progression.
In the present study, we first found that egg
consumption was significantly associated with reduced risk of
bladder cancer when using unadjusted estimates. However, this
association became insignificant when models were adjusted for
potential confounding variables. In subgroup analyses, the pooled
analysis from the cohort studies, which have the advantage of being
less vulnerable to selection and recall bias than case-control
studies, also suggested no association. When subgroup analysis was
conducted by egg cooking methods, we found a strong positive
association between fried egg intake and bladder cancer risk,
although the analysis was combined from only two studies. The
elevated risk could be explained by formation of heterocyclic
amines, which are known to be involved in bladder carcinogenesis by
occupational exposure and smoking, during high-temperature cooking
of eggs. We also observed a significantly negative association
among studies using self-administered questionnaires but no
association among studies using interviewing techniques. This
contrast may be a consequence of response bias due to different
assessment techniques or to chance alone. Notably, in subgroup
analysis by study population, we noted a significant association of
egg consumption with decreased risk of bladder cancer ws for
Japanese, and this result was somewhat homogeneous (p=0.485;
I2=0). However, increased risk was observed in
Uruguayans. We are currently not able to identify a plausible
explanation for the difference. A possible role of ethnic
differences in genetic backgrounds might be taken into account.
Our study has several important limitations. First,
the number of studies included was limited and we did not search
for unpublished studies or original data, and therefore potential
publication bias might influence the findings. However, no
publication bias was indicated visually or in formal statistical
testing. Second, residual confounders are always of concern in
observational studies. Although the majority of included studies
was adjusted for a wide range of potential confounders for bladder
cancer, we were unable to exclude the possibility that other
unmeasured or inadequately measured factors confounded the true
association. Third, smoking is one of the major risk factors of
bladder cancer, but we are unable to conduct stratified analyses
adjusted by smoking, due to the lack of sufficient data from the
included studies.
In summary, we did not find a significant
association between overall egg intake and bladder cancer risk.
However, this association varied significantly across different
populations. Our findings have significant public health
implications for high egg consumption worldwide. Given the small
number of studies included in this meta-analysis, large prospective
studies are required to confirm this association.
References
|
1
|
Genkinger JM, Hunter DJ, Spiegelman D, et
al: A pooled analysis of 12 cohort studies of dietary fat,
cholesterol and egg intake and ovarian cancer. Cancer Causes
Control. 17:273–285. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Takeyama Y: Dietary intake as a risk
factor for pancreatic cancer in Japan: high cholesterol and low
vitamin C diet. J Gastroenterol. 40:324–325. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Key TJ, Schatzkin A, Willett WC, Allen NE,
Spencer EA and Travis RC: Diet, nutrition and the prevention of
cancer. Public Health Nutr. 7:187–200. 2004.PubMed/NCBI
|
|
4
|
Franceschi S, Favero A, Conti E, et al:
Food groups, oils and butter, and cancer of the oral cavity and
pharynx. Br J Cancer. 80:614–620. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nishimoto IN, Hamada GS, Kowalski LP, et
al: Risk factors for stomach cancer in Brazil (I): a case-control
study among non-Japanese Brazilians in Sao Paulo. Jpn J Clin Oncol.
32:277–283. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Marchand JL, Luce D, Goldberg P, Bugel I,
Salomon C and Goldberg M: Dietary factors and the risk of lung
cancer in New Caledonia (South Pacific). Nutr Cancer. 42:18–24.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ito LS, Inoue M, Tajima K, et al: Dietary
factors and the risk of gastric cancer among Japanese women: a
comparison between the differentiated and non-differentiated
subtypes. Ann Epidemiol. 13:24–31. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chow WH, Schuman LM, McLaughlin JK, et al:
A cohort study of tobacco use, diet, occupation, and lung cancer
mortality. Cancer Causes Control. 3:247–254. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
De Stefani E, Boffetta P, Ronco AL, et al:
Dietary patterns and risk of cancer of the oral cavity and pharynx
in Uruguay. Nutr Cancer. 51:132–139. 2005.
|
|
10
|
DerSimonian R and Laird N: Meta-analysis
in clinical trials. Control Clin Trials. 7:177–188. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Higgins JP, Thompson SG, Deeks JJ and
Altman DG: Measuring inconsistency in meta-analyses. BMJ.
327:557–560. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Begg CB and Mazumdar M: Operating
characteristics of a rank correlation test for publication bias.
Biometrics. 50:1088–1101. 1994. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Steineck G, Norell SE and Feychting M:
Diet, tobacco and urothelial cancer. A 14-year follow-up of 16,477
subjects. Acta Oncol. 27:323–327. 1988.PubMed/NCBI
|
|
14
|
La Vecchia C, Negri E, Decarli A, D’Avanzo
B, Liberati C and Franceschi S: Dietary factors in the risk of
bladder cancer. Nutr Cancer. 12:93–101. 1989.PubMed/NCBI
|
|
15
|
Steineck G, Hagman U, Gerhardsson M and
Norell SE: Vitamin A supplements, fried foods, fat and urothelial
cancer. A case-referent study in Stockholm in 1985–87. Int J
Cancer. 45:1006–1011. 1990.PubMed/NCBI
|
|
16
|
Chyou PH, Nomura AM and Stemmermann GN: A
prospective study of diet, smoking, and lower urinary tract cancer.
Ann Epidemiol. 3:211–216. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nagano J, Kono S, Preston DL, et al:
Bladder-cancer incidence in relation to vegetable and fruit
consumption: a prospective study of atomic-bomb survivors. Int J
Cancer. 86:132–138. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wakai K, Takashi M, Okamura K, et al:
Foods and nutrients in relation to bladder cancer risk: a
case-control study in Aichi Prefecture, Central Japan. Nutr Cancer.
38:13–22. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Balbi JC, Larrinaga MT, De Stefani E, et
al: Foods and risk of bladder cancer: a case-control study in
Uruguay. Eur J Cancer Prev. 10:453–458. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wakai K, Hirose K, Takezaki T, et al:
Foods and beverages in relation to urothelial cancer: case-control
study in Japan. Int J Urol. 11:11–19. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sakauchi F, Mori M, Washio M, et al:
Dietary habits and risk of urothelial cancer incidence in the JACC
Study. J Epidemiol. 15(Suppl 2): 190–195. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Radosavljevic V, Jankovic S, Marinkovic J
and Dokic M: Diet and bladder cancer: a case-control study. Int
Urol Nephrol. 37:283–289. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Baena AV, Allam MF, Del Castillo AS, et
al: Urinary bladder cancer risk factors in men: a Spanish
case-control study. Eur J Cancer Prev. 15:498–503. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Aune D, De Stefani E, Ronco AL, et al: Egg
consumption and the risk of cancer: a multisite case-control study
in Uruguay. Asian Pac J Cancer Prev. 10:869–876. 2009.
|
|
25
|
Brinkman MT, Buntinx F, Kellen E, et al:
Consumption of animal products, olive oil and dietary fat and
results from the Belgian case-control study on bladder cancer risk.
Eur J Cancer. 47:436–442. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Le Marchand L, Wilkens LR, Hankin JH,
Kolonel LN and Lyu LC: A case-control study of diet and colorectal
cancer in a multiethnic population in Hawaii (United States):
lipids and foods of animal origin. Cancer Causes Control.
8:637–648. 1997.PubMed/NCBI
|
|
27
|
Steinmetz KA and Potter JD: Egg
consumption and cancer of the colon and rectum. Eur J Cancer Prev.
3:237–245. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zheng W, Blot WJ, Shu XO, et al: Diet and
other risk factors for laryngeal cancer in Shanghai, China. Am J
Epidemiol. 136:178–191. 1992.PubMed/NCBI
|
|
29
|
Levi F, Pasche C, La Vecchia C, Lucchini
F, Franceschi S and Monnier P: Food groups and risk of oral and
pharyngeal cancer. Int J Cancer. 77:705–709. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Richman EL, Kenfield SA, Stampfer MJ,
Giovannucci EL and Chan JM: Egg, red meat, and poultry intake and
risk of lethal prostate cancer in the prostate-specific
antigen-era: incidence and survival. Cancer Prev Res (Phila).
4:2110–2121. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hu J, La Vecchia C, de Groh M, Negri E,
Morrison H and Mery L: Dietary cholesterol intake and cancer. Ann
Oncol. 23:491–500. 2012. View Article : Google Scholar
|
|
32
|
Ferretti G, Bacchetti T, Negre-Salvayre A,
Salvayre R, Dousset N and Curatola G: Structural modifications of
HDL and functional consequences. Atherosclerosis. 184:1–7. 2006.
View Article : Google Scholar : PubMed/NCBI
|