Introduction
Pulmonary surfactant is a compound comprising
phospholipids and proteins. Phospholipids (mainly dipalmitoyl
phosphatidyl choline, DPPC) account for 70–80% these pulmonary
surfactants, while pulmonary surfactant-associated proteins
including SP-A, SP-B, SP-C and SP-D account for 10% and the
remaining 10% are the neutral fat (mainly cholesterol). Pulmonary
surfactant is crucial in the reduction of the surface tension at
the air-water interface where pulmonary surfactant is degraded and
recycled. SP-B is an important pulmonary surfactant-associated
protein capable of reducing or altering the surface tension by
changing the surface area, preventing alveolar collapse (1–3).
Studies have shown SP-B protein deficiency to be associated with
the pathogenesis of neonatal respiratory distress syndrome (RDS)
(4,5). The protein expression is known to be
regulated by upstream genes. To explore whether the SP-B mRNA
expression is associated with the pathogenesis of neonatal RDS,
in situ hybridization was conducted to detect the SP-B mRNA
expression in the lung of neonates, who were treated in the
neonatal intensive care unit (NICU) between July, 2006 and October,
2010 and succumbed to RDS.
Materials and methods
Patients and samples
A total of 60 unrelated Han neonates in Beijing who
succumbed to RDS were recruited from the NICU between July, 2006
and October, 2012 and then subdivided into ≤32-,
32–36+6- and ≥37-week group (n=20 per group) on the
basis of gestational age. In addition, 60 neonates who succumbed to
other diseases, including congenital heart diseases,
bronchopulmonary dysplasia and persistent pulmonary hypertension,
were enrolled as controls. The controls were also subdivided into
gestational age-matched groups (P>0.05). The neonates developed
RDS within 30 min to 6 h after birth and progressive dyspnea was
the major clinical manifestation. Blood gas analysis showed
hypercapnia and hypoxemia. These findings together with the chest
X-ray results were employed to confirm the diagnosis of grade III
or IV RDS. During the hospitalization, RDS patients were repeatedly
treated with pulmonary phospholipids (200 mg/kg) from swine (a
total of 3 or 4 times), with high frequency oscillatory mechanical
ventilation being performed simultaneously. However, these patients
died within 14 days. Informed consent was obtained from relatives
and the whole study was approved by the Ethics Committee of General
Hospital of Beijing Military Region.
Main reagents
A DAB kit, an in situ hybridization kit for
SP-B (Wuhan Boster Biotech Co., China), and other domestic reagents
(analytically pure) were used in the present study.
Digoxin-conjugated oligonucleotide probes targeting human SP-B were
synthesized at the Department of Molecular Genetics as follows: i)
5′-ATGATGCCAGGTGTGTAGCC-3′; ii) 5′-AGAACCTCCCCATTGGAGC-3′; iii)
5′-GGCCTTGT GTCCAGGGAC-3′.
Sample collection
Lung tissues were collected within 30 min after
death. Samples were collected from the five lobes of RDS patients.
In the control group, the lung tissues were randomly selected. The
lung tissues were fixed in 4% paraformaldehyde, embedded in
paraffin and consecutively cut into 5-μm sections.
Detection of SP-B mRNA expression
In situ hybridization was conducted to
measure the mRNA expression of SP-B in the lung according to the
manufacturer’s instructions, with modification. Sections were
routinely deparaffinized and dehydrated and then treated with 3%
methanol in H2O2 for 20 min. After washing in
distilled water three times (5 min for each), sections were treated
with pepsin in 3% citric acid at 37°C for 2 min. The sections were
then washed three times in PBS for in situ hybridization (5
min for each) and in distilled water once. Sections were fixed in
1% paraformaldehyde/0.1 MPBS (pH 7.2–7.6) at room temperature for
10 min. The sections were treated with pre-hybridization solution
at 42°C for 4 h and then with digoxin-conjugated probes at 42°C for
20 h subsequent to washing three times in distilled water. After
washing twice with 2X SSC at 37°C (5 min for each), 0.5X SSC at
37°C for 15 min and 0.2X SSC at 37°C for 10 min, the sections were
incubated at 37°C for 30 min. Subsequently, these sections were
treated with biotin-conjugated mouse anti-digoxin antibody at 37°C
for 90 min. The sections were washed four times in PBS for in
situ hybridization (5 min for each) and treated with SABC at
37°C for 30 min. The sections were then incubated with
biotin-conjugated peroxidase at 37°C for 30 min after washing three
times in PBS for in situ hybridization (5 min for each).
Subsequent to washing in PBS for in situ hybridization four
times (5 min for each), the sections were visualized with DAB for 5
min, followed by washing in water and counterstaining with
hematoxylin for 1 min. The sections were treated with 1%
hydrochloric acid in alcohol followed by dehydration and
transparentization, and mounted. The cell nucleus was stained blue
and the sections were observed under a microscope. Representative
images were captured. Of note, no probe or antibody was used, in
the negative control group.
Detection of SP-B mRNA-positive
cells
Positive cells had yellow brown granules in the
cytoplasm following staining. Positive cells were counted at a
magnification of x400. Three sections were selected from each
sample and 10 fields were randomly selected from each section. A
total of 30 positive cells was counted in each sample, and the
number of SP-B mRNA-positive cells was determined in each
subgroup.
Determination of SP-B mRNA
deficiency
The lower limit of normal of the number of SP-B mRNA
positive cells was the mean - 2 standard deviations (−2SD). The
sample with the number of cells lower than the lower limit of
normal of the control group was defined as SP-B mRNA
deficiency.
Statistical analysis
Statistical analysis was performed with SPSS version
13.0. Data were shown as the mean ± standard deviation (±SD). The
paired t-test was employed to determine comparisons between the two
groups, while the one way analysis of variance was performed to
determine comparisons among different groups. SP-B mRNA deficiency
was analyzed with χ2 test. P<0.05 was considered
statistically significant.
Results
Clinical data
RDS developed within 30 min to 12 h after birth and
dyspnea progressed rapidly. Results of the blood gas analysis
showed hypercapnia and hypoxemia. These findings together with the
chest X-ray results confirmed the diagnosis of RDS (grade III or
IV). During the hospitalization, pulmonary phospholipids from the
swine were repeatedly administered at 200 mg/kg (3–4 times). High
frequency oscillatory mechanical ventilation was simultaneously
performed. Although comprehensive therapy was also performed, these
patients died naturally or refused further treatment due to
economic concern. The neonates with grade IV RDS generally died
within 7 days after birth.
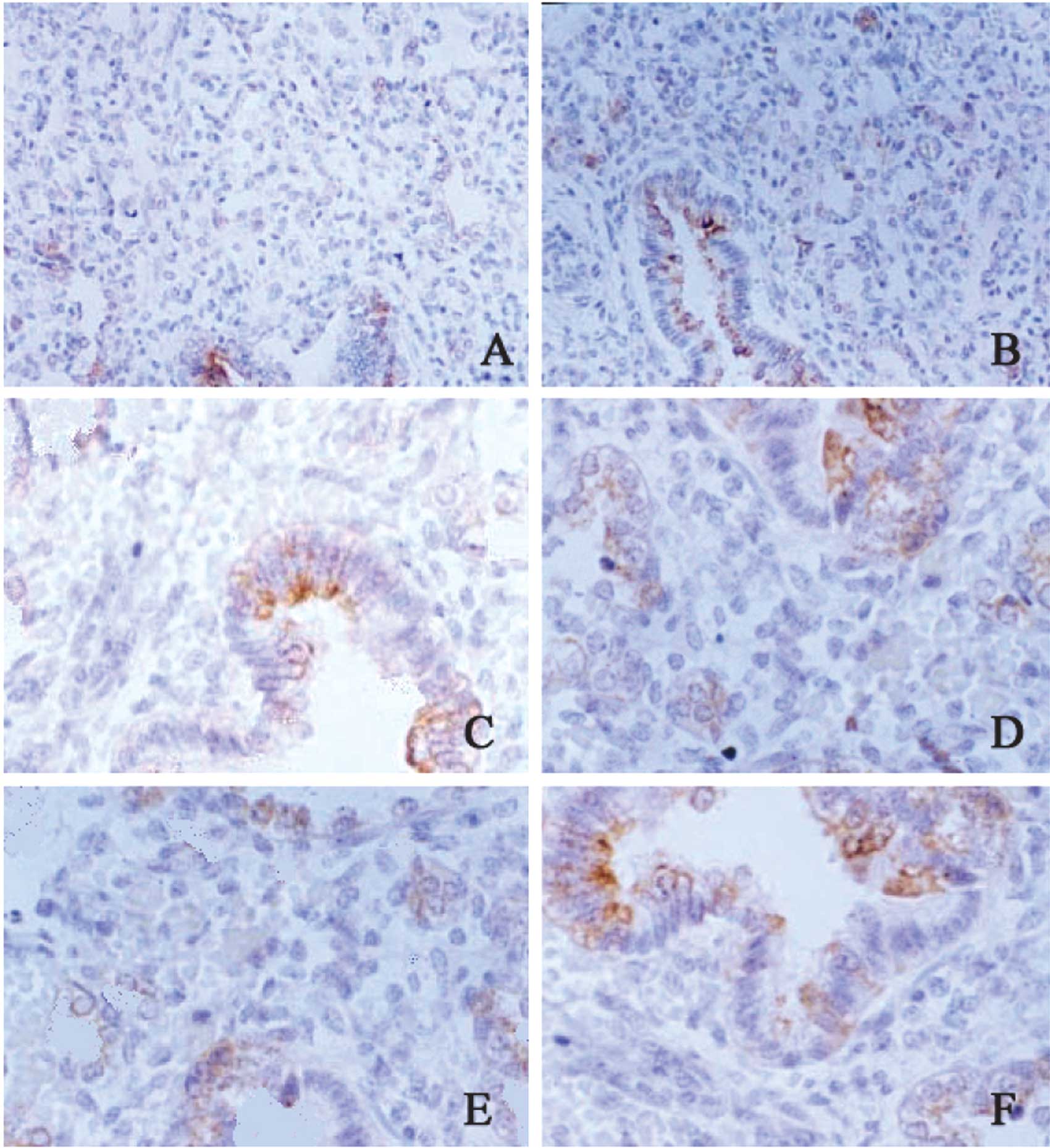
SP-B mRNA expression in the lung
The SP-B mRNA was mainly found in the cytoplasm,
stained yellow. The color and the extent of staining were
different, varying in the neonates in different groups, with
different gestational age and with different severities of RDS. Of
60 patients with RDS, 5 had a gestational age of 24 weeks, 4 had 26
weeks, 4 had 31 weeks, 7 had 34 weeks, 5 had 36 weeks, 4 had 38
weeks and 6 had 42 weeks. The SP-B mRNA expression in the lung in
the RDS neonates was markedly lower than that in the gestational
age-matched controls. Four neonates with a gestational age of 38
weeks were diagnosed with RDS of grade IV and the SP-B mRNA
expression was lower than that in the remaining neonates. In the
control group, 5 neonates with a gestational age of 24 weeks and 3
with 26 weeks had a lower SP-B mRNA expression as compared to the
lower limit of normal (Fig.
1).
Number of SP-B mRNA-positive cells
The number of SP-B mRNA-positive cells was
34.106±15.85 in the RDS group and 53.82±11.44 in the control group,
showing a significant difference (t=7.812, P<0.001). Among the
RDS groups, the number of SP-B mRNA-positive cells was comparable
among RDS patients with a different gestational age (F=2.348,
P>0.105). However, among the controls, the number of SP-B
mRNA-positive cells were found to be elevated with the increase in
gestational age (F=50.124, P<0.001). In the ≤32-week group, the
number of SP-B mRNA-positive cells in RDS patients was markedly
reduced as compared to that of the controls (t=3.185, P<0.01).
In the 32–36+6-week group, the SP-B mRNA-positive cells
in RDS patients were significantly reduced when compared to those
of the controls (t=9.342, P<0.001). In the ≥37-week group, the
number of SP-B mRNA-positive cells in RDS patients was markedly
lower than that in the controls (t=4.238, P<0.001) (Table I).
 | Table INumber of cells positive for SP-B mRNA
in the lung of different groups (mean ± SD). |
Table I
Number of cells positive for SP-B mRNA
in the lung of different groups (mean ± SD).
| Group | ≤32-week
subgroup |
32–36+6-week subgroup | ≥37-week
subgroup | F | P-value |
|---|
| Control | 41.20±9.84 | 58.50±3.74 | 61.8±6.04 | 50.124 | <0.001 |
| RDS | 32.2±7.81 | 30.00±13.12 | 40.10±22.09 | 2.348 | >0.105 |
| t | 3.185 | 9.342 | 4.238 | | |
| P-value | <0.01 | <0.001 | <0.001 | | |
SP-B mRNA deficiency
In the RDS group, there were 13 neonates in the
≤32-week group, 12 neonates in the 34–36+6-week group
and 10 neonates in the ≥37-week group with a lower SP-B mRNA level
as compared to the lower limit of normal in the control group, with
a frequency of SP-B mRNA deficiency of 58.3% (35/60). In the
control group, there were 5 neonates in the ≤32-week group and 3
neonates in the 34–36+6-week group with a lower SP-B
mRNA level as compared to the lower limit of normal, with a
frequency of SP-B mRNA deficiency of 13.3% (8/60). Results of the
statistical analysis revealed a significant difference in the
frequency of SP-B mRNA deficiency in the RDS and control groups
(χ2=26.421, P<0.001).
Discussion
The deficiency of pulmonary surfactant of any cause
is known to potentially cause neonate RDS. Although the SP-B
protein accounts for 1–2% of PS compound, SP-B is a crucial SP in
the maintenance of normal surfactant function (6). SP-B has the potential to facilitate
the spread of the pulmonary surfactant on the air-water interface,
promote the entry of pulmonary surfactant into the interface and
enhance the function of pulmonary surfactant, thereby reducing the
alveolar surface tension and preventing alveolar collapse (7). Findings of recent studies have shown
that SP-B reduces the alveolar surface tension and prevents
alveolar collapse and also exerts an antibacterial effect (6,8). The
SP-B gene located in 2p12-2p11.2, with a length of 950 bp,
comprises 11 exons, while the pre-SP-B is encoded by 10 exons
(9). The mature human SP-B is the
product of a single gene and is encoded by exons VI and VII
deriving from its precursor with a molecular weight of 42 kDa. The
mature human SP-B is a lung specific protein comprising 79 amino
acids with a molecular weight of 8 kDa (10). In 1981, Teja et al (11) identified the SP-B deficiency as an
autosomal recessive disease for the first time. The SP-B deficiency
has family specificity and the SP-B gene mutation varies among
different races, populations and diseases. Results of another study
have shown the incidence of SP-B deficiency in African Americans to
be higher than in Caucasian Americans (12). The SP-B gene mutation has been
shown to be associated with some respiratory diseases, including
neonatal RDS, acute respiratory distress syndrome, congenital
pulmonary alveolar proteinosis, adult chronic obstructive pulmonary
emphysema and chronic lung diseases in children (13–15).
In the present study, the SP-B mRNA expression in
the lung was detected in RDS and non-RDS neonates. Results showed
that SP-B mRNA was mainly found in the cytoplasm and the SP-B mRNA
level varied in different groups, including neonates with different
gestational ages and those with RDS of different severities. Of 60
neonates with RDS, the SP-B mRNA expression in the lung was not
enhanced with the increase in gestational age. In 5 neonates with a
gestational age of 24 weeks, 4 with 26 weeks, 4 with 31 weeks, 7
with 34 weeks, 5 with 36 weeks, 4 with 38 weeks and 6 with 42
weeks, the SP-B mRNA expression was markedly lower than that in the
gestational age-matched controls. In addition, four neonates with a
gestational age of 38 weeks were diagnosed with RDS of grade IV,
with the SP-B mRNA expression being lower than that in the
remaining RDS group. Of 60 controls, 5 with a gestational age of 24
weeks and 3 with 26 weeks exhibited a lower SP-B mRNA expression
than the lower limit of normal. Furthermore, the frequency of SP-B
mRNA deficiency was determined to be 58.3% in the RDS group and
13.3% in the control group. The statistical analysis showed the
frequency of SP-B mRNA deficiency in RDS neonates to be markedly
higher than that in the controls. Thus, the SP-B protein deficiency
may be attributed to the reduced transcription of SP-B mRNA, which
is indirectly involved in the pathogenesis of RDS. This finding may
be explained as follows: the transcription of SP-B mRNA is reduced,
and then the SP-B protein expression is decreased. Thus, the
formation of lamellar bodies or tubular myelin is disrupted, the
mature SP-B is reduced, and the secondary mature SP-C is also
decreased. Consequently, the spread and adherence of the pulmonary
surfactant on the air-water interface is reduced, and the activity
of pulmonary surfactant is compromised. Subsequently, the ability
of the pulmonary surfactant to reduce the alveolar surface tension
was compromised resulting in alveolar collapse. The fluid exudates
from the capillary into the alveoli. In addition, the reduction of
SP-B reduces resistance, and the antimicrobial activity of SP-B in
the lung is attenuated or disappears resulting in the presence of
neonate RDS. In the present study, we confirmed the hypothesis of
our previous study (16). The
alteration in the quality and quantity of SP-B protein suggests the
changes in the upstream genes. In addition, SP-B mRNA expression
was higher than the SP-B protein expression, suggesting a potential
interruption in SP-B gene translation, resulting in the reduction
of the SP-B protein. Therefore, SP-B in neonatal RDS should be
further investigated.
Numerous studies have been conducted to investigate
the correlation between SP-B deficiency and some diseases. Results
have shown that SP-B deficiency is correlated with certain
pulmonary diseases including neonatal RDS and chronic obstructive
pulmonary (17,18). Recent ongoing studies have
investigated this correlation yielding primary results (19). However, the mechanism underlying
the reduction in SP-B protein should be examined. Studies on the
genetic SP-B deficiency may therefore provide evidence for the
clinical diagnosis and treatment of diseases in neonates.
Acknowledgements
This study was sported by the grant
from National Natural Science Foundation of China (30871397).
References
|
1
|
Nkadi PO, Merritt TA and Pillers DA: An
overview of pulmonary surfactant in the neonate: genetics,
metabolism, and the role of surfactant in health and disease. Mol
Genet Metab. 97:95–101. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mazela J, Merritt TA, Gadzinowski J and
Sinha S: Evolution of pulmonary surfactants for the treatment of
neonatal respiratory distress syndrome and paediatric lung
diseases. Acta Paediatr. 95:1036–1048. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen MG, Atkins CL, Bruce SR, Khan AM, Liu
Y and Alcorn JL: Infant formula alters surfactant protein A (SP-A)
and SP-B expression in pulmonary epithelial cells. Pediatr
Pulmonol. 46:903–912. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yin XJ, Luo FP, Li AH, et al: Relationship
between reduced expression of surfactant protein B and neonatal
respiratory distress syndrome in twenty Han ethnic group neonates
in China. Chin J Pediatr. 46:9–12. 2008.
|
|
5
|
Lu WC, Xiang W, Wu M, et al: Relationship
between pulmonary surfactant-associated protein B polymorphisms and
the susceptibility to neonatal respiratory distress syndrome.
Zhongguo Dang Dai Er Ke Za Zhi. 14:24–27. 2012.(In Chinese).
|
|
6
|
Chroneos ZC, Sever-Chroneos Z and Shepherd
VL: Pulmonary surfactant: an immunological perspective. Cell
Physiol Biochem. 25:13–26. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schürch D, Ospina OL, Cruz A and Pérez-Gil
J: Combined and independent action of proteins SP-B and SP-C in the
surface behavior and mechanical stability of pulmonary surfactant
films. Biophysical J. 99:3290–3299. 2010.PubMed/NCBI
|
|
8
|
Orgeig S, Hiemstra PS, Veldhuizen EJ, et
al: Recent advances in alveolar biology: evolution and function of
alveolar proteins. Respir Physiol Neurobiol. 173(Suppl): S43–S54.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Puthothu B, Forster J, Heinze J, Heinzmann
A and Krueger M: Surfactant protein B polymorphisms are associated
with severe respiratory syncytial virus infection, but not with
asthma. BMC Pulm Med. 7:6–8. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tafel O, Latzin P, Paul K, Winter T,
Woischnik M and Griese M: Surfactant proteins SP-B and SP-C and
their precursors in bronchoalveolar lavages from children with
acute and chronic inflammatory airway disease. BMC Pulm Med.
8:62008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Teja K, Cooper PH, Squires JE and
Schnatterly PT: Pulmonary alveolar proteinosis in four siblings. N
Engl J Med. 305:1390–1392. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hamvas A, Nogee LM, Wegner DJ, et al:
Inherited surfactant deficiency cause by uniparental disomy of rare
mutations in the surfactant protein-B and ATP binding cassette,
subfamily a, member 3 genes. J Pediatr. 155:854–859. 2009.
View Article : Google Scholar
|
|
13
|
Sumita Y, Sugiura T, Kawaguchi Y, et al:
Genetic polymorphisms in the surfactant proteins in systemic
sclerosis in Japanese: T/T genotype at 1580 C/T (Thr131IIe) in the
SP-B gene reduces the risk of interstitial lung disease.
Rheumatology. 47:289–291. 2008. View Article : Google Scholar
|
|
14
|
Woodworth BA, Wood R, Bhargave G, Cohen
NA, Baatz JE and Schlosser RJ: Surfactant protein B detection and
gene expression in chronic rhinosinusitis. Laryngoscope.
117:1296–1301. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lyra PP, Vaz FA, Moreira PE, Hoffmann JW,
Demello DE and Diniz EM: Comparison of surfactant protein B
polymorphisms of healthy term newborns with preterm newborns having
respiratory distress syndrome. Braz J Med Biol Res. 40:779–786.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yin XJ, Li LH, Wang Y, et al: A study on
expression of surfactant protein B in neonatal respiratory distress
syndrome. Chin J Neonatol. 26:336–339. 2011.
|
|
17
|
Lyra PP, Diniz EM, Abe-Sandes K, Angelo
AL, Machado TM and Cardeal M: Surfactant protein B gene
polymorphism in preterm babies with respiratory distress syndrome.
Braz J Med Biol Res. 44:66–72. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bakvad-Hansen M, Nordestgaard BG and Dahl
M: Surfactant protein B polymorphisms, pulmonary function and COPD
in 10231 individuals. Eur Respir J. 37:791–799. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yin XJ, Xie L, Chi JH, et al: SP-B
deficiency induced neonatal respiratory distress symdrome: a report
of 3 cases. Chin J Neonatol. 26:2682011.
|