Introduction
The hypoxia-responsive element (HRE) is the minimal
indirect cis-regulatory element transactivated by
hypoxia-inducible factor (HIF). Data from over 70 genes suggest
that endogenous HREs are composite regulatory elements comprising
the conserved HIF-binding site (HBS) with an A/GCGTG core sequence
and a highly variable flanking sequence. A single HBS is essential
but not sufficient for activation by hypoxia. Binding sites of
transcription factors provided by the flanking sequence are not
necessary for hypoxia regulation but are required to amplify the
hypoxic response or make the HRE tissue-specific (1). In contrast to regulation of the HIF
pathway, our knowledge of the fundamental structural features of
HREs is quite limited. In this study, we firstly synthesized the
minimal HRE and then introduced NT4-TAT-His-PR39 to HRE-regulated
adeno-associated virus (AAV) to investigate the expression in
hypoxic vascular endothelial cells (VEC) of human umbilical vein
(CRL-1730 cell lines) and the angiogenesis-promoting effect on
myocardial cells in pigs with acute myocardial infarction
(AMI).
Materials and methods
Cell line, plasmid, tool enzyme, cell
and cloning vector
Escherichia coli TOPIO, T/T AT-His vector,
pBV220/NT4 vector, AAV vector (pSSHG-CMV), helper virus pAAV/Ad,
helper packaging plasmid PFG140 and biological enzyme were
purchased from the Xi’an Sino-American Biotechnology Co., Ltd.
Hypoxic (1% O2) incubator was provided by the Department
of Pathology, School of Basic Medical Sciences, Fourth Military
Medical University. CRL-1730 cells were provided by the Department
of Pharmacology, Xian Jiaotong University. CoCl2 was
provided by the Department of Molecular Genetics, Fourth Military
Medical University. Recombinant virus
pSS-HRE-CMV-NT4-TAT-6His-PR39-PolyA-AAV was packed. Experimental
miniature pigs were purchased from the Animal Center, Fourth
Military Medical University (Chengdu Dossy Biological Technology
Co., Ltd.). Pentobarbital was purchased from the Reagent Center,
Fourth Military Medical University. Suminaxin injection was
manufactured by the Jilin Huamu Animal Health Product Co., Ltd. The
experimental procedures in the present study were approved by the
local ethics committee and the animals were treated according to
guidelines set by our University’s Animal Laboratory (The Fourth
Military Medical University, Shaanxi, China).
Synthesis and cloning of infusion gene
HRE-CMV-MCS-PolyA
Design and synthesis of primers. According to
data in the literature and databases, the minimal HRE sequence was
CTGCACGTA CTGCACGTA CTGCACGTA CTGCACGTA and 35 bp sequences spacing
in the TA Box. Based on nucleotide sequences in GenBank,
XbaI-recognition sequence TCTAGA (cohesive end) was added to
the 5′ and 3′ ends and the protective base G was introduced to the
5′ end of forward and reverse primers. Using the DNAsis software,
forward and reverse primers were designed as follows and were
synthesized by the Shanghai Generay Biotech Co., Ltd.: F1, ‘-g
5′-TCTAGA CTGCACGTA CTGCACGTA CTGCACGTA CTGCACGTA atcaattacg
gggtcattag-3′; ‘-g 5′-TCTAGA acgcgttaag atacattgat gag-3′.
The complete pGEM-T-HRE-CMV-MCS-PolyA was obtained by PCR
amplification and this sequence was completely consistent with that
of gene expression box designed, as proved by DNAsis software.
Construction of pSS-HRE-CMV-MCS-PolyA-AAV
vector. pSS-HRE-CMV was digested with both E. co 721 and
BamHI. The digested HRE-CMV segment was connected with the
human pBV220/NT4-TAT-His-PR39 vector with the method
aforementioned. The recombinant T/TAT-His-PR39 was harvested and
digested by both NaeI and BamHI. The digested segment
was connected with the human pBV220/NT4 vector. The recombinant
pBV220/NT4-TAT-His-PR39 was harvested and sent to Sangon Biotech
(Shanghai) Co., let for gene sequencing.
Package of recombinant virus. Using the
calcium phosphate precipitation method, HEK-293 cells were
co-transfected with plasmid pssHG-9H-CMV-NT4-PR39 helper packaging
plasmid pAAV/Ad with cloning Rep and Cap genes, and adenoviral
plasmid PFG140 to produce the recombinant AAV-9H-CMV-NT4-PR39.
Recombinant AAV under HRE promoter
control for myocardium of ischemic heart disease (IHD) pigs
Construction of myocardial infarction in
miniature pigs. Eighteen miniature pigs were randomized into
the experimental group (HRE-AAV-PR39 group, n=8), control group 1
(physical saline group, n=4) and 2 (EV group, n=4). Every pig was
fasted for 8 h and then injected intramuscularly with 2 ml of
Suxinmian injection and 18 ml of 5% pentobarbital (1 ml/kg). Under
electrocardiographic (ECG) monitoring, left ventriculography was
performed by introducing the guide wire and 6F arterial sheath;
subsequently, 6F Amplatz guide catheter was exchanged to conduct
coronary angiography. The occluded site of the left anterior
descending artery (LAD) was identified. Next, a PTCA balloon
catheter was introduced into the distal LAD artery with the aid of
a microcatheter and microguide wire. The balloon (2.0 or 2.5) was
placed 0.5–1.0 cm below the first diagonal branch of LAD and
inflated with 4–6 times of atmospheric pressure to occlude LAD for
90 min. If LAD was completely occluded, the AMI model was
successfully constructed. One milliliter of AAV/HRE-PR39 or 1 ml of
physical saline/control virus was injected through the
microcatheter. After that, the catheter and sheath were removed.
Magnetic resonance imaging (MRI) was performed preoperatively and
24 h, 1, 2 and 3 weeks postoperatively.
MRI scanning post AMI. In this study, a
magnetom triotim 3.0-T magnetic resonance scanner (Siemens, German)
was used to perform MRI scanning under both the conventional MR
sequence and a novel sequence sensitive to myocardium edema
(T2-weighted TrueFISP imaging of myocardium).
Additionally, myocardium delayed perfusion scanning was also
performed under the TrueFISP-PSIR sequence. Changes in MR images of
MI area over time were observed. Bright-blood sequence: scanning
was performed using the novel sequence sensitive to myocardium
edema (T2-weighted TrueFISP imaging of myocardium).
Scanning parameters were as follows: TR/TE, 281.95/1.09 msec;
section thickness, 6 mm; gap, 0; matrix, 256x256; FOV, 360 mm;
RFOV, 75%; acquisition window, 715 msec; 2 trigger pulse, 2;
trigger delay, 433 msec and Flip, 90°C. For the TrueFISP-PSIR
sequence to performed myocardium delayed perfusion scanning, 2D
TrueFISP-PSIR sequence was used. The contrast agent was injected at
a rate of 0.1 mmol/kg. Scanning was started 10 min later. Scanning
parameters were as follows: TR/TE, 82.24/1.09 msec; section
thickness, 6 mm; gap, 0; matrix, 256x256; FOV, 360 mm; RFOV, 75%;
acquisition window, 750 msec; 2 trigger pulse, 2; trigger delay,
300 msec and Flip, 90°C.
Molecular assessment after AMI
A pig of every group was sacrificed respectively 1,
2 and 3 weeks postoperatively. The fresh heart was harvested for
immunohistochemical examination to measure expression of HIF-1α at
the area of ischemia. The myocardium tissue was fixed with
paraform, dehydrated with 60–100% alcohol solution, and was
embedded in soft, neutral and hard paraffin wax. Sections were made
followed by dewaxing in 170–100%. Ischemia myocardium samples of
each pig were collected for SABC immunohistochemical staining to
observe expression of HIF-1α around the area of infarction and
compare expression levels between the experimental and control
groups.
Results
Expression of HRE-triggering AAV-PR39
under hypoxia
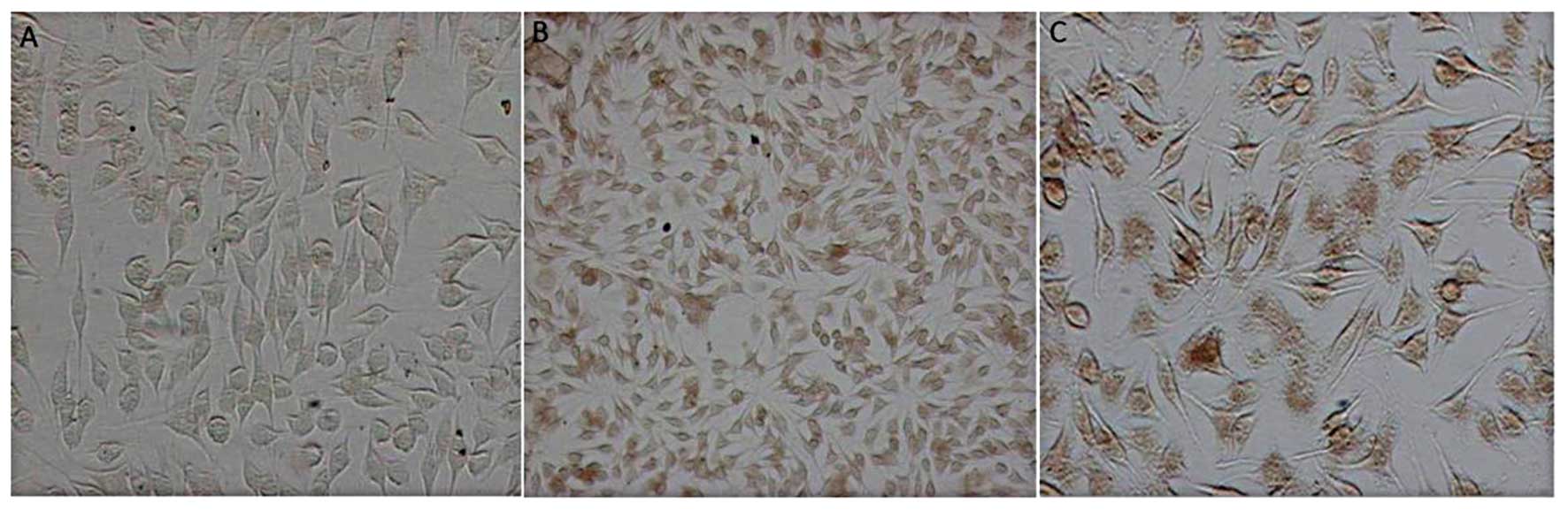
The OD of the 6xHis staining area was calculated
with the IPP Image Processing software, which represented the level
of PR39 protein. In the AAV/HRE-PR39 group, massive brown area was
present in and out of the cytoplasm under hypoxia but only in the
cytoplasm under common oxygen; in non-viral cells, a small quantity
of brown area was observed in the cytoplasm (a trace of 6xHis
background staining was present in the normal cytoplasm). The
average OD value for positive 6xHis was 132.3±8.5 in control group
2, 23.3±5.4 in the experimental group and 48.5±7.3 in control group
1 (control group 2 vs. experimental group, P<0.05; control group
1 vs. 2, P<0.05), illustrating that HRE triggers the expression
of NT4-TAT-His-PR39 of hypoxic CRL-1730 cells (Fig. 1).
Imaging results
Delayed perfusion MR images in different stages of
AMI models could significantly distinguish normal and infarcted
myocardium. The area of infarction was displayed clearly.
Significant differences in myocardium changes over time post AMI
were noted between the experimental and control group. The area of
infarction in the control group changed slightly over time (no
inflammatory edema period was taken into account). In the
experimental group, the area of infarction decreased over time and
the difference was statistically significant, as measured by the
eFilm software. The left ventricular ejection fraction post AMI
also differed significantly between the two groups.
Pathological results
Pathological examinations showed that the cardiac
apex, the anterior wall of the left ventricle, and the anterior
septal were infarcted. The infarcted myocardium was crisp and white
and significantly decreased over time in the experimental group.
The white myocardium was collected for paraffin sectioning.
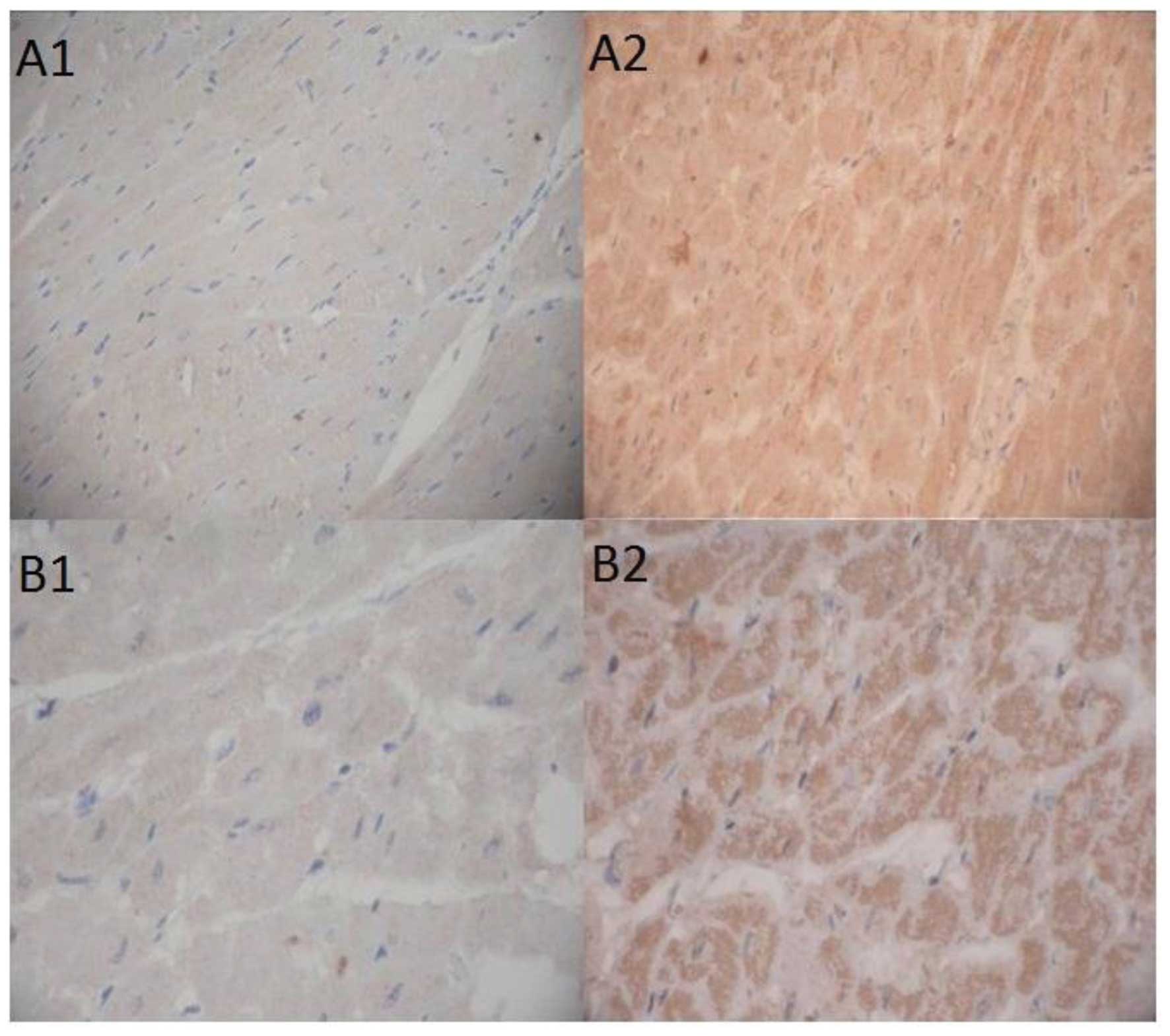
Expression of HIF-1α in myocardial cells was assayed using
immunohistochemistry, as described previously. Digital
microphotographs showed that the average OD value for positive
HIF-1α was 5469±395.7 in the experimental group and 1686±423.2 in
the control group (P<0.001), respectively, suggesting that rAAV
expressing PR39 could significantly increase the level of HIF-1α
proteins in hypoxic myocardial cells (Fig. 2).
Discussion
The molecular biological mechanism of cell hypoxia
inhibiting responsive regulation suggests that intracellular HIF is
activated and highly expressed under hypoxia; furthermore, it can
bind the expression regulatory element of hypoxic reactive genes,
HRE, triggering and enhancing expression of hypoxic reactive
proteins and cytokines via cis-activation (2–6).
Various HRE-controlled expression vectors have been constructed
since 1991 when Semenza et al (7) discovered HRE. However, HREs differ
greatly in the structure, sequence and hypoxic inducible ability
with hypoxic responsive genes (9).
A single HRE is not enough to induce a hypoxic response and
hypoxic-responsive regulatory vectors often require tandem repeat
HREs. However, multiple repeats necessarily increase the sequence
length of regulatory elements and the number of genetic elements
introduced in the human body and cells.
In 2008, Kaluz et al (10) analyzed HRE and HBS of multiple
hypoxic responsive genes and the structure and sequence of the
artificial-synthesized minimal HRE (36 bp) with the monomer,
forward/reverse tandem sequences and point mutation method, which
effectively regulated hypoxic expression. It was proven that the
36-bp HRE had the optimal distance with the TATA Box of the CMV
promoter and that its hypoxic-inducible ability was higher than
4xEPO HRE and comparable with the CMV promoter.
If the CMV promoter of cytomegalovirus was adopted
as the promoter of adenovirus, the downstream PR39 gene will
continuously express under any environment (hypoxia or normal
oxygen), inducing excessive expression of VEGF protein. VEGF will
accumulate in the systemic circulation, causing hemangioma
(11,12). In this study, the 9HRE segment was
added to the upstream of the AAV promoter CMV, which rapidly
triggers the expression of PR39 in myocardial cells under hypoxia
and timely terminates the expression of PR39 when normal oxygen
returns. Thus, some adverse effects associated with continuous high
expression of VEGF are prevented and thus, PR39 is more safe and
effective in clinical gene therapy.
According to previous literature, the minimal HRE is
planned to insert to the site of 35 bp in the upstream of CMV.
However, there is no digestion site in the upstream of the CMV
promoter (13). Therefore, the
synthetic 36 bp HRE and the 35 bp sequence in the upstream of the
CMV promoter (TATA Box) are amplified to the HRE-regulated CMV
promoter with PCR technique utilizing the plasmid containing the
CMV promoter as the template in accordance with the base
complementary principle. The complete HRE-CMV-MCS-PolyA gene
sequence is 918 bp and the minimal HRE is the four repeats of
CTGCACGTA, easily resulting in the palindrome structure and other
mismatching modes during PCR synthesis. In our study, the reactant
amount and reaction conditions of the PCR reactive system were
adjusted multiple times and HRE-CMV-MCS-PolyA was successfully
synthesized and verified by gene sequencing.
In this study, AAV was used as the viral vector
carrying PR39, which could stably express PR39 in the host cell
during a long period. Recovery of AMI is time enduring. Hence,
AAV-PR39 is superior to the conventional method for treating AMI
and also valuable in the prevention and management of AMI. It is
promising for thr gene therapy of IHD.
The HRE-CMV-MCS-PolyA gene segment is a
hypoxia-regulatory promoter requiring increasing research for
hypoxia-regulation. This promoter has more potent hypoxia-inducible
ability and is more minor than current hypoxia-regulatory
promoters. It offers a minor promoter with the regulating ability
for constructing thr rAAV vector, i.e. it offers a relatively large
remaining space to insert the objective gene for the 4-kb space
which can be inserted in the endogenous gene. We believe that
HRE/CMV can be valuable in future gene therapy.
To better investigate the effect of HRE-regulated
PR39 on IHD, AMI was modeled. Since miniature pigs have a coronary
artery similar to that of humans with less collateral circulation
(55,58), it is used for modeling. We constructed AMI models by
introducing a balloon to block the coronary artery and verified the
model by monitoring ECG and measuring troponin I and creatine
kinase isoenzymes at different stages.
Delayed perfusion MR images at different stages of
AMI models significantly distinguish normal and infracted
myocardium. The infarcted area was displayed clearly. Significant
differences in changes of myocardium over time post AMI were noted
between the experimental and control group. The area of infarction
in the control group changed slightly over time (no inflammatory
edema period was taken into account). In the experimental group,
the area of infarction decreased over time and the difference was
statistically significant, as measured by the EFilm software. The
left ventricular ejection fraction post AMI was also significantly
different between the two groups.
Myocardial cells of the AMI miniature pigs receiving
recombinant viruses and physical saline were analyzed using
immunohistochemistry and the results showed that rAAV secreting
PR39 could significantly increase the level of HIF-1α protein in
hypoxic myocardial cells, thereby upregulating the expression of
VEGF and promoting angiogenesis.
These animal experiments utilized only imaging data
to show the ability of the recombinant virus to reduce the
infarcted myocardial area and increase the HIF-1α protein in
hypoxic myocardial cells. In this study, PR39 was administered
immediately after AMI modeling and only pathological and
immunohistochemical examinations were performed. However, in
clinical practice, most AMI patients are not treated in a timely
manner but are treated several hours post onset. Therefore,
quantitative studies of the effects of the recombinant virus on IHD
gene therapy should be performed to investigate the efficacy of the
recombinant virus in this study following longer periods of
AMI.
Acknowledgements
This study was supported by the
National Natural Science Foundation of China (grant no.
30871041).
References
|
1
|
Wenger RH, Stiehl DP and Camenish G:
Integration of oxygen signaling at the consensus HRE. Sci STKE.
306:re122005.PubMed/NCBI
|
|
2
|
Beck T, Weinmann R and Caro G:
Characterization of hypoxia-responsive enhancer in the human
erythropoietin gene shows presence of hypoxia-inducible 120-kD
nuclear DNA-binding protein in erythropoietin-producing and
nonproducing cells. Blood. 82:704–711. 1993.
|
|
3
|
Shen GM, Zhao YZ, Chen MT, et al:
Hypoxia-inducible factor-1 (HIF-1) promotes LDL and VLDL uptake
through inducing VLDLR under hypoxia. Biochem J. 441:675–683. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fijalkowska I, Xu W, Comhair SA, et al:
Hypoxia inducible-factor1alpha regulates the metabolic shift of
pulmonary hypertensive endothelial cells. Am J Pathol.
176:1130–1138. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Su H, Arakawa-Hoyt J and Kan YW:
Adeno-associated viral vector-mediated hypoxia response
element-regulated gene expression in mouse ischemic heart model.
Proc Natl Acad Sci USA. 99:9480–9485. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shen F, Fan Y, Su H, et al:
Adeno-associated viral vector-mediated hypoxia-regulated VEGF gene
transfer promotes angiogenesis following focal cerebral ischemia in
mice. Gene Ther. 15:30–39. 2008. View Article : Google Scholar
|
|
7
|
Semenza GL, Roth PH, Fang HM and Wang GL:
Transcriptional regulation of genes encoding glycolytic eazymes by
hypoxia-inducible factor-1. J Biol Chem. 269:23757–23763.
1994.PubMed/NCBI
|
|
8
|
Hu HW, Li XK, Zheng RY, Xiao J, Zeng JQ
and Hou ST: bFGF expression mediated by a hypoxia-regulated
adenoviral vector protects PC12 cell death induced by serum
deprivation. Biochem Biophys Res Commun. 390:115–120. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shi Q, Zhang P, Zhang J, Chen X, Lu H,
Tian Y, Parker TL and Liu Y: Adenovirus-mediated brain-derived
neurotrophic factor expression regulated by hypoxia response
element protects brain from injury of transient middle cerebral
artery occlusion in mice. Neurosci Lett. 465:220–225. 2009.
View Article : Google Scholar
|
|
10
|
Kaluz S, Kaluzová M and Stanbridge EJ:
Rational design of minimal hypoxia-inducible enhancers. Biochem
Biophys Res Commun. 370:613–618. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Springer ML, Chen AS, Kraft PE, et al:
VEGF gene delivery to muscle: potential role for vasculogenesis in
adults. Mol Cell. 2:549–558. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Park MS, Ravi V and Araujo DM: Inhibiting
the VEGF-VEGFR pathway in angiosarcoma, epithelioid
hemangioendothelioma, and hemangiopericytoma/solitary fibrous
tumor. Curr Opin Oncol. 22:351–355. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lalwani AK, Walsh BJ, Carvalho GJ,
Muzyczka N and Mhatre AN: Expression of adenoassociated virus
integrated transgene within the mammalian vestibular organs. Am J
Otol. 19:390–395. 1998.PubMed/NCBI
|