Introduction
Testicular cancer (TC) is a relatively rare type of
cancer that may often leads to metastasis. Approximately 8,590 new
cases are likely to be diagnosed in the United States in 2012, of
which 360 individuals are expected to succumb to the disease
(1). Although the disease is not
common, as the likelihood of developing testicular cancer is
approximately 1 in 270, the rate of testicular cancer has been on
the increase in the United States and many other countries, with
the increase mostly in seminomas (1). Caucasians have 5 times the risk of
developing the disease compared to African-Americans and 3 times
that of Asian-Americans (1). As
yet, no rationale for the increase has been found. TC affects males
of all ages although 90% of cases occur in men between the ages of
20 and 54 years. Factors that increase the risk of developing
testicular cancer include undescended testicle, Kleinfelder
syndrome, family history of testicular cancer, HIV infection,
particularly in those with AIDS, carcinoma in situ, and
cancer of the other testicle (1).
TC is considered to be one of the most curable forms of cancer.
However, if the cancer has metastasized beyond the lymph nodes, the
5-year survival is reduced to 71% (1). The primary modality of spread of TC
is through the lymph node system to the retroperitoneum and in
certain cases to other lymph nodes along the mid-line of the body.
Spread continues through the bloodstream, common in patients with
advanced germ cell tumors or those with choriocarcinoma or
embryonal carcinoma elements. The main sites for blood-borne
metastatic tumors are the lungs, followed by the liver, bone and
brain (2,3).
Metastatic malignant melanoma cells, specifically
B16, have been successfully utilized for experimental metastasis to
study the effectiveness of anticancer agents, since melanoma cells
are extremely aggressive and metastasize to secondary areas of the
body, such as lymph nodes, lungs, liver, brain or bone (4). Hart and Fidler (4) studied the role of organ selectivity
in the determination of metastatic patterns of B16 melanoma and
concluded that the outcome of metastasis was dependent on tumor
cell properties and host factors, supporting the ‘seed and soil’
hypothesis to explain the non-random pattern of cancer metastasis.
For example, although the circulatory mode of spread leads to the
dissemination of a number of malignant cells, it cannot fully
explain the patterns of distribution of numerous tumors, such as
the infrequent metastatic development in organs including the
spleen or skeletal muscle with highly developed vasculature. In
their study, Zeidman and Busso (5)
reported that tumor cells from different tumors interacted
differently with the capillary bed of a given organ, while
Sugarbaker et al (6) found
that tumor cell suspensions from different types of tumors injected
into the same site in rats established different patterns of
metastases. Experimental data have indicated that melanoma B16
cells preferentially metastasize to specific organs, such as the
lungs and liver (4,7,8).
A nutrient mixture (NM) containing lysine, proline,
ascorbic acid and green tea extract has demonstrated anticancer
activity in a number of human cancer cell lines, inhibiting cancer
cell growth, MMP secretion, invasion, metastasis and angiogenesis
(9). In a previous study, we
demonstrated that NM was effective in inhibiting the pulmonary
metastasis of B16FO melanoma cells injected into the tail vein of
C57BL/6 mice, especially when nutrients were delivered
intravenously or intraperitoneally (7). We also demonstrated the effectiveness
of dietary supplementation with NM to prevent experimental hepatic
metastasis by studying its effect on the intrasplenic injection of
B16FO cells into athymic nude mice (10).
The aim of this study was to investigate the effect
of NM on the experimental metastasis of melanoma cells by the
intratesticular injection of B16FO cells into male nude mice. This
experimental model of metastasis was selected to study the
effectiveness of nutrients against metastasis to the lungs and
other organs from the testes since melanoma cells are aggressive
enough to result in significant metastasis in mice, particularly
pulmonary metastasis, a common end organ of metastasis for
testicular cancer. Furthermore, the intratesticular model was found
to be an effective model for studying mechanisms of metastasis and
evaluating treatment strategies due to the stable formation of
tumors with metastatic potential (11).
Materials and methods
Cancer cell line and culture
Murine melanoma B16FO cells obtained from ATCC
(American Type Culture Collection, Rockville, MD, USA) were
maintained in Dulbecco’s modified Eagle’s medium (DMEM)
supplemented with 10% fetal bovine serum, 100 U/ml penicillin and
100 μg/ml streptomycin. The media and sera used were obtained from
ATCC, while the antibiotics (penicillin and streptomycin) were
purchased from Gibco-BRL (Long Island, NY, USA).
Animals
Male nude mice, approximately 9–11 weeks of age on
arrival, were purchased from Simonsen Laboratories (Gilroy, CA,
USA), and kept in microisolator cages under pathogen-free
conditions on a 12-h light/dark schedule for a week. The animals
were cared for in accordance with institutional guidelines for the
care and use of experimental animals.
Diet
The regular rodent diet was obtained from Purina
Mills (Gray Summit, MO, USA). The NM 1% supplemented diet mix was
milled and pressed by Purina Mills, LLC, and generated by Vitatech
(Tustin, CA, USA). The NM 1% diet comprised the following in the
ratio indicated: vitamin C (as ascorbic acid and as Mg, Ca, and
palmitate ascorbate) 700 mg; L-lysine 1000 mg; L-proline 750 mg;
L-arginine 500 mg; N-acetyl cysteine 200 mg; standardized green tea
extract (80% polyphenol) 1000 mg; selenium 30 μg; copper 2 mg;
manganese 1 mg.
Experimental design
Male athymic mice (n=12), 10–12 weeks of age, were
anesthetized by inhalation utilizing isofluorane USP (Abbott
Laboratories, Chicago, IL, USA). The right side of the abdomen
overlying the testis was sterilely prepped and a skin incision of 1
cm was made to expose the right testis. Mice were inoculated with
5×105 B16FO melanoma cells in 100 μl of PBS into the
right testis, while the left testis was left untreated. The
cavities were sutured and clamped. After inoculation, the mice were
randomly divided into 2 groups. The control group (n=6) was fed
regular Purina mouse chow diet, while the mice in the NM 1% group
(n=6) were fed the same diet, but supplemented with 1% NM. The
quantity of diet provided to the mice was unrestricted, however,
the mice consumed, on average, 4 g of their respective diets/day.
Thus, the supplemented mice received ∼40 mg of NM/day, indicating
that they received the following amounts of NM components/day:
ascorbic acid 7 mg, L-lysine 10 mg, green tea extract 10 mg,
L-proline 7.5 mg, L-arginine 5 mg, N-acetyl cysteine 2 mg, selenium
0.3 μg, copper 0.02 mg, manganese 10 μg. Four weeks later the mice
were sacrificed, the abdominal cavity was opened and testes, lungs,
kidneys, livers and spleens were excised from all the animals and
examined. Since growth of testes in the control animals expanded
profoundly into the peritoneum making it difficult to determine the
limits of the organs, measurements of volume and weight were not
carried out. Growth of melanoma colonies in testicles were
evaluated by sectioned tissue. Lung metastasis was evaluated by
observation of the melanoma colonies. A control mouse was
sacrificed and examined at 1 week. All procedures were performed
according to humane and customary care and use of experimental
animals and conducted under protocols approved by the Internal
Animal Care and Use Committee (IACUC).
Histopathology
Testicular tissues were fixed in 10% buffered
formalin, embedded in paraffin and cut at 4–5 microns. Sections
were deparaffininzed through xylene and graduated alcohol series to
water, and stained with hematoxylin and eosin (H&E) for
microscopic evaluation by IDEXX Reference Laboratories.
Results
Melanoma growth in testes and peritoneal
metastasis
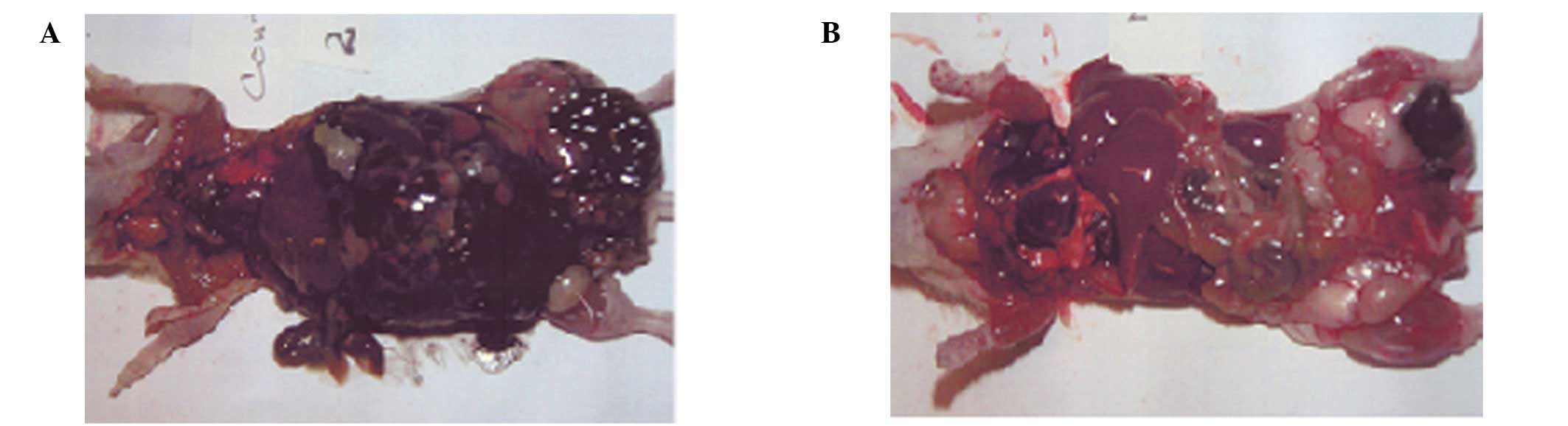
The mice (6/6) in the control group exhibited
extensive metastasis in the peritoneal cavity, which was totally
masked by B16FO melanoma cells, in contrast to the NM 1% group,
which showed no peritoneal metastasis in 3 mice and mild metastasis
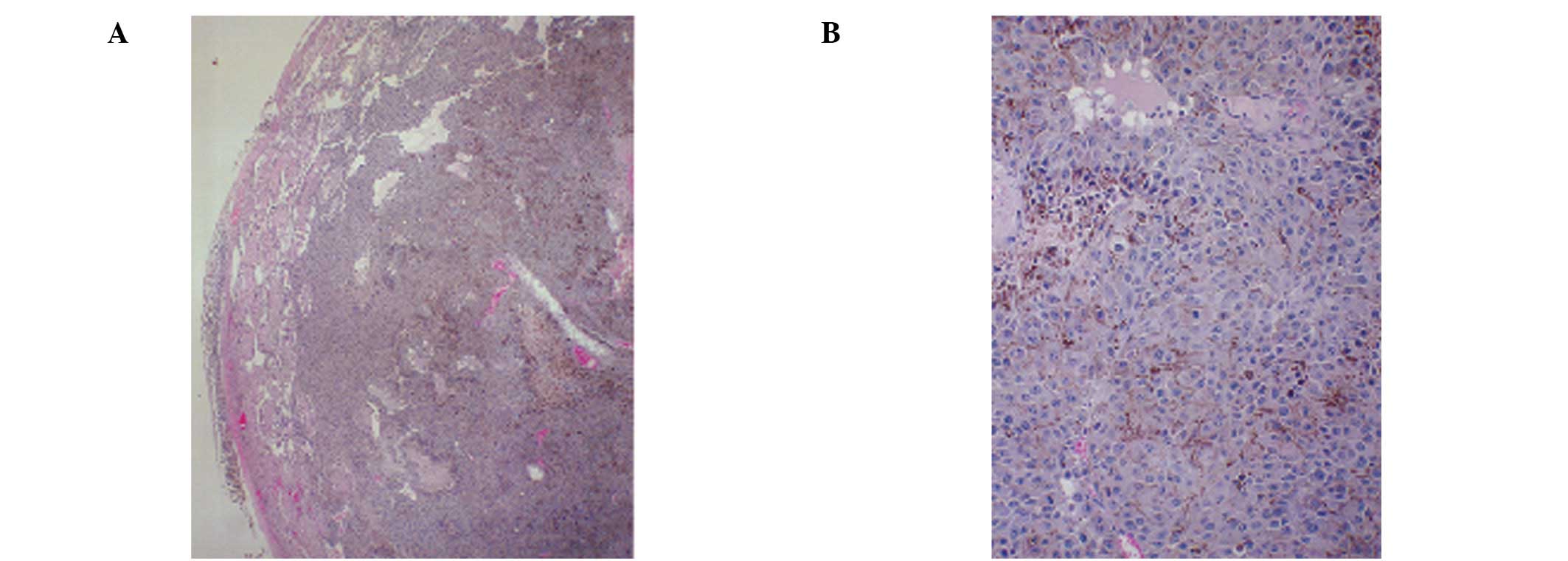
in 3 mice. Representative images of the peritoneum in the two
groups are shown in Fig. 1. The
right testis in the control group was severely enlarged and
replaced by invading malignant melanoma cells and the remaining
testicular tissue was represented by necrotic seminiferous tubules.
The capsular region of the testis was severely infiltrated with a
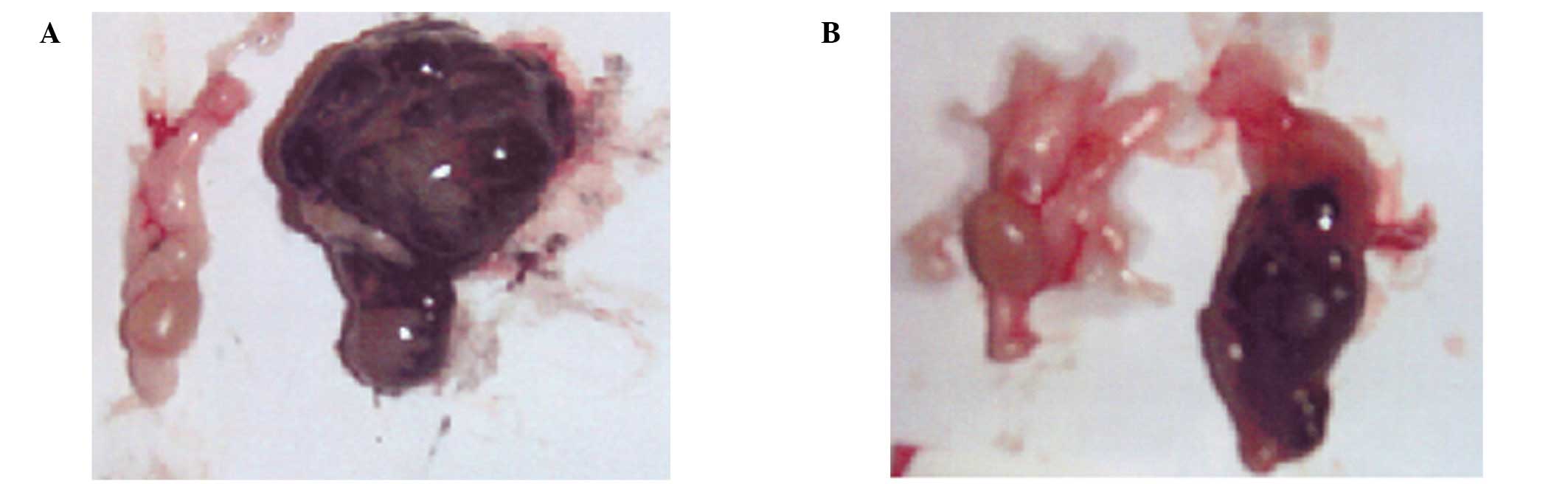
population of mixed cells. By contrast, in the NM 1% group, the
testes were slightly enlarged and the seminiferous tubules in the
area of invasion showed evidence of degeneration. The left testes
of the two groups shows no evidence of melanoma colonies; however,
the right testes of the control group of nude mice shows extensive
melanoma invasion, while the NM 1% group shows mild invasion of
melanoma cells (Fig. 2). Profound
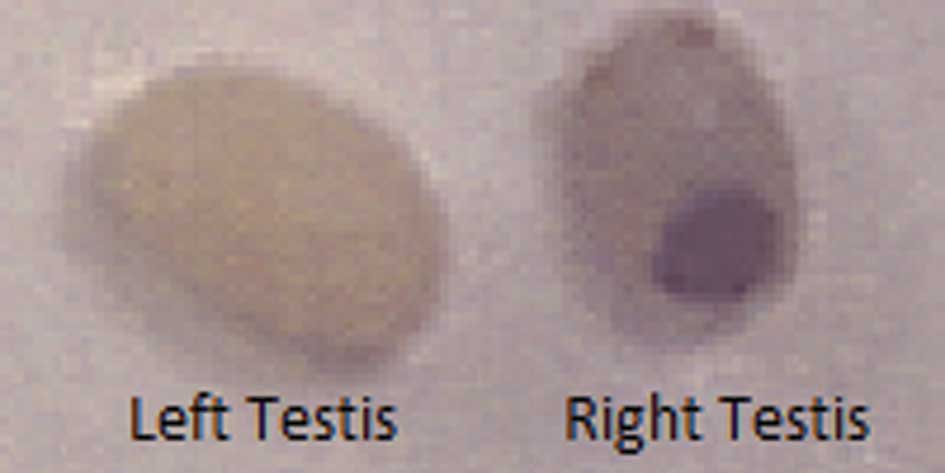
enlargement of the right testis was observed in the control group
mice. By contrast, some enlargement of the right testis was evident
in the NM 1% group, although it was much smaller than that observed
in the control group. Representative gross images of the left and
right testes in the control group at 1 week post-injection are
shown in Fig. 3.
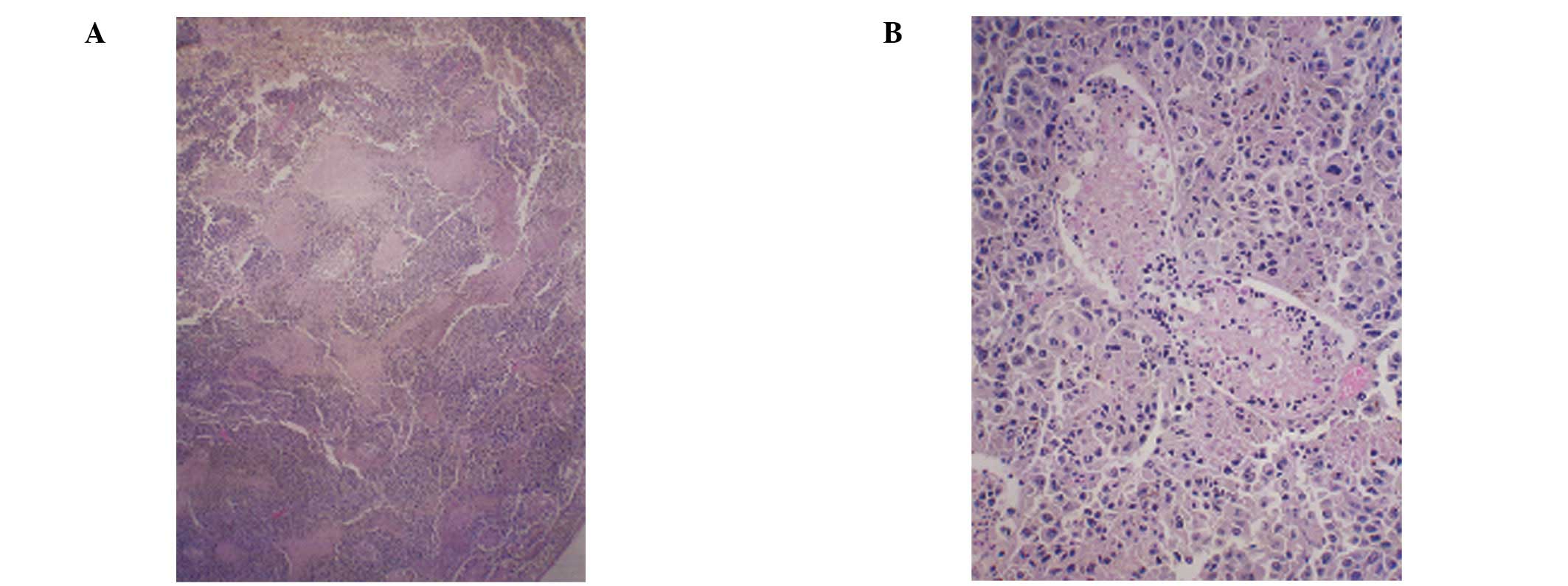
Histopathology of representative
testicular sections
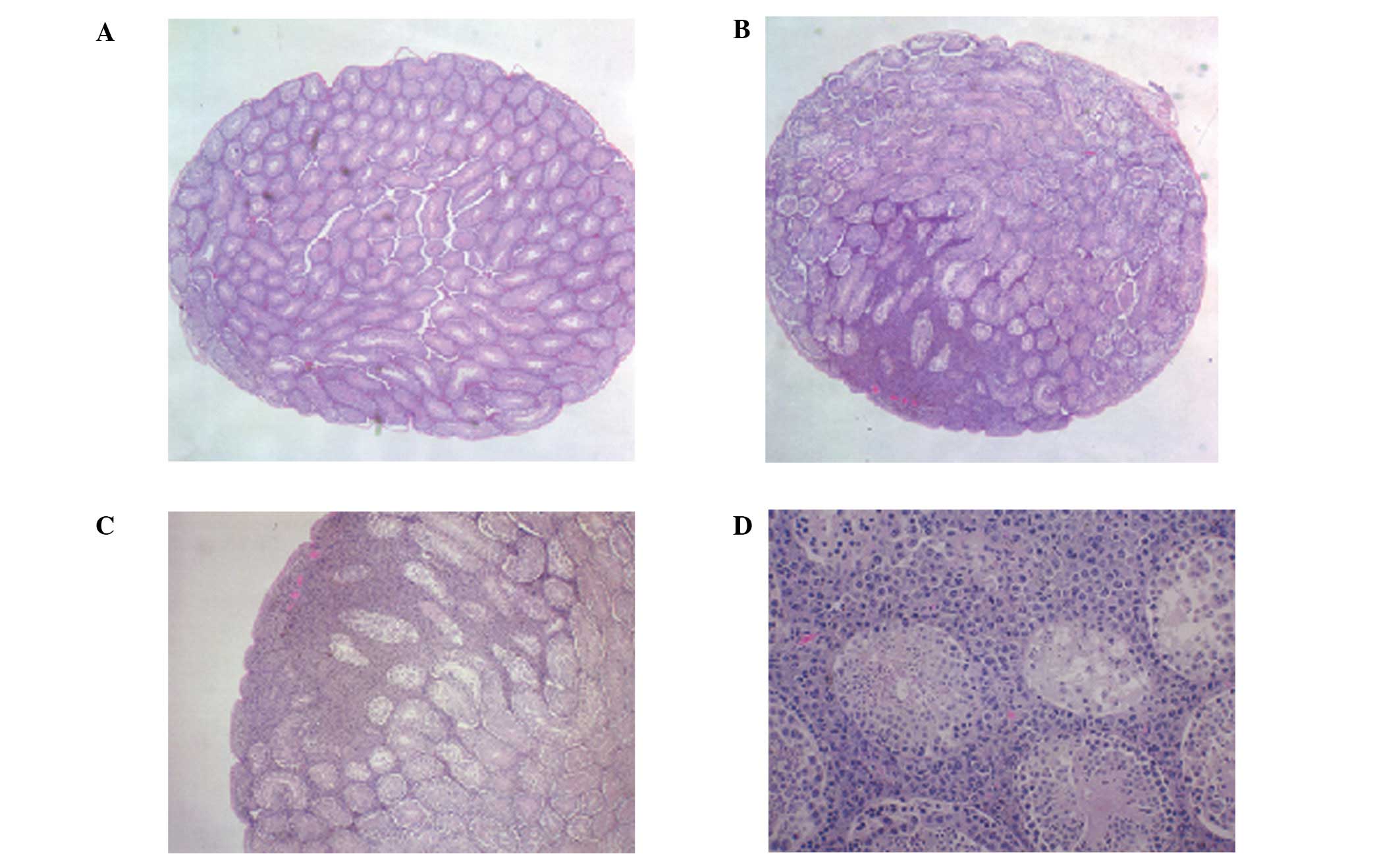
The right testes in the two groups showed overgrowth
of melanoma cells. All of the control mice showed testicular
metastasis, while in the NM diet group, only 3 mice showed mild
metastasis and 3 mice showed no metastasis. A week after the
melanoma injection, the left testis (untreated) of the control
mouse was normal, while the right testis showed a focal area of
melanoma invasion (Fig. 4). The
magnified section (x200) of the right testis (Fig. 4D) shows melanoma cells surrounding
the seminiferous tubules at 1 week post-injection. At 4 weeks
post-injection, the control mice showed significant testicular
invasion by melanoma cells (Fig.
5) in contrast to the less pronounced metastasis in the NM 1%
group of mice (Fig. 6).
Lung metastasis
No metastasis to the liver, kidney or spleen was
detected in either group. Lung metastasis was observed in 2 of 6
mice in each group. However, severe lung metastasis was observed in
the control group, while mild metastasis was detected in the NM 1%
group (Fig. 7).
Mean initial and final weights of
mice
No significant difference was found between the
initial and final mean weights within the two groups. The mean
initial weight of the control group was 37.2±1.3 g and the mean
final weight was 38.4±1.6 g. The mean initial weight of the NM 1%
group was 36.6±1.5 g and the final weight was 36.7±1.1 g.
Discussion
The aim of the present study was to investigate the
effect of a nutrient mixture on melanoma B16FO growth and
metastasis from intratesticular injection into nude mice,
representing the lymphatic and hematogenous dissemination of
melanoma malignancy. In our study, supplementation with the
nutrient mixture suppressed B16FO melanoma cell growth in the
testes and metastasis to the peritoneum and lungs after
intratesticular injection. All of the mice receiving the control
diet exhibited extensive metastasis in the peritoneal cavity, in
contrast to the NM 1% diet group, which showed no metastasis in 50%
of mice and mild peritoneal metastasis in the remaining mice. No
metastasis to liver, kidney or spleen was evident in either of the
two groups. Lung metastasis was observed in 2 of 6 mice in each
group, with severe lung metastasis being observed in the control
group and mild metastasis in the NM 1% group.
Notably, the melanoma cells invaded the peritoneum
and metastasized to the lungs from the right testes (injection site
of B16FO cells), but did not metastasize to the left testes
(untreated), suggesting the testes are not common sites for
melanoma B16FO cell metastasis. Previously, we showed that
intraperitoneal injection of B16FO melanoma cells into C57BL/6 mice
demonstrated intraperitoneal growth and ascites, but did not result
in metastasis to other organs (10). In regards to testicular tumor
growth, we demonstrated that supplementation with dietary NM
significantly suppressed murine melanoma B16FO tumor growth in
immune-impaired (athymic) mice. Previous in vitro studies
have demonstrated significant inhibition of melanoma B16FO and
A2058 cell proliferation and strong induction of apoptosis at 500
μg/ml NM, suggesting that inhibition of tumor growth was due
probably to induction of apoptosis (12). These findings are in agreement with
our in vivo findings that exposure of melanoma cells for 18
h to NM before injecting them into mice completely prevented the
formation of metastatic lung tumor modules (7).
Degradation of the extracellular matrix (ECM) by
matrix metalloproteinases (MMPs) plays a critical role in the
formation of tumors and metastasis (13). Findings of studies have shown that
highly metastatic melanoma and other cancer cells secrete higher
amounts of MMPs as compared to poorly metastatic cells,
demonstrating that the invasive and metastatic abilities of these
cancer cells correlate with MMP expression, particularly MMP-9 and
-2 (14–18). Type IV collagenases MMP-2 and -9
have been the focus of research as type IV collagen is a major
structural protein for ECM and basement membrane, and MMP-2 and -9
expression is associated with cancer cell invasion and elevated in
a variety of malignancies (19,20).
Previous in vitro studies have shown that NM significantly
inhibited melanoma and other cancer cell MMP-2 and -9 secretion and
Matrigel invasion (21). In
addition, ECM synthesized by normal fibroblasts treated with NM
exhibited increased stability and significantly reduced the
osteosarcoma cell growth rate, invasive activity (MMP-2 and -9
secretion and Matrigel invasion) and adhesion to collagen I and
other substrates, suppressing tumor growth independently of the
immune system function and inhibiting critical steps in cancer
metastasis (22).
Rath and Pauling (23) suggested the use of nutritional
components, such as vitamin C and lysine and lysine analogues to
target plasmin-mediated connective tissue degradation as a
universal approach to controlling common pathomechanisms in cancer
progression. Lysine interferes with the activation of plasminogen
into plasmin by tissue plasminogen activator (tPA) by binding to
plasminogen active sites, thereby affecting the plasmin-induced MMP
activation cascade (23).
Subsequent studies have confirmed this approach and resulted in
identifying a novel formulation (NM) comprising lysine, ascorbic
acid, proline and green tea extract, and other micronutrients that
have shown significant anticancer activity against a large number
(∼40) of cancer cell lines, blocking cancer growth, tissue invasion
and MMP expression both in vitro and in vivo
(9).
NM is a mixture of nutrients that addresses critical
physiological targets in cancer progression and metastasis, such as
ECM integrity and MMP activity. Optimal ECM formation and structure
is dependent upon adequate supplies of ascorbic acid and the amino
acids lysine and proline, which ensure proper synthesis and
hydroxylation of collagen fibers. Manganese and copper are also
essential for collagen formation. Lysine, a natural inhibitor of
plasmin-induced proteolysis, is crucial in supporting ECM stability
(23,24). Green tea extract has been shown to
be a potent agent in controlling cancer cell growth, metastasis,
angiogenesis, and other aspects of cancer progression (25–29).
N-acetyl cysteine has been observed to inhibit MMP-9 activity
(30) and invasive activities of
tumor cells (31). Selenium
inhibits MMP secretion and tumor invasion (32), as well as migration of endothelial
cells through ECM (31). In
addition to addressing ECM properties, some nutrients are critical
in inducing cancer cell death. Findings of a previous study
confirmed that ascorbic acid inhibits cell division and growth
through the production of hydrogen peroxide (33). Since arginine is a precursor of
nitric oxide (NO), any deficiency of arginine is capable of
limiting the production of NO, which has been shown to
predominantly act as an inducer of apoptosis, as in breast cancer
cells (34).
In conclusion, the results of the present study have
shown that the nutrient mixture was effective in significantly
reducing melanoma B16FO cell testicular tumor growth and peritoneal
and lung metastasis in male nude mice injected with melanoma cells
intratesticularly. These findings together with our earlier results
clearly indicate the anticancer potential of NM. Furthermore, use
of the nutrient mixture is not likely to pose any toxic effect
clinically, especially in the relevant doses, as demonstrated by
in vivo safety studies. During an in vivo study on
possible toxicity from NM, we found that NM did not have any
adverse effect on vital organs, such as the heart, liver and
kidney, nor on the associated functional serum enzymes (35).
Acknowledgements
The present study was funded by the Dr
Rath Health Foundation (Santa Clara, CA, USA), a non-profit
organization. Consulting pathologist Alexander de Paoli, the IDEXX
Reference Laboratories provided histopathology slides of B16FO
tumors.
References
|
1
|
Cancer.org: Testicular cancer: What are
the key statistics about testicular cancer? http://www.cancer.org/Cancer/TesticularCancer/DetailedGuide/testicular-cancer-key-statisticsuri.
Accessed March 19, 2012.
|
|
2
|
Kinkade S: Testicular Cancer. Am Fam
Physician. 59:2539–2544. 1999.PubMed/NCBI
|
|
3
|
Nichols C: Testicular Cancer. Testicular
Cancer Resource Center. http://tcrc.acor.org/iu.htmluri.
Updated May 25, 2010.
|
|
4
|
Hart IR and Fidler IJ: Role of organ
selectivity in the determination of metastatic patterns of B16
melanoma. Cancer Res. 40:2281–2297. 1980.PubMed/NCBI
|
|
5
|
Zeidman I and Busso JM: Transpulmonary
passage of tumor cell emboli. Cancer Res. 12:731–733.
1952.PubMed/NCBI
|
|
6
|
Sugarbaker ED: The organ selectivity of
experimentally induced metastases in rats. Cancer. 5:606–612. 1952.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Roomi MW, Ivanov V, Kalinovsky T,
Niedzwiecki A and Rath M: Inhibition of pulmonary metastasis of
melanoma B16FO cells in C57BL/6 mice by a nutrient mixture
consisting of ascorbic acid, lysine, proline, arginine, and green
tea extract. Exp Lung Res. 32:517–530. 2006. View Article : Google Scholar
|
|
8
|
Gheorgheosu D, Dehelean C, Cristea M and
Muntean D: Development of the B16 murine melanoma model. Annals of
RSCB. 16:148–152. 2011.
|
|
9
|
Niedzwiecki A, Roomi MW, Kalinovsky T and
Rath M: Micronutrient synergy - a new tool in effective control of
metastasis and other key mechanisms of cancer. Cancer Metastasis
Rev. 29:529–543. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Roomi MW, Kalinovsky T, Roomi NW,
Monterrey J, Rath M and Niedzwiecki A: Suppression of growth and
hepatic metastasis of murine B16FO melanoma cells by a novel
nutrient mixture. Oncol Rep. 20:809–817. 2008.PubMed/NCBI
|
|
11
|
Koshida K, Konaka H, Imao T, Egawa M,
Mizokami A and Namiki M: Comparison of two in vivo models for
prostate cancer: orthotopic and intratesticular inoculation of
LNCaP or PC-3 cells. Int J Urol. 11:1114–1121. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Roomi MW, Kalinovsky T, Niedzwiecki A and
Rath M: Anticancer effects of a micronutrient mixture on melanoma:
modulation of metastasis and other critical parameters.
Breakthroughs in Melanoma Research. Tanaka Y: In Tech Publisher;
ISBN: ISBN 978-953-307-291-32011, View
Article : Google Scholar
|
|
13
|
Fidler IJ: Critical factors in the biology
of human cancer metastasis: twenty-eighth G.H.A. Clowers memorial
award lecture. Cancer Res. 50:6130–6138. 1990.PubMed/NCBI
|
|
14
|
Fang W, Li H, Kong L, Niu G, Gao Q, Zhou
K, Zheng J and Wu B: Role of matrix metalloproteinases (MMPs) in
tumor invasion and metastasis: serial studies on MMPs and TIMPs.
Beijing Da Xue Xue Bao. 35:441–443. 2003.(In Chinese).
|
|
15
|
Liotta LA, Tryggvason K, Garbisa A, Hart
I, Foltz CM and Shafie S: Metastatic potential correlates with
enzymatic degradation of basement membrane collagen. Nature.
284:67–68. 1980. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Stetler-Stevenson WG: The role of matrix
metalloproteinases in tumor invasion, metastasis and angiogenesis.
Surg Oncol Clin N Am. 10:383–392. 2001.PubMed/NCBI
|
|
17
|
Duffy MJ: The role of proteolytic enzymes
in cancer invasion and metastasis. Clin Exp Metastasis. 10:145–155.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Stetler-Stevenson WG, Hewitt R and
Corcoran M: Matrix metalloproteinases and tumor invasion from
correlation and causality to the clinic. Semin Cancer Biol.
7:174–154. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kleiner DL and Stetler-Stevenson WG:
Matrix metalloproteinases and metastasis. Cancer Chemother
Pharmacol. 43(Suppl): S42–S51. 1999. View Article : Google Scholar
|
|
20
|
Chambers AF and Matrisian LM: Changing
views on the role of matrix metalloproteinases in metastasis. J
Natl Cancer Inst. 89:1260–1270. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Roomi MW, Monterrey JC, Kalinovsky T, Rath
M and Niedzwiecki A: Inhibition of invasion and MMPs by a nutrient
mixture in human cancer cell lines: a correlation study. Exp Oncol.
32:243–248. 2010.PubMed/NCBI
|
|
22
|
Ivanov V, Ivanova S, Roomi MW, Kalinovsky
T, Niedzwiecki A and Rath M: Naturally produced extracellular
matrix inhibits growth rate and invasiveness of human osteosarcoma
cancer cells. Med Oncol. 24:209–217. 2007. View Article : Google Scholar
|
|
23
|
Rath M and Pauling L: Plasmin-induced
proteolysis and the role of apoprotein(a), lysine and synthetic
analogs. J Orthomolecular Med. 7:17–23. 1992.
|
|
24
|
Sun Z, Chen YH, Wang P, Zhang J, Gurewich
V, Zhang P and Liu JN: The blockage of high-affinity lysine binding
sites of plasminogen by EACA significantly inhibits
prourokinase-induced plasminogen activation. Biochem Biophys Acta.
1596:182–192. 2002.
|
|
25
|
Valcic S, Timmermann BN, Alberts DS,
Wachter GA, Krutzsch M, Wymer J and Guillen JM: Inhibitory effect
of six green tea catechins and caffeine on the growth of four
selected human tumor cell lines. Anticancer Drugs. 7:461–468. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mukhtar H and Ahmed N: Tea polyphenols:
prevention of cancer and optimizing health. Am J Clin Nutr.
71(Suppl 6): S1698–S1702. 2000.PubMed/NCBI
|
|
27
|
Yang GY, Liao J, Kim K, Yurtow EJ and Yang
CS: Inhibition of growth and induction of apoptosis in human cancer
cell lines by tea polyphenols. Carcinogenesis. 19:611–616. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Taniguchi S, Fujiki H, Kobayashi H, Go H,
Miyado K, Sadano H and Shimokawa R: Effect of (−) epigallocatechin
gallate, the main constituent of green tea, on lung metastasis with
mouse B16 melanoma cell lines. Cancer Lett. 65:51–54. 1992.
|
|
29
|
Hare Y: Green Tea: Health Benefits and
Applications. Marcel Dekker; New York, Basel: 2001, View Article : Google Scholar
|
|
30
|
Kawakami S, Kageyama Y, Fujii, Kihara K
and Oshima H: Inhibitory effects of N-acetyl cysteine on invasion
and MMP 9 production of T24 human bladder cancer cells. Anticancer
Res. 21:213–219. 2001.PubMed/NCBI
|
|
31
|
Morini M, Cai T, Aluigi MF, Noonan DM,
Masiello L, De Flora S, D’Agostini F, Albini A and Fassina G: The
role of the thiol N-acetyl cysteine in the prevention of tumor
invasion and angiogenesis. Int J Biol Markers. 14:268–271.
1999.PubMed/NCBI
|
|
32
|
Yoon SO, Kim MM and Chung AS: Inhibitory
effects of selenite on invasion of HT 1080 tumor cells. J Biol
Chem. 276:20085–20092. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Maramag C, Menon M, Balaji KC, Reddy PG
and Laxmanan S: Effect of vitamin C on prostate cancer cells in
vitro: effect on cell number, viability and DNA synthesis.
Prostate. 32:188–195. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cooke JP and Dzau VJ: Nitric oxide
synthase: Role in the genesis of vascular disease. Annu Rev Med.
48:489–509. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Roomi MW, Ivanov V, Netke SP, Niedzwiecki
A and Rath M: Serum markers of the liver, heart, and kidney and
lipid profile and histopathology in ODS rats treated with nutrient
synergy. J AM Coll Nutr. 22:477(Abs. 86),. 2003.
|