Introduction
Primary liver cancer is the most common primary
cancer of the liver, accounting for approximately 6% of all human
cancers. It is estimated that half a million cases are diagnosed
worldwide annually, making primary liver cancer the fifth and ninth
most common malignancy in males and females, respectively (1–6).
Hepatocellular carcinoma (HCC) accounts for 85–90% of primary liver
cancers (7) and the age-adjusted
HCC mortality rate has increased in recent decades in Japan
(8). Similarly, a trend of
increasing rates of HCC has been reported in several developed
countries in North America, Europe and Asia (9,10).
HCC often develops in patients with liver cirrhosis caused by
hepatitis B virus (HBV) or hepatitis C virus (HCV) infection,
excessive alcohol consumption or non-alcoholic fatty liver disease.
Of the hepatitis viruses that cause HCC, HCV is predominant in
Japan (11–14).
α-fetoprotein (AFP) is a tumor marker of HCC and is
also reported to reflect the effectiveness of long-term low-dose
interferon (IFN) therapy in HCV patients with chronic liver disease
(15). The correlation between AFP
levels and the incidence of HCC has been discussed over a long
period. We investigated whether high levels of AFP at the time of
diagnosis were associated with an increased incidence of HCC in
patients with HCV.
Patients and methods
Study population
Between 1976 and 2010, 107 patients were diagnosed
with liver cirrhosis due to HCV infection at the Department of
Gastroenterology and Hepatology, Nagasaki University Hospital
(Nagasaki, Japan). The diagnosis of liver cirrhosis was based on
biopsy and/or clinical findings. Sera were stored at −80°C until
they were used for assays. The diagnosis of chronic HCV infection
was based on the presence of anti-HCV antibodies (HCV Abs;
microparticle enzyme immunoassay; Abbott Laboratories, Chicago, IL,
USA) and HCV RNA, as detected by the polymerase chain reaction. The
diagnosis of chronic HBV infection was excluded on the basis of the
presence of hepatitis B surface antigen (HBsAg; enzyme-linked
immunosorbent assay; Abbott Laboratories). Serum AFP was measured
using a radioimmunoassay (Abbott Laboratories). The patient alcohol
intake histories were obtained from their medical records. Habitual
drinking was defined as an average daily consumption of an amount
equivalent to 80 g of pure ethanol for a period of >10
years.
Follow-up of patients and diagnosis of
HCC
Following the initial diagnosis of patients with
liver cirrhosis and HCV infection, the patients underwent
measurement of AFP levels and liver function biochemistry every 1
to 3 months during the follow-up period and ultrasonography (USG)
was performed every 3 to 6 months. The diagnosis of HCC was based
on imaging techniques, including USG, computerized tomography (CT),
magnetic resonance imaging (MRI), hepatic angiography (HAG) and/or
liver biopsy. The diagnostic criteria for HCC included confirmative
liver biopsy, neovascularization in HAG and/or CT.
The end date of the present study was December 2010,
detection of HCC or the time of patient mortality. If a patient had
not been monitored in the hospital for >1 year, the patient was
considered lost to the follow-up. The median observation period was
3.8 years (IQR, 5.0).
IFN therapy
During the observation period, 43 (40%) of the 107
patients received IFN monotherapy, PEGylated (PEG)-IFN monotherapy
or combination therapy with IFN and ribavirin or PEG-IFN and
ribavirin. A sustained virological response (SVR) was defined as
the absence of detectable HCV RNA at the end of treatment
persisting for >6 months, while a failure to meet these criteria
was defined as non-SVR. There were no relapses of viremia in the
SVR patients after 6 months.
Statistical analysis
The HCC development rate was analyzed using the
Kaplan-Meier technique and differences in the curves were studied
using the log-rank test. Independent risk factors associated with
the rate of HCC development were identified using the stepwise
method of non-time-dependent Cox regression analysis. Parametric
comparisons were performed using analysis of variance (ANOVA). The
significance of individual differences was evaluated using the
Scheffe test. Data analysis was performed with SPSS version 16.0
for Windows. P<0.05 was considered to indicate a statistically
significant result.
Results
Clinical features of the studied
patients
Patient characteristics at the time of the cirrhosis
diagnosis are shown in Table I.
There were 54 male (51%) and 53 female (49%) cirrhosis patients
(median age, 62.5 years). Habitual drinkers and diabetic patients
were 10% (11 of 107) and 44% (41 of 107) of all cases,
respectively. Child-Pugh grade A was recorded in 52% (56 of 107) of
patients, grade B in 41% (44 of 107) and grade C in 7% (7 of 107).
Of the studied patients, 40% (43 of 107) underwent IFN therapy and
60% (64 of 107) were followed closely without receiving IFN
treatment. The proportion of IFN-treated patients exhibiting an SVR
was 25.6% (11/43). The patients were classified into 3 categories
according to the level of AFP. The AFP levels were <6 ng/ml in
34 (32%) patients, between 6 and 19 ng/ml in 38 (35%) and ≥20 ng/ml
in 35 (33%).
 | Table ICharacteristics of 107 studied
hepatitis C patients with liver cirrhosis. |
Table I
Characteristics of 107 studied
hepatitis C patients with liver cirrhosis.
| Characteristic | Value |
|---|
| Number of
patients | 107 |
| Age (years), median
(IQR) | 62.5 (13.3) |
| Gender, n (%) | |
| Male | 54 (51) |
| Female | 53 (49) |
| Height (m), median
(IQR) | 1.58 (0.2) |
| Weight (kg), median
(IQR) | 56.4 (13.3) |
| BMI
(kg/m2), median (IQR) | 22.6 (4.2) |
| Alcohol
consumption, n (%) | |
| Excessive | 11 (10) |
| Not
excessive | 96 (90) |
| Diabetes mellitus,
n (%) | |
| + | 44 (41) |
| - | 63 (59) |
| Diagnosis, n
(%) | |
| Histological | 80 (75) |
| Clinical | 27 (25) |
| Child-Pugh grade, n
(%) | |
| A | 56 (52) |
| B | 44 (41) |
| C | 7 (7) |
| Platelet count
(103/μl), median (IQR) | 100 (6.5) |
| AST (IU/l), median
(IQR) | 71 (64) |
| ALT (IU/l), median
(IQR) | 60 (61) |
| γ-GTP (IU/l),
median (IQR) | 45 (58) |
| Bilirubin (mg/dl),
median (IQR) | 1.0 (0.9) |
| Albumin (mg/dl),
median (IQR) | 3.8 (0.9) |
| TC (mg/dl), median
(IQR) | 152 (44) |
| TG (mg/dl), median
(IQR) | 92 (57 |
| AFP (ng/ml), median
(IQR) | 11 (24) |
| <6, n (%) | 34 (32) |
| 6–19, n (%) | 38 (35) |
| ≥20, n (%) | 35 (33) |
| BCAA, n (%) | |
| + | 39 (36) |
| - | 68 (64) |
| Interferon therapy,
n (%) | |
| SVR | 11 (10) |
| Non-SVR | 32 (30) |
| No therapy | 64 (60) |
Risk factors for HCC
Cox regression analysis was performed on variables,
including age, gender, alcohol consumption, experience of IFN
therapy and biochemical parameters. The following factors were
identified as exhibiting an increased risk of HCC by univariate
analysis: aspartate transaminase (AST) ≥71 IU/l, alanine
transaminase (ALT) ≥60 IU/l, AFP ≥6 ng/ml and IFN therapy (Table II). Multivariate analysis
identified the etiology of the AFP level [6–19 ng/ml: hazard ratio
(HR), 2.22; P=0.006 and ≥20 ng/ml: HR, 2.09; P=0.003] as
independent and significant risk factor for the development of HCC
(Table III).
 | Table IIFactors increasing the risk of
hepatocellular carcinoma (HCC) determined by univariate
analysis. |
Table II
Factors increasing the risk of
hepatocellular carcinoma (HCC) determined by univariate
analysis.
| Parameters | Hazard ratio | P-value |
|---|
| Age (years) | | |
| >62 | 1.29 | 0.291 |
| Gender | | |
| Male | 0.80 | 0.360 |
| BMI
(kg/m2) | | |
| >25 | 0.88 | 0.636 |
| Alcohol
consumption | | |
| Excessive | 1.40 | 0.211 |
| Diabetes mellitus
(%) | | |
| + | 1.10 | 0.712 |
| Child-Pugh
grade | | |
| A | 1 | - |
| B | 1.20 | 0.474 |
| C | 0.94 | 0.925 |
| Platelet
(103/μl) | | |
| <100 | 1.07 | 0.788 |
| AST (IU/l) | | |
| ≥71 | 1.83 | 0.016 |
| ALT (IU/l) | | |
| ≥60 | 1.80 | 0.020 |
| γ-GTP (IU/l) | | |
| ≥45 | 1.25 | 0.970 |
| Bilirubin
(mg/dl) | | |
| ≥1.0 | 0.72 | 0.189 |
| Albumin
(mg/dl) | | |
| <3.8 | 0.85 | 0.520 |
| TC (mg/dl) | | |
| ≥152 | 0.66 | 0.095 |
| TG (mg/dl) | | |
| ≥92 | 0.76 | 0.269 |
| AFP (ng/ml) | | |
| <6 | 1 | - |
| 6–19 | 2.54 | 0.006 |
| ≥20 | 2.71 | 0.003 |
| BCAA | | |
| + | 1.59 | 0.063 |
| Interferon therapy
(%) | | |
| No therapy | 1 | - |
| Non-SVR | 0.77 | 0.366 |
| SVR | 0.26 | 0.031 |
 | Table IIIFactors increasing the risk for
hepatocellular carcinoma (HCC), determined by multivariate
analysis. |
Table III
Factors increasing the risk for
hepatocellular carcinoma (HCC), determined by multivariate
analysis.
| Parameters | Hazard ratio | 95% CI | P-value |
|---|
| AST (IU/l) | | | |
| ≥71 | 1.27 | 0.72–2.26 | 0.411 |
| ALT (IU/l) | | | |
| ≥60 | 1.40 | 0.81–2.43 | 0.229 |
| AFP (ng/ml) | | | |
| <6 | 1 | - | - |
| 6–19 | 2.22 | 1.13–4.38 | 0.006 |
| ≥20 | 2.09 | 1.03–4.23 | 0.003 |
| Interferon therapy
(%) | | | |
| No therapy | 1 | - | - |
| Non-SVR | 0.99 | 0.55–1.80 | 0.989 |
| SVR | 0.46 | 0.14–1.57 | 0.218 |
Development of HCC
During the follow-up period, HCC developed in 68
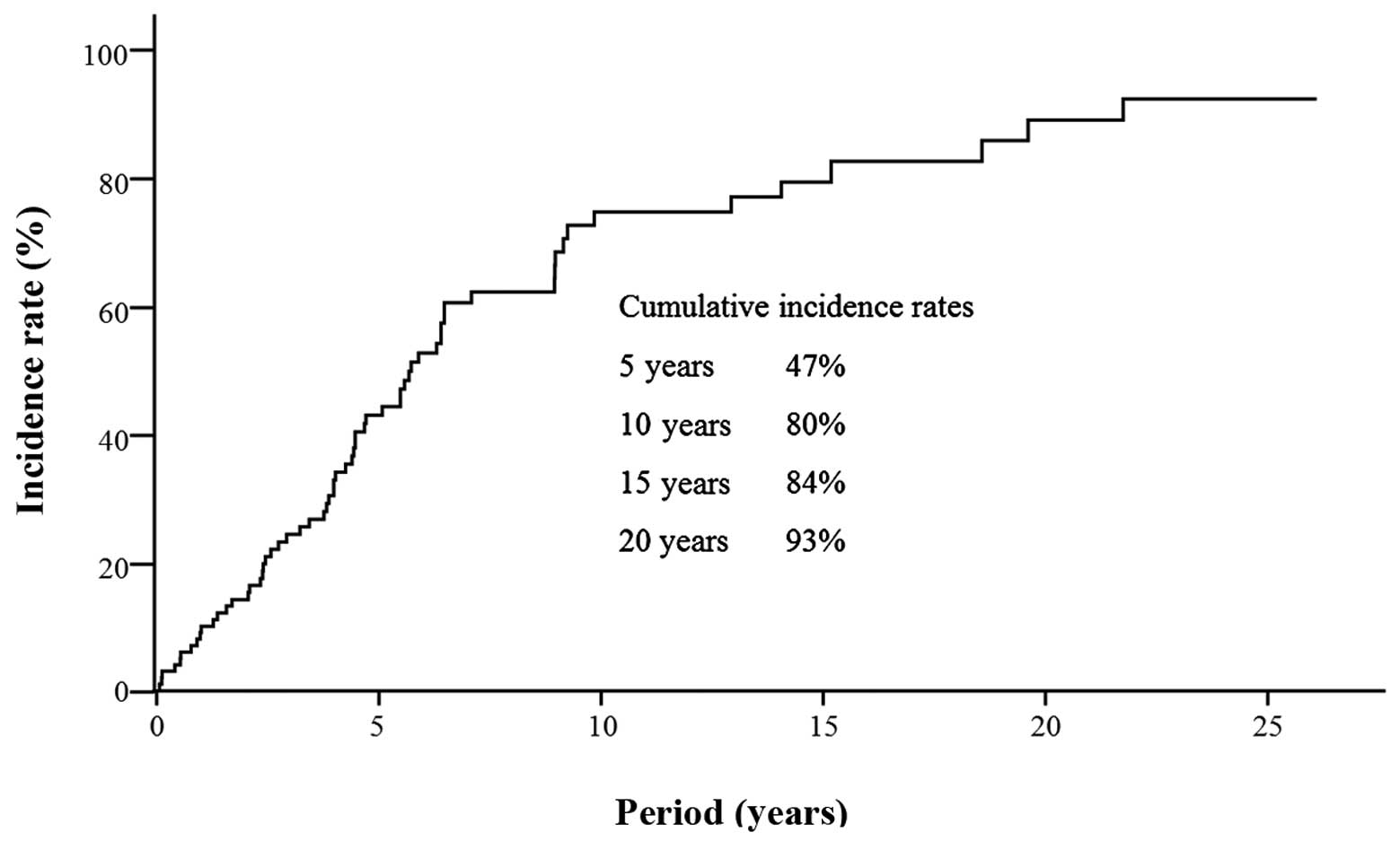
(63.6%) patients. Kaplan-Meier estimates of the cumulative risk of
HCC are shown in Fig. 1. The
10-year cumulative incidence rate of HCC was 80%. The cumulative
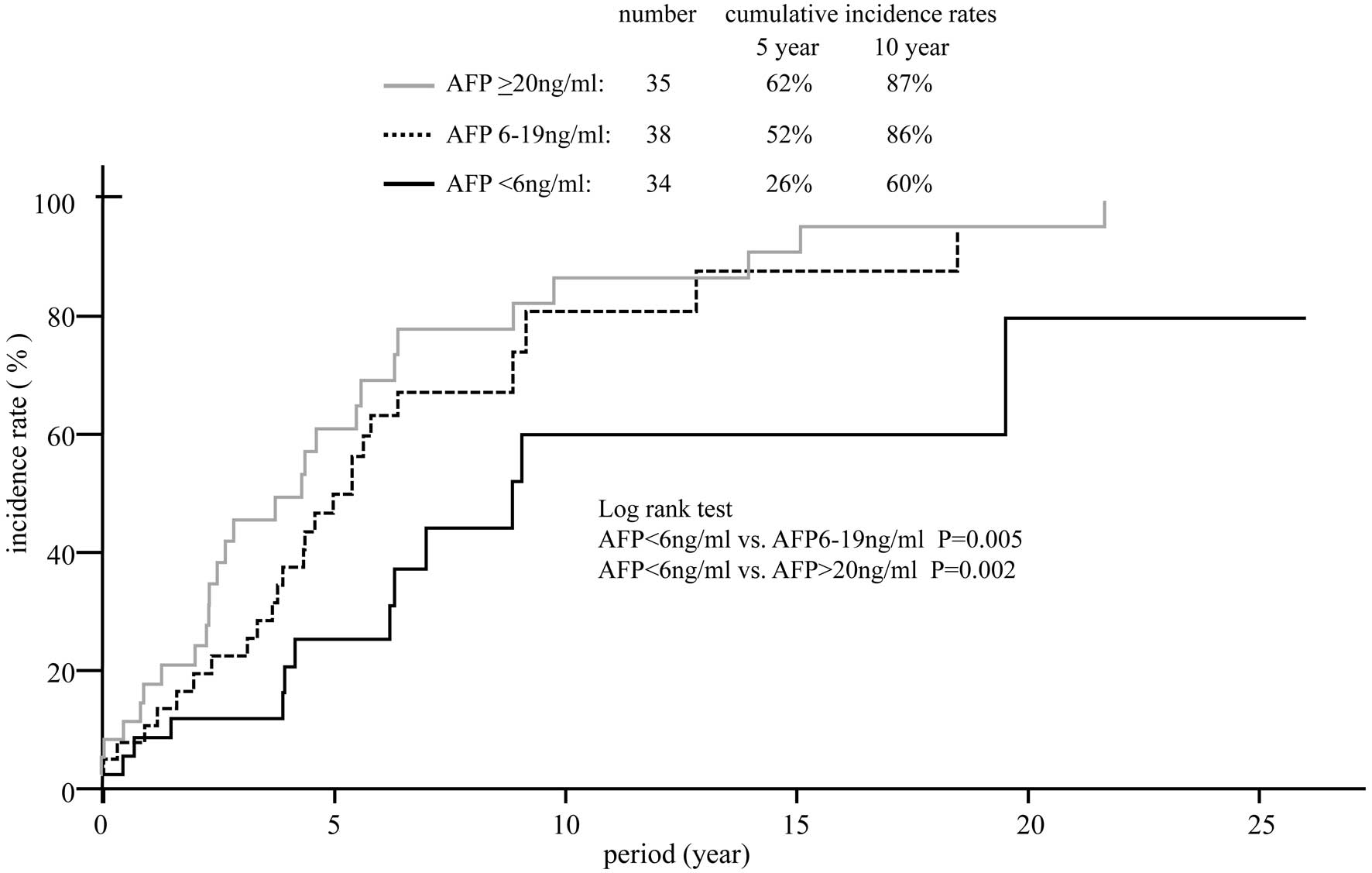
incidence of HCC in patients with various AFP levels is shown in
Fig. 2. The 10-year cumulative
risk of HCC was 60% in the 34 patients with AFP levels <6 ng/ml
at study entry, 86% in the 38 patients with AFP levels between 6
and 19 ng/ml and 87% in the 34 patients with AFP levels ≥20 ng/ml.
Significant differences were observed in the HCC incidence between
those with AFP level <6 ng/ml and those with an AFP level
between 6 and 19 ng/ml and ≥20 ng/ml.
Discussion
In the present study, the AFP level was identified
as a risk factor for HCC in HCV patients with liver cirrhosis.
Notably, patients with high (≥20 ng/ml) and elevated AFP levels
(between 6 and 19 ng/ml) had an increased risk of HCC development.
This deviated slightly from the serum AFP levels of healthy adults
reported to range between 0.1 and 5.8 ng/ml (16). In the present study, analyses were
performed by setting various AFP cut-off levels to evaluate their
performance as risk factors. In HCV patients with cirrhosis, an AFP
level ≥6 ng/ml was observed to be associated with the development
of HCC in the multivariate analysis.
AFP is used as a serological marker of HCC and
employed in combination with USG for HCC screening (17,18).
Numerous studies have demonstrated an elevated AFP level to be a
risk factor for the development of HCC in HCV patients (19–26).
There is extensive evidence demonstrating that AFP is functionally
an embryonic and fetal carrier/transport molecule for a number of
of ligands, including fatty acids, bilirubin, heavy metals,
steroids, retinoids, drugs, dyes and antibiotics (27). However, the biological and
pathophysiological roles of the association of AFP with an
increased risk of HCC development remain unclear. Tateyama et
al reported that AFP levels were elevated in parallel with
advanced fibrosis stages and correlated well with the fibrosis
stage (26). Since the patients
with slightly elevated AFP levels (between 6 and 19 ng/ml) had
moderately advanced liver fibrosis stages, these AFP levels may
indicate an elevated risk of HCC in patients with chronic HCV
infection. Li et al identified a functional link between
cytoplasmic AFP and the PTEN/AKT signalling pathway and provided
further evidence for the understanding of the novel role of
cytoplasmic AFP in the maintenance of tumor cell growth (28). The silencing of AFP expression by a
knockdown of its gene may play a role in growth arrest and
apoptosis in human HCC cells (28–31).
IFN has been used to treat patients with HCV
infection. A failure to achieve an SVR with IFN-based therapies,
pre-existing advanced hepatic fibrosis and/or cirrhosis are major
predictors of HCC (20,32–35).
Numerous Japanese cohort studies have demonstrated that IFN therapy
reduces the incidence of HCC, not only in sustained virological
responders but also in transient responders in whom the elimination
of HCV has failed (32,36–40).
In cirrhotic patients, Nishiguchi et al reported that the
relative risk of patients receiving IFN-α treatment developing HCC
was 0.067 in comparison with the control group (34). By contrast, Valla et al were
unable to demonstrate any significant benefit for the prevention of
HCC in patients with or without IFN treatment (41). Cammà et al suggested a
slight preventive effect of IFN on HCC development in patients with
HCV-related cirrhosis (42).
Shiffman et al reported that continuous IFN therapy led to a
decline in hepatic fibrosis despite the persistence of viremia
(43). In addition, Nomura et
al reported that the AFP level was significantly decreased at 3
months following the start of low-dose long-term IFN treatment
(15). Murashima et al
demonstrated that IFN therapy, but not Stronger Neo-Minophagen C
(SNMC), universally reduced basic AFP levels (44). In an in vitro study of the
effects of IFN on an HCC cell line, IFN exhibited antitumor effects
(45). Taken together, these
findings suggest that AFP levels may aid the prediction of the
development of HCC during IFN-based treatments, including long-term
low-dose IFN therapy.
In conclusion, AFP is a non-invasive predictive
marker of the development of HCC in HCV patients. The present study
indicates that high (≥20 ng/ml), and slightly elevated (between 6
and 19 ng/ml) AFP levels, may suggest a substantial risk of HCC
development, complementing the fibrosis stage. By contrast, AFP
levels <6 ng/ml indicate a low risk of HCC development.
References
|
1
|
El-Serag HB and Mason AC: Risk factors for
the rising rates of primary liver cancer in the United States. Arch
Intern Med. 160:3227–3230. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
El-Serag HB: Epidemiology of
hepatocellular carcinoma. Clin Liver Dis. 5:87–107. vi2001.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
El-Serag HB, Hampel H, Yeh C and Rabeneck
L: Extrahepatic manifestations of hepatitis C among United States
male veterans. Hepatology. 36:1439–1445. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
El-Serag HB: Hepatocellular carcinoma and
hepatitis C in the United States. Hepatology. 36(Suppl 1): S74–S83.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
El-Serag HB: Hepatocellular carcinoma: an
epidemiologic view. J Clin Gastroenterol. 35(Suppl 2): S72–S78.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hassan MM, Frome A, Patt YZ and El-Serag
HB: Rising prevalence of hepatitis C virus infection among patients
recently diagnosed with hepatocellular carcinoma in the United
States. J Clin Gastroenterol. 35:266–269. 2002. View Article : Google Scholar
|
|
7
|
El-Serag HB and Rudolph KL: Hepatocellular
carcinoma: epidemiology and molecular carcinogenesis.
Gastroenterology. 132:2557–2576. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kiyosawa K and Tanaka E: Characteristics
of hepatocellular carcinoma in Japan. Oncology. 62:5–7. 2002.
View Article : Google Scholar
|
|
9
|
McGlynn KA, Tsao L, Hsing AW, Devesa SS
and Fraumeni JF Jr: International trends and patterns of primary
liver cancer. Int J Cancer. 94:290–296. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bosch FX, Ribes J, Díaz M and Cléries R:
Primary liver cancer: worldwide incidence and trends.
Gastroenterology. 127:S5–S16. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hamasaki K, Nakata K, Tsutsumi T, et al:
Changes in the prevalence of hepatitis B and C infection in
patients with hepatocellular carcinoma in the Nagasaki Prefecture,
Japan. J Med Virol. 40:146–149. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kato Y, Nakata K, Omagari K, et al: Risk
of hepatocellular carcinoma in patients with cirrhosis in Japan.
Analysis of infectious hepatitis viruses. Cancer. 74:2234–2238.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shiratori Y, Shiina S, Imamura M, et al:
Characteristic difference of hepatocellular carcinoma between
hepatitis B- and C- viral infection in Japan. Hepatology.
22:1027–1033. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shiratori Y, Shiina S, Zhang PY, et al:
Does dual infection by hepatitis B and C viruses play an important
role in the pathogenesis of hepatocellular carcinoma in Japan?
Cancer. 80:2060–2067. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nomura H, Kashiwagi Y, Hirano R, et al:
Efficacy of low dose long-term interferon monotherapy in aged
patients with chronic hepatitis C genotype 1 and its relation to
alpha-fetoprotein: A pilot study. Hepatol Res. 37:490–497. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Taketa K: Alpha-fetoprotein: reevaluation
in hepatology. Hepatology. 12:1420–1432. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Di Bisceglie AM: Hepatitis C and
hepatocellular carcinoma. Hepatology. 26(Suppl 1): 34S–38S.
1997.
|
|
18
|
Sherman M: Hepatocellular carcinoma:
epidemiology, risk factors, and screening. Semin Liver Dis.
25:143–154. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rodríguez-Díaz JL, Rosas-Camargo V,
Vega-Vega O, et al: Clinical and pathological factors associated
with the development of hepatocellular carcinoma in patients with
hepatitis virus-related cirrhosis: a long-term follow-up study.
Clin Oncol (R Coll Radiol). 19:197–203. 2007.
|
|
20
|
Bruix J and Sherman M; Practice Guidelines
Committee: American Association for the Study of Liver Disease:
Management of hepatocellular carcinoma. Hepatology. 42:1208–1236.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Colombo M, de Franchis R, Del Ninno E, et
al: Hepatocellular carcinoma in Italian patients with cirrhosis. N
Engl J Med. 325:675–680. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tsukuma H, Hiyama T, Tanaka S, et al: Risk
factors for hepatocellular carcinoma among patients with chronic
liver disease. N Engl J Med. 328:1797–1801. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Oka H, Tamori A, Kuroki T, Kobayashi K and
Yamamoto S: Prospective study of alpha-fetoprotein in cirrhotic
patients monitored for development of hepatocellular carcinoma.
Hepatology. 19:61–66. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ganne-Carrié N, Chastang C, Chapel F, et
al: Predictive score for the development of hepatocellular
carcinoma and additional value of liver large cell dysplasia in
Western patients with cirrhosis. Hepatology. 23:1112–1118.
1996.PubMed/NCBI
|
|
25
|
Sangiovanni A, Colombo E, Radaelli F, et
al: Hepatocyte proliferation and risk of hepatocellular carcinoma
in cirrhotic patients. Am J Gastroenterol. 96:1575–1580. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tateyama M, Yatsuhashi H, Taura N, et al:
Alpha-fetoprotein above normal levels as a risk factor for the
development of hepatocellular carcinoma in patients infected with
hepatitis C virus. J Gastroenterol. 46:92–100. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mizejewski GJ, Dias JA, Hauer CR,
Henrikson KP and Gierthy J: Alpha-fetoprotein derived synthetic
peptides: assay of an estrogen-modifying regulatory segment. Mol
Cell Endocrinol. 118:15–23. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li M, Li H, Li C, et al: Alpha fetoprotein
is a novel protein-binding partner for caspase-3 and blocks the
apoptotic signaling pathway in human hepatoma cells. Int J Cancer.
124:2845–2854. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li M, Li H, Li C, et al:
Alpha-fetoprotein: a new member of intra-cellular signal molecules
in regulation of the PI3K/AKT signaling in human hepatoma cell
lines. Int J Cancer. 128:524–532. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang X, Zhang Y, Zhang L, Zhang L and Mao
J: Silencing alpha-fetoprotein expression induces growth arrest and
apoptosis in human hepatocellular cancer cell. Cancer Lett.
271:281–293. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li M, Li H, Li C, et al: Cytoplasmic
alpha-fetoprotein functions as a co-repressor in RA-RAR signaling
to promote the growth of human hepatoma Bel 7402 cells. Cancer
Lett. 285:190–199. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yoshida H, Shiratori Y, Moriyama M, et al:
Interferon therapy reduces the risk for hepatocellular carcinoma:
national surveillance program of cirrhotic and noncirrhotic
patients with chronic hepatitis C in Japan. IHIT Study Group
Inhibition of Hepatocarcinogenesis by Interferon Therapy. Ann
Intern Med. 131:174–181. 1999. View Article : Google Scholar
|
|
33
|
Fattovich G, Stroffolini T, Zagni I and
Donato F: Hepatocellular carcinoma in cirrhosis: incidence and risk
factors. Gastroenterology. 127(Suppl 1): S35–S50. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nishiguchi S, Kuroki T, Nakatani S, et al:
Randomised trial of effects of interferon-alpha on incidence of
hepatocellular carcinoma in chronic active hepatitis C with
cirrhosis. Lancet. 346:1051–1055. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yu ML, Huang CF, Dai CY, Huang JF and
Chuang WL: Long-term effects of interferon-based therapy for
chronic hepatitis C. Oncology. 72(Suppl 1): 16–23. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Imai Y, Kawata S, Tamura S, et al:
Relation of interferon therapy and hepatocellular carcinoma in
patients with chronic hepatitis C. Osaka Hepatocellular Carcinoma
Prevention Study Group. Ann Intern Med. 129:94–99. 1998. View Article : Google Scholar
|
|
37
|
Kasahara A, Hayashi N, Mochizuki K, et al:
Risk factors for hepatocellular carcinoma and its incidence after
interferon treatment in patients with chronic hepatitis C. Osaka
Liver Disease Study Group. Hepatology. 27:1394–1402. 1998.
View Article : Google Scholar
|
|
38
|
Ikeda K, Saitoh S, Arase Y, et al: Effect
of interferon therapy on hepatocellular carcinogenesis in patients
with chronic hepatitis type C: A long-term observation study of
1,643 patients using statistical bias correction with proportional
hazard analysis. Hepatology. 29:1124–1130. 1999. View Article : Google Scholar
|
|
39
|
Okanoue T, Itoh Y, Kirishima T, et al:
Transient biochemical response in interferon therapy decreases the
development of hepatocellular carcinoma for five years and improves
the long-term survival of chronic hepatitis C patients. Hepatol
Res. 23:62–77. 2002.
|
|
40
|
Hino K and Okita K: Interferon therapy as
chemoprevention of hepatocarcinogenesis in patients with chronic
hepatitis C. J Antimicrob Chemother. 53:19–22. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Valla DC, Chevallier M, Marcellin P, et
al: Treatment of hepatitis C virus-related cirrhosis: a randomized,
controlled trial of interferon alpha-2b versus no treatment.
Hepatology. 29:1870–1875. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Cammà C, Di Bona D and Craxì A: The impact
of antiviral treatments on the course of chronic hepatitis C: an
evidence-based approach. Curr Pharm Des. 10:2123–2130.
2004.PubMed/NCBI
|
|
43
|
Shiffman ML, Hofmann CM, Contos MJ, et al:
A randomized, controlled trial of maintenance interferon therapy
for patients with chronic hepatitis C virus and persistent viremia.
Gastroenterology. 117:1164–1172. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Murashima S, Tanaka M, Haramaki M, et al:
A decrease in AFP level related to administration of interferon in
patients with chronic hepatitis C and a high level of AFP. Dig Dis
Sci. 51:808–812. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yano H, Iemura A, Haramaki M, et al:
Interferon alpha receptor expression and growth inhibition by
interferon alpha in human liver cancer cell lines. Hepatology.
29:1708–1717. 1999. View Article : Google Scholar : PubMed/NCBI
|