Introduction
Lung cancer is the leading cause of cancer-related
death in the world, and non-small cell lung cancer (NSCLC) accounts
for 80–85% of lung cancer cases. Patients with early-stage NSCLC
have relatively high long-term survival rates after surgical
resection, but a substantial majority of patients, ∼80%, present in
advanced or metastatic stages. Over the past decade,
third-generation agents such as vinorelbine, taxanes and
gemcitabine have been introduced for the treatment of NSCLC
(1). Combination of one or more of
these agents with a platinum compound has resulted in high response
rates and prolonged overall survival. Today, doublet chemotherapies
consisting of platinum plus one of the third-generation agents have
become the current standard regimen, the first line of chemotherapy
(2). Many studies have examined
short-term survival rates in patients receiving such treatment, yet
current evidence regarding long-term survival in advanced-stage
NSCLC, particularly in stages IIIB and IV, is limited. This study
aimed to determine the long-term results of cisplatin plus
third-generation (vinorelbine or gemcitabine) cytotoxic
chemotherapy in patients with locally advanced and advanced
NSCLC.
Materials and methods
Patients
The patients with stage IIIB and IV NSCLC were
evaluated at the Department of Pulmonary Medicine, Ondokuz Mayis
University's Faculty of Medicine, between January 2001 and
September 2004. The patient data, which included demographic,
clinical, radiological, disease characteristics and therapy
regimens were retrospectively obtained from the files of the
patients in departmental archives. We followed up the patients from
the time of diagnosis until death, and all of the patients had
succumbed to causes related to lung cancer. A total of 196 patients
were enrolled in the study, however, 55 (28%) were excluded due to
factors rendering them inappropriate for the study and thus 141
patients were included. Approval from the patients and the
institution was obtained in order to use their records for our
study.
Eligibility criteria
Performance status was classified in accordance with
the criteria of the European Cooperative Oncology Group (ECOG).
Staging was conducted by evaluation of imaging methods, chest
X-ray, thoracic computed tomography, abdominal computed tomography,
abdominal ultrasonography (USG), cranial computed tomography and
bone scintigraphy. The criteria for eligibility included
pathologically confirmed NSCLC, radiologically measurable lesions,
an age of at least 18 years, adequate hematological function (as
indicated by a white cell count of at least 4,000/ml3
and a platelet count of at least 100,000/ml3), hepatic
function (as indicated by a bilirubin level that did not exceed 1.5
mg/dl, and by AST and ALT levels being <3 times the normal
values), renal function (as indicated by a creatinine level that
did not exceed 1.5 mg/dl), and ECOG performance status (PS) ≤2.
Criteria for exclusion from the study were as follows: insufficient
hematological, renal or hepatic functions; unstable brain
metastasis; history of prior chemotherapy and/or radiotherapy;
presence of uncontrolled infections; presence of an additional
malignancy; presence of a systemic disease contradicting
administration of chemotherapy; pregnancy; ECOG PS >3; and
unfitness for follow-up due to psychological, familial,
sociological or geographical reasons.
Treatment plan
The main treatment regimen consisted of chemotherapy
for patients in stage IV of the disease, and sequential
chemoradiotherapy was used for patients in stage IIIB. Vinorelbine
at a dose of 30 mg/m2 or gemcitabine at a dose of 1,250
mg/m2 on Days 1 and 8 and cisplatin at a dose of 80
mg/m2 on Day 1 were administered on a three-week cycle.
The cycle was repeated every three weeks. At least two cycles were
administered to the patients who were considered assessable for
response. Patients who responded to the treatment and did not show
signs of toxicity or progression received four to six cycles.
Dosage was adjusted according to hematological, neurological, renal
and hepatic functions. Dosage was decreased by 25% for patients who
were classified as Grade III or Grade IV in accordance with WHO
toxicity criteria. Curative radiotherapy was administered to all
patients in stage IIIB of the disease who responded to chemotherapy
after three cycles of chemotherapy regimens. A final one to three
cycles were administered between three weeks and one month after
administration of radiotherapy. Standard ECOG response criteria
were used. The response was evaluated by thorax CT scan after two
cycles of chemotherapy and at the end of the treatment. Briefly, a
complete response was defined as the absence of disease at all
known sites for at least four weeks. A partial response was defined
as a 50% reduction in the sum of the perpendicular diameters of all
measurable lesions, lasting at least four weeks. Progressive
disease was defined as either a 25% increase in the area of any one
lesion over the prior measurement or the development of one or more
new lesions. Survival was calculated from the date of diagnosis
until the date of death.
Statistical analysis
Data were evaluated with SPSS 13.0 (SPSS, Inc.,
Chicago, IL, USA). Survival of the patients was calculated from the
date of diagnosis to the date of death. Response rates were
calculated for patients with complete or partial responses. Median
age, smoking habits, performance status, response rates and
toxicity results of the groups were compared with the Mann-Whitney
U and Pearson Chi-square tests. The survival rates were calculated
by the Kaplan-Meier method.
Results
The median age was 59.1±9.9 years and the
male-to-female ratio was 124/17. Most of the patients had smoked
>21 packs/year (81.6%). In the distribution of ECOG PS, 46.1% of
patients were in ECOG 0–1, 43.3% of patients in ECOG 2 and 10.6% of
patients in ECOG 3–4. It was observed that 62.4% of the patients
had stage IIIB disease and 37.6% of patients had stage IV disease.
Furthermore, 69.6% of patients had squamous cell carcinoma, 17.7%
of patients had adenocarcinoma and 12.7% of patients were
classified as having undifferentiated NSCLC. The metastatic sites
were bone (15%), brain (10.5%), liver (6%) and adrenal gland (4.5%)
in the metastatic patients (Table
I). The median number of chemotherapy cycles was 3.7 for
cisplatin plus third-generation (vinorelbine or gemcitabine)
agents. The overall response rate was 32.6%. Respectively, 32.9 and
18% of patients received curative and palliative radiotherapy, and
13.5% of patients received second-line chemotherapy. The median
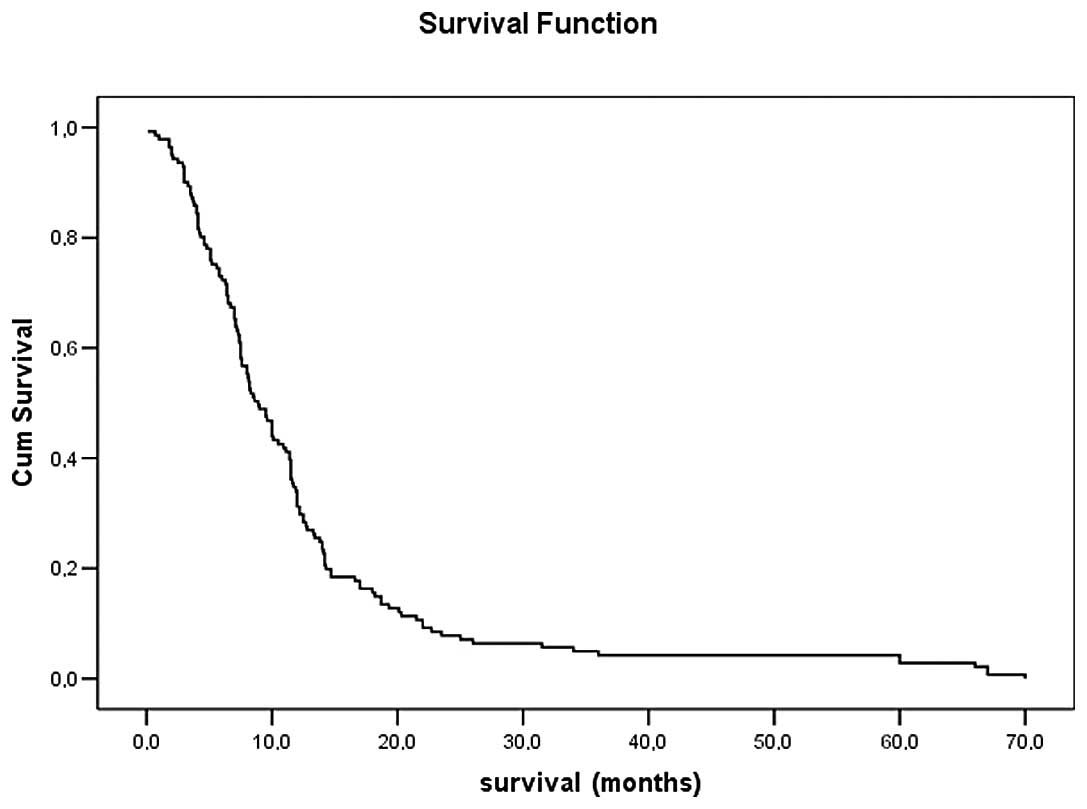
survival time was 12.3 months (95% CI, 10.2–14.5). The median
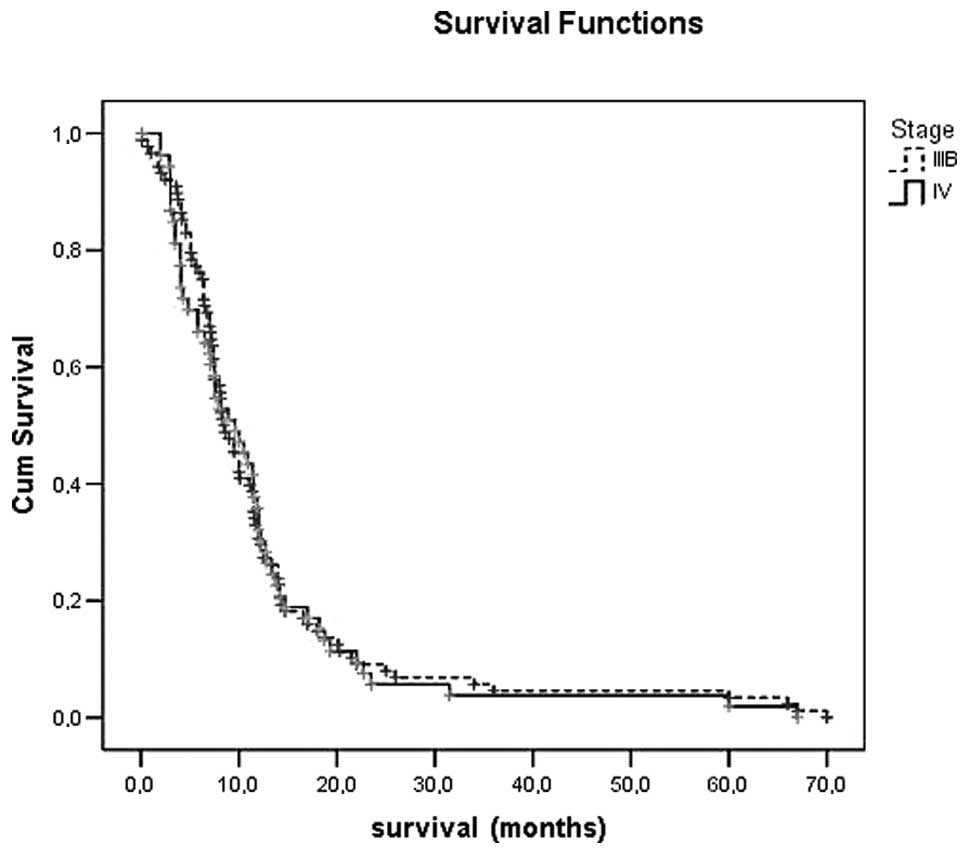
survival times for stages IIIB and IV were 12.6±1.4 and 11.9±1.7
months, respectively. The 1-, 2-, 3- and 5-year survival rates were
33, 7.5, 4.3 and 2.8%, respectively (Table II). All patients were evaluated for
toxicity. The major hematological toxicities encountered in this
study were neutropenia, febrile neutropenia, thrombocytopenia and
anemia. Percentages of grades 1–2 and 3–4 toxicity for anemia were
43.9 and 8.1%, for neutropenia 40.2 and 9.6%, for thrombocytopenia
8.9 and 5.1% and for nausea and vomiting 61.9 and 3.6%,
respectively. The rate of febrile neutropenia was 4.9% (Table III). No serious hemorrhagic events
were noted with either regimen. Kaplan-Meier survival curves of all
patients, stage III and IV are shown in Figs. 1 and 2.
 | Table IBaseline characteristics of the
patients. |
Table I
Baseline characteristics of the
patients.
| Characteristics | n=141 |
|---|
| Age (median ±
SD) | 59.1±9.9 |
| Gender (%) | |
| Male | 87.9 |
| Female | 12.1 |
| Smoking status
(%) | |
| Nonsmoker | 5.0 |
| <10
packs/year | 5.0 |
| 10–20
packs/year | 8.5 |
| 21–30
packs/year | 20.6 |
| >30
packs/year | 61.0 |
| ECOG performance
status (%) | |
| 0–1 | 46.1 |
| 2 | 43.3 |
| 3–4 | 10.6 |
| Disease stage
(%) | |
| IIIB | 62.4 |
| IV | 37.6 |
| Histological type
(%) | |
| Squamous cell | 69.5 |
| Adenocarcinoma | 17.7 |
| Undifferentiated
NSCLC | 12.7 |
| Metastatic sites
(%) | |
| Bone | 15.0 |
| Brain | 10.5 |
| Liver | 6.0 |
| Adrenal | 4.5 |
 | Table IIOutcomes of the treatments. |
Table II
Outcomes of the treatments.
| Variables | n=141 |
|---|
| Response (%) | |
| Complete
response | 6.8 |
| Partial
response | 26.1 |
| Stable disease | 40.5 |
| Progressive
disease | 26.6 |
| Overall response rate
(%) | 32.6 |
| Radiotherapy (%) | |
| Curative | 32.9 |
| Palliative | 18.0 |
| Second-line
chemotherapy (%) | 13.5 |
| Overall survival
[months (95% CI)] | 12.3 (10.2–14.5) |
| Stage IIIB median
survival (months) | 12.6±1.4 |
| Stage IV median
survival (months) | 11.9±1.7 |
| Survival (%) | |
| 1-year
survival | 33.0 |
| 2-year
survival | 7.5 |
| 3-year
survival | 4.3 |
| 5-year
survival | 2.8 |
 | Table IIIToxic effects. |
Table III
Toxic effects.
| Type of toxicity | n=141 |
|---|
| Anemia (%) | |
| Grade 1–2 | 43.9 |
| Grade 3–4 | 8.1 |
| Neutropenia (%) | |
| Grade 1–2 | 40.2 |
| Grade 3–4 | 9.6 |
| Febrile neutropenia
(%) | 4.9 |
| Thrombocytopenia
(%) | |
| Grade 1–2 | 8.9 |
| Grade 3–4 | 5.1 |
| Nausea and vomiting
(%) | |
| Grade 1–2 | 61.9 |
| Grade 3–4 | 3.6 |
Discussion
According to reported studies, the long-term
survival rate of patients with locally advanced and advanced NSCLC,
varies by disease characteristics but is generally low, with 5-year
survival rates for all stages ranging from 9 to 15%. The reported
survival rates in stages IIIA (14.1%), IIIB (4.6%) and IV (4.2%)
are closely comparable to those reported from other centers for
IIIA (8–11%), IIIB (1–5%) and IV (1–5%) (3–5).
Hagerty et al (6) emphasized the importance of patient
preferences in the case of metastatic disease from a variety of
cancers, with patients more frequently wanting to know the longest
survival time with treatment rather than the 5-year survival rate.
However, data are sparse concerning the 5-year survival rates and
5-year survival advantages of patients with stage IIIB and IV NSCLC
who receive chemotherapy or chemoradiotherapy.
Okamoto et al (7) reported that 7.7% of 222 metastatic
NSCLC patients survived for >2 years. Satoh et al
(8) reported that 19.4 and 13.9%
of advanced NSCLC patients survived for >2 or 3 years with
cisplatin-based chemotherapy, respectively. In this study, all
patients received platinum-based chemotherapy as a first-line
chemotherapy, and the response rate was found to be 42.8%. Kaira
et al (9) reported a 20%
response rate with first-line chemotherapy and 8% of patients
survived for >5 years. These findings are similar to the
findings of our study. Wang et al (5) evaluated 56 patients with stage III
and IV NSCLC who had survived for 5 years or longer. Only one
(1.7%) patient with stage IV NSCLC survived for 5 years treated
with chemotherapy alone.
There is a wide variety in the toxicity rates
reported in previous studies, with grade 3–4 anemia in 7–24% and
20–30% of the patients receiving cisplatin and vinorelbine and the
patients receiving cisplatin and gemcitabine, respectively; grade
3–4 neutropenia in 5.4–38.5% and 13.8–81%; grade 3–4
thrombocytopenia in 2.5–20% and 2.5–6%; and grade 3–4 nausea and
vomiting in 0–58% and 3.2–39%, respectively (10–13).
Our toxicity results were consistent with those of previous
studies.
In conclusion, the effects of chemotherapy in
advanced-stage NSCLC patients have been controversial since the
1990s. However, the cisplatin-based new-generation cytotoxic agents
for combined modality therapy offer increased hope of long-term
survival of patients with locally advanced and advanced NSCLC.
There is a continued need to follow up the outcomes of such
patients over long periods of time.
References
|
1
|
Molina JR, Adjei AA and Jett JR: Advances
in chemotherapy of non-small cell lung cancer. Chest.
130:1211–1219. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pfister DG, Johnson DH, Azzoli CG, Sause
W, Smith TJ, Baker S Jr, et al: American Society of Clinical
Oncology treatment of unresectable non-small-cell lung cancer
guideline: update 2003. J Clin Oncol. 22:330–353. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
De Cos Escuin JS, Vecente CD, Penafiel JC,
Miranda JAR, Gonzalez MAS and Jimenez JFM: Overall long-term
survival in lung cancer analyzed in 610 unselected patients. Arch
Bronconeumol. 40:268–274. 2004.PubMed/NCBI
|
|
4
|
Fry WA, Phillips JL and Menck HR: Ten-year
survey of lung cancer treatment and survival in hospitals in the
United States: a national cancer data base report. Cancer.
86:1867–1876. 1999.PubMed/NCBI
|
|
5
|
Wang T, Nelson RA, Bogardus A and Granis
FW Jr: Five-year lung cancer survival: which advanced stage
nonsmall cell lung cancer patients attain long-term survival?
Cancer. 116:1518–1525. 2010.PubMed/NCBI
|
|
6
|
Hagerty RG, Butow PN, Ellis PA, et al:
Cancer patient preferences for communication of prognosis in the
metastatic setting. J Clin Oncol. 22:1721–1730. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Okamoto T, Maruyama R, Shoji F, Asoh H,
Ikeda J, Miyamoto T, et al: Long-term survivors in stage IV
non-small cell lung cancer. Lung Cancer. 47:85–91. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Satoh H, Ishikawa H, Ohara G, Kagohashi K,
Kurishima K, Ohtsuka M and Hizawa N: Long-term survivors after
chemotherapy in advanced non-small cell lung cancer. Anticancer
Res. 27:4457–4460. 2007.PubMed/NCBI
|
|
9
|
Kaira K, Takahashi T, Murakami H, Tsuya A,
Nakamura Y, Naito T, Endo M and Yamamoto N: Long-term survivors of
more than 5 years in advanced non-small cell lung cancer. Lung
Cancer. 67:120–123. 2010.PubMed/NCBI
|
|
10
|
Southern Italy Cooperative Oncology Group
(SICOG): Gemcitabine plus cisplatin combinations in advanced
non-small cell lung cancer. Anticancer Drugs. 11:S23–S27. 2000.
|
|
11
|
Huisman C, Giaccone G, van Groeningen CJ,
Sutedja G, Postmus PE and Smit EF: Combination of gemcitabine and
cisplatin for advanced non-small cell lung cancer: a phase II study
with emphasis on scheduling. Lung Cancer. 33:267–275. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Crino L, Scagliotti GV, Ricci S, De
Marinis F, Rinaldi M, Gridelli C, et al: Gemcitabine and cisplatin
versus mitomycin, ifosfamide, and cisplatin in advanced
non-small-cell lung cancer: a randomized phase III study of the
Italian Lung Cancer Project. J Clin Oncol. 17:3522–3530.
1999.PubMed/NCBI
|
|
13
|
Cardenal F, Lopez-Cabrerizo M, Anton A,
Alberola V, Massuti B, Carrato A, et al: Randomized phase III study
of gemcitabine-cisplatin versus etoposide-cisplatin in the
treatment of locally advanced or metastatic non-small-cell lung
cancer. J Clin Oncol. 17:12–18. 1999.PubMed/NCBI
|