Introduction
Epithelial-mesenchymal transition (EMT) is a
critical developmental process that plays a central role in the
formation and differentiation of multiple tissues and organs.
During EMT, epithelial cells lose cell-cell adhesion and apical
polarity and acquire mesenchymal features, including motility,
invasiveness and resistance to apoptosis (1). One of the key hallmarks of EMT is
loss of E-cadherin, a cell-adhesion protein that is regulated by
multiple transcription factors, including Snail, Slug and Twist.
These transcription factors act as E-box repressors and block
E-cadherin transcription (2).
Transforming growth factor (TGF)-β1 induces EMT in epithelial cells
through the upregulation of Snail1 in Smad-dependent signaling
(3). The inhibition of Snail1 in
mesenchymal cells results in decreased Nanog promoter luciferase
activity and loss of self-renewal characteristics in vitro.
BMP-7 induces mesenchymal-epithelial transition (MET) through
Snail1 and Nanog downregulation. In mesenchymal cells post-EMT,
Snail1 directly regulates Nanog expression and loss of Snail1
causes liver fibrosis.
Snail1 and Snail2 belong to the Snail superfamily of
zinc finger (ZF) transcription factors (3) and have emerged as important
repressors of E-cadherin and inducers of EMT (4). Vertebrate Snail1 and Snail2 factors
share a high degree of homology at the DNA-binding C-terminal
region, containing four and five C2H2 ZFs, respectively, and at the
N-terminal region that contains the SNAG transactivation domain
(5). Snail1 and Snail2 present a
similar modular organization of nuclear import sequences,
distributed among several ZFs (6).
Snail factors have emerged as essential regulators of physiological
and pathological EMT processes (7). Post-translational modifications of
mammalian Snail have been shown to modulate Snail1 stability and
functional repressor activity. In particular, phosphorylation by
GSK3β or PKD1 plays a negative role (8), while phosphorylation by PAK1, CK2,
PKC or Lats2 or interaction with lysyl oxidase-like 2/3 (LOXL2/3),
exerts a positive effect on Snai1 functionality (9).
Our hypothesis is that mesenchymal cells acquire
liver fibrosis traits after EMT through Snail1-dependent
mechanisms. In this study, we demonstrate that BMP-7 induces MET
through Snail1 in rat liver fibrosis cells (post-EMT).
Materials and methods
Animals
Adult gender-matched (n=20 each) C57BL rats weighing
200±10.2 g were purchased from Tongji University Laboratories
(Shanghai, China) and fed with a commercial diet and water. All
animal experiments were performed according to the National
Institutes of Health (NIH) guidelines for the ethical care and use
of laboratory animals, and the experimental protocol was approved
by the Tongji Animal Care and Use Committee of China.
Rat liver fibrosis models
A total of 40 adult numbered rats were sorted into
liver fibrosis model and normal control groups. A total of 20 rats
in the liver fibrosis group received intraperitoneal injections of
40% CCl4 and olive oil admixture (0.5 ml/100 mg,
Sigma-Aldrich, St. Louis, MO, USA) tert as previously described
(4). Rats were sacrificed after 8
weeks of treatment.
Chemicals and materials
Glass slides (75×25 mm2) were obtained
from Gibco (Carlsbad, CA, USA). (3-Acryloxypropyl) trichlorosilane
was purchased from Gelest, Inc. (Morrisville, PA, USA).
Streptavidin-conjugated Alexa 546, AlexaFluor 488 anti-mouse IgG,
BMP-7 and Snail were obtained from Sigma-Aldrich. Mouse
anti-E-cadherin antibody was purchased from BD Biosciences
(Franklin Lakes, NJ, USA). Concentrated phosphate-buffered saline
(10X PBS) was purchased from Lonza (China). Minimal essential
medium (MEM), sodium pyruvate, non-essential amino acids, fetal
bovine serum (FBS), Superscript III, RNaseOut (RNase inhibitor) and
dNTPs were purchased from Lonza. The 384-well polypropylene
microarray plates were obtained from Genetix (China). Goat anti-rat
cross-adsorbed albumin antibody was obtained from Sigma-Aldrich.
Formalin was purchased from Fisher Scientific (China). ApopTag Red
in situ Apoptosis Detection kit was obtained from Chemicon
(China). DAPI stain mounting media were purchased from Vectorshield
(China).
Cell culture and transfections
Established LEPC cells were obtained from the ATCC
collection (LGC Standards-SLU, Barcelona, Spain). Cell lines were
maintained in DMEM supplemented with 10% FBS and antibiotics (100
μg/ml ampicillin, 32 μg/ml gentamicin; Sigma-Aldrich).
Stable and transient transfections were performed using
Lipofectamine reagent (Invitrogen, Carlsbad, CA, USA) according to
the manufacturer’s instructions for the generation of stable
clones.
Immunoblot analysis, immunocytochemistry
and immuno-precipitation
Tissue and cell lysates were prepared and immunoblot
analysis was performed as described previously (10). Band intensity was determined using
ImageMaster 2D Elite version 4.01 software (Amersham/GE Healthcare,
Uppsala, Sweden). For immunoprecipitation, after liver cells were
treated with Snail or BMP-7 for 48 h, the cells were lysed in
buffer [50 mM Tris-HCl, pH 8.0, 150 mM NaCl, 5 mM
ethylenediaminetetraacetic acid (EDTA) and 0.5% Nonidet P-40
(NP-40)] and centrifuged at 16,000 × g for 15 min to remove debris.
Cleared lysates were subjected to immunoprecipitation with
antibodies. For immunocytochemistry, cells were fixed in 4%
paraformaldehyde at room temperature for 15 min, permeabilized in
5% Triton X-100 for 5 min and then stained using pAbs. The
secondary antibodies used were anti-mouse Alexa Fluor 594 dye
conjugate and anti-rabbit Alexa Fluor 488 dye conjugate (Molecular
Probes/Life Technologies, Carlsbad, CA, USA). Nuclei were stained
with 4′,6-diamidino-2-phenylindole (DAPI Blue; Molecular
Probes/Life Technologies). After mounting, the cells were
visualized using a multiphoton confocal laser-scanning microscope
(Carl Zeiss, Thornwood, NY, USA).
Co-immunoprecipitation and western blot
assays
Briefly, LEPC cells were transiently transfected
with the indicated vectors for 48 h. Lysates were then obtained in
immunoprecipitation buffer (50 mM Tris-HCl, pH 8.0, 150 mM NaCl, 5
mM EDTA, 0.5% NP-40) containing protease and phosphatase inhibitors
(2 μg/ml aprotinin, 1 μg/ml leupeptin, 1 mM PMSF, 1
mM Na3VO4, 10 mM NaF) and precleared with
Sepharose G-beads. Supernatants were subjected to overnight
incubation with anti-HA affinity matrix (Roche Diagnostics,
Indianapolis, IN, USA) or Sepharose G-beads coated with anti-rat
IgG as an immunoprecipitation control. Immunoprecipitates were
resolved by PAGE on 7.5–12% SDS gels, transferred to membranes and
incubated with the indicated antibodies. The membranes were then
developed using ECL reagent following the manufacturer’s
instructions (Amersham Pharmacia Biotech, Piscataway, NJ, USA).
Blots were incubated with rat anti-HA (Roche Diagnostics; 1:100) or
mouse anti-flag (Sigma-Aldrich; 13:000). The secondary antibodies
used were HRP-coupled goat anti-rat (Pierce Biotechnology, Inc.,
Rockford, IL, USA; 110:000) or sheep anti-mouse (Pierce
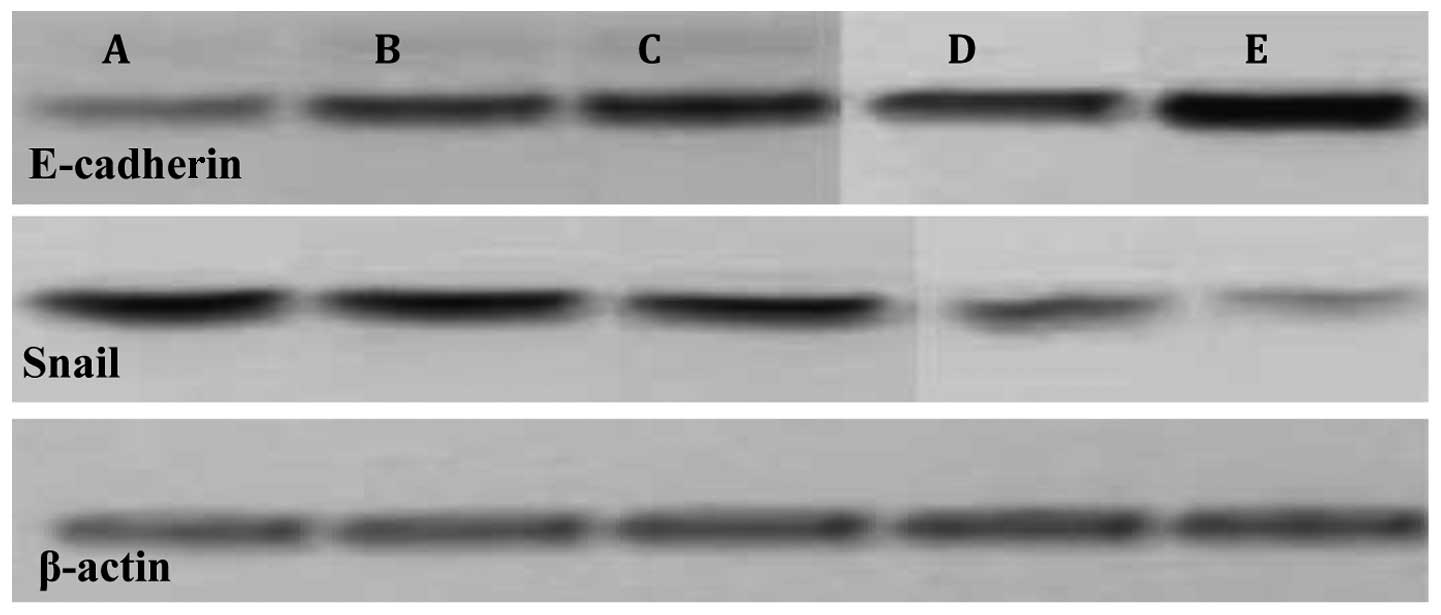
Biotechnology, Inc.; 11:000). For detection of E-cadherin, α-smooth
muscle actin (α-SMA) and Snail expression, western blotting was
performed on whole-cell lysates using rat anti-E-cadherin ECCD2 mAb
(1:200, produced in our laboratory from the ECCD2 hybridoma, a gift
of M. Takeichi, Ricken Center, Japan), mouse anti-α-SMA (1:500,
Dako, Carpinteria, CA, USA) or rat anti-Snail (Roche Diagnostics),
followed by HRP-coupled secondary antibodies.
Real-time quantitative PCR (RT-PCR)
analysis
RT-PCR analysis of cDNA samples was performed with
specific primers designed using Primer Express software (Applied
Biosystems, Foster City, CA, USA). The primers used for Snail were
5′-AAGGATCTCCAGGCTCGAAAG-3′ (forward) and
5′-GCTTCGGATGTGCATCTTGA-3′ (reverse) and those used for β-actin
were 5′-GCAAAGACCTGTACGCCAACA-3′ (forward) and
5′-TGCATCCTGTCGGCAATG-3′ (reverse). Total RNA was extracted from
cultured cells using an RNeasy kit (Qiagen, Hilden, Germany)
according to the manufacturer’s instructions. cDNA was synthesized
using 1 μg of RNA with avian myeloblastosis virus reverse
transcriptase (Promega, Madison, WI, USA) and oligo(dT) primers.
Transcript levels were assessed by RT-PCR (ABI 7300; Applied
Biosystems) and all experiments were normalized to β-actin.
In vivo ubiquitination assay
The cells were treated with 10 μM MG132 for 6
h, 24 h after transfection. The treated cells were then harvested
with PBS containing 10 mM N-ethylmaleimide (NEM) and 1 mM
dithiothreitol (DTT). The cells were washed with PBS, centrifuged
and subjected to one freeze-thaw cycle. Cell pellets were then
resuspended in 200 μl buffer 1 [10 mM Tris-HCl, pH 7.5, 10
mM NaCl, 0.5% NP-40, 5 mM EDTA, 1 mM ethylene glycol tetraacetic
acid (EGTA), 10 mM NEM, 1 mM DTT, 5 mM NaF, 1 mM
Na3VO4 and protease inhibitor cocktail] and
sonicated in a water bath (Bioruptor; Diagenode, Denville, NJ,
USA). Next, 500 μl buffer 2 (20 mM Tris-HCl, pH 7.5, 0.5 M
NaCl, 0.5% NP-40, 5 mM EDTA, 1 mM EGTA, 10 mM NEM, 1 mM DTT, 5 mM
NaF, 1 mM Na3VO4 and protease inhibitor
cocktail) was added and the extracts were subjected to a 30-min
rotation at 4°C. The extracts were then centrifuged. We added 2
μg of anti-Flag M2 antibody and protein A/G beads to the
supernatant, which was then incubated for 2 h. The beads were then
washed three times, resuspended in loading buffer and boiled.
Immunoblotting was performed as described above.
Groups for a role for Snail in rat liver
fibrosis
A total of 15 adult numbered rats were randomly
sorted into 3 groups: i) normal control group: 5 rats received
intraperitoneal injections of olive oil (0.5 ml/100 mg) twice per
week; ii) liver fibrosis model group: 5 rats received
intraperitoneal injections of 40% CCl4 and olive oil
admixture (0.5 ml/100 mg); iii) BMP-7-treated group: 5 rats
received intraperitoneal injections of 40% CCl4 and
olive oil admixture (0.5 ml/100 mg) twice per week and BMP-7 (300
μg/kg) at same time. Primary rat hepatocytes
(1×106/dish) were cultured for 48 h in F12 medium
containing 10% fetal bovine serum and 2 μg/ml insulin until
they had adhered, which was marked as ‘−’; Primary rat hepatocytes
(1×106/dish) were cultured for 48 h in F12 medium
containing 10% fetal bovine serum and 2 μg/ml insulin until
they had adhered. To induce EMT, the media were replaced with F12
media supplemented with 0.5% fetal bovine serum and 200
mg/μl insulin containing Snail for 96 h, which was marked as
‘+’.
Statistical analysis
All results shown in the bar graphs are expressed as
the fold ratio relative to untreated or control cells. Statistical
analysis was performed using SPSS version 17 statistical software
(SPSS Inc., Chicago, IL, USA). Student’s t-test was used when
comparing two groups. One-way ANOVA was used when comparing
multiple groups, followed by Tukey’s post-hoc test. P<0.001 was
considered to indicate a statistically significant result.
Results
General remarks and groups
None of the animals died during the study period.
Body weight gain was lower in the Snail-treated compared to the
control rats (data not shown). A total of 40 adult numbered rats
were randomly sorted into 4 groups: i) normal control group: 5 rats
received intraperitoneal injections of olive oil (0.5 ml/100 mg)
twice every week; ii) liver fibrosis model group: 10 rats received
intraperitoneal injections of 40% CCl4 and olive oil
admixture (0.5 ml/100 mg) tert as previously described (4); iii) Snail-treated group: 10 rats
received intraperitoneal injections of 40% CCl4 and
olive oil admixture (0.5 ml/100 mg) twice every week and Snail (500
μg/kg) at the same time; iv) BMP-7-treated group, 10 rats
received intraperitoneal injections of 40% CCl4 and
olive oil admixture (0.5 ml/100 mg) twice every week and BMP-7 (300
μg/kg) at the same time. The rats were sacrificed after 8
weeks of treatment.
A role for Snail in rat liver
fibrosis
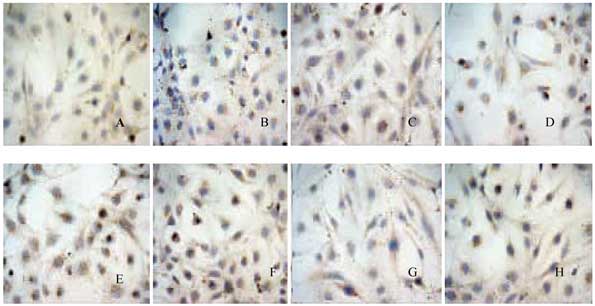
The present study demonstrated that treatment with
Snail induced EMT and increased liver injury during
CCl4-induced liver fibrosis in rats. This was
accompanied by increased expression of liver fibrosis mesenchymal
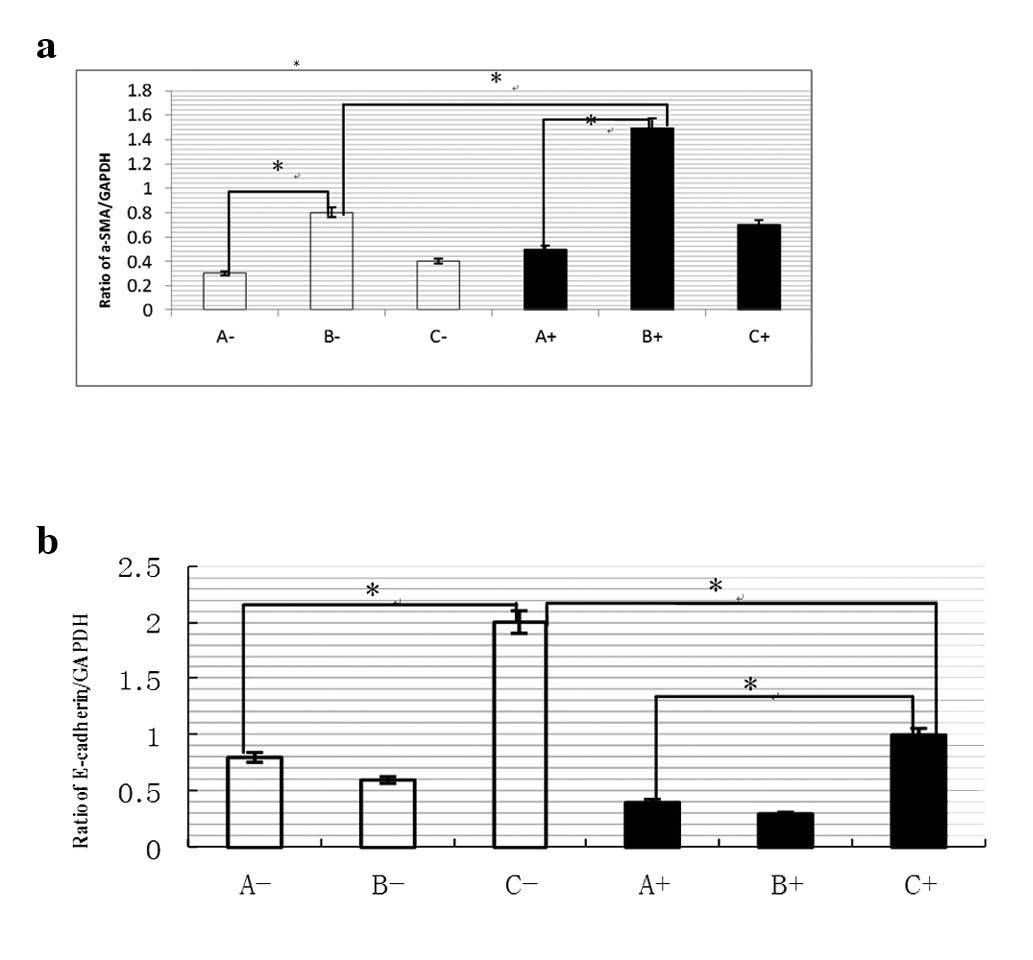
markers, including α-SMA, but inhibition of E-cadherin (P<0.001;
Figs. 1–5). The ratio of α-SMA/GAPDH in liver
fibrosis model group was higher than that in control group, with
200 mg/μl insulin containing Snail-treated for 96 h, ratio
of α-SMA increased significantly in liver fibrosis model group than
control group. But this was reverse in ratio of E-cadherin/GAPDH
(P<0.001; Fig. 5).
A role for Snail in rat liver
fibrosis
The present study demonstrated that rats treated
with Snail had increased hepatic fibrosis in
CCl4-induced liver injury. This was accompanied by
increased expression of hepatic fibrosis mesenchymal markers,
including α-SMA, but repression of E-cadherin (Figs. 1–4).
A role for BMP-7 in rat liver
fibrosis
We demonstrated that CCl4-induced
fibrosis is reversed in rats treated with BMP-7. Significantly more
BMP-7 and less Snail mRNA were expressed in the hepatic fibrosis
model group than in the controls (P<0.001; Figs. 1–4). This was accompanied by reduced
expression of hepatic fibrosis mesenchymal markers, including
α-SMA, but increased expression of E-cadherin (Fig. 1). Thus, a strategy that
specifically increased BMP-7 in myofibroblasts from cirrhotic
livers tended to reverse the myofibroblastic phenotype and caused
the cells to acquire a more quiescent and epithelial phenotype.
Discussion
BMP-7 induces MET through Snail1, which represses
α-SMA by binding to E-box promoter elements (11). In the present study, Snail
stimulation of epithelial liver fibrosis cells resulted in a
mesenchymal phenotype with fibroblastoid appearance and loss of
E-cadherin. However, the underlying mechanism has not yet been
elucidated. Based on our results, we hypothesize that these liver
fibrosis characteristics are Snail-dependent. Inhibition of Snail1
causes the downregulation of Nanog and CD44 and loss of
self-renewal, as evidenced by decreased liver fibrosis formation.
Liver fibrosis cells are more mesenchymal in character, with
increased Snail1, Zeb1 and Zeb2 mRNA expression and decreased
E-cadherin expression. Notably, although Smads and Snail proteins
are known to play a central role in liver fibrosis cell growth,
Notch signaling is also capable of inhibiting liver fibrosis growth
through the induction of EMT (12). Notch1 is known to regulate Snail
and Slug mRNA levels, but efforts have not been made to examine
alternative functions of NICD and Snail expression in liver
fibrosis (13). In addition,
Notch1 is involved in the mesenchymal program by activating Snail
expression in liver fibrosis development (10). However, since Notch signals and
cellular functions vary according to cell type and cellular
environment, these inconsistencies may be caused by the different
cell types and conditions. We hypothesized that ROS stress
upregulated Snail mRNA and protein expression (14). E-cadherin expression decreased in
Snail-overexpressed cells compared with control cells (P<0.001).
Our study provides one clue for understanding the complex
regulation mechanism of p53, MDM2, Notch1 and Snail in the EMT
process. The regulation of these proteins and their physiological
contribution to EMT require further investigation. However, the
mechanism that we have described presents substantial evidence of
cross-interference between the Notch and Snail signaling pathways,
which may be mediated by BMP-7. In addition, Snail1 is one of a
number of regulators of EMT, and thus manipulation of multiple
factors may be required to fully inhibit liver fibrosis
initiation.
Acknowledgements
The authors are grateful to Professor
Guo-Tong Xu and Professor Jue Li for histological evaluation and Dr
Shu-Chang Xu for technical assistance. This study was supported by
a grant from the National Natural Science Foundation of China
(no.81070343) and Shanghai Excellent Academic Leaders Program (no.
08D14045).
References
|
1
|
Thenappan A, Li Y, Kitisin K, Rashid A,
Shetty K, Johnson L and Mishra L: Role of transforming growth
factor beta signaling and expansion of progenitor cells in
regenerating liver. Hepatology. 51:1373–1382. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
You H, Ding W and Rountree CB: Epigenetic
regulation of cancer stem cell marker CD133 by transforming growth
factor-beta. Hepatology. 51:1635–1644. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bi WR, Yang CQ and Shi Q: Transforming
growth factor-β1 induced epithelial-mesenchymal transition in
hepatic fibrosis. Hepatogastroenterology. 59:1960–1963. 2012.
|
|
4
|
Lin Y, Wu Y, Li J, Dong C, Ye X, et al:
The SNAG domain of Snail1 functions as a molecular hook for
recruiting lysine-specific demethylase 1. EMBO J. 29:1803–1816.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lin T, Ponn A, Hu X, Law BK and Lu J:
Requirement of the histone demethylase LSD1 in Snail1-mediated
transcriptional repression during epithelial-mesenchymal
transition. Oncogene. 29:4896–4904. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
MacPherson MR, Molina P, Souchelnytskyi S,
Wernstedt C, Martin-Pérez J, et al: Phosphorylation of serine 11
and serine 92 as new positive regulators of human Snail1 function:
potential involvement of casein kinase-2 and the cAMP-activated
kinase protein kinase A. Mol Biol Cell. 21:244–253. 2010.
View Article : Google Scholar
|
|
7
|
Du C, Zhang C, Hassan S, Biswas MH and
Balaji KC: Protein kinase D1 suppresses epithelial-to-mesenchymal
transition through phosphorylation of Snail. Cancer Res.
70:7810–7819. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang K, Rodriguez-Aznar E, Yabuta N, Owen
RJ, Mingot JM, et al: Lats2 kinase potentiates Snail1 activity by
promoting nuclear retention upon phosphorylation. EMBO J. 31:29–43.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nieto MA: The ins and outs of the
epithelial to mesenchymal transition in health and disease. Annu
Rev Cell Dev Biol. 10:347–376. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Viñas-Castells R, Beltran M, Valls G,
Gómez I, García JM, et al: The hypoxia-controlled FBXL14 ubiquitin
ligase targets SNAIL1 for proteasome degradation. J Biol Chem.
285:3794–3805. 2010.PubMed/NCBI
|
|
11
|
Lander R, Nordin K and LaBonne C: The
F-box protein Ppa is a common regulator of core EMT factors Twist,
Snail, Slug and Sip1. J Cell Biol. 194:17–25. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang ZX, Song SH, Teng F, Wang GH, Guo WY,
Shi XM, et al: A single-center retrospective analysis of liver
transplantation on 255 patients with hepatocellular carcinoma. Clin
Transplant. 24:752–757. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lim SO, Park YM, Kim HS, Quan X, Yoo JE,
Park YN, et al: Notch1 differentially regulates oncogenesis by
wild-type p53 overexpression and p53 mutation in grade III
hepatocellular carcinoma. Hepatology. 53:1352–1362. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Luna-Zurita L, Prados B, Grego-Bessa J,
Luxán G, del Monte G, Benguría A, et al: Integration of a
Notch-dependent mesenchymal gene program and Bmp2-driven cell
invasiveness regulates murine cardiac valve formation. J Clin
Invest. 120:3493–3507. 2010. View
Article : Google Scholar : PubMed/NCBI
|