Introduction
Hepatic encephalopathy (HE) has been defined as a
disturbance in central nervous system function due to hepatic
insufficiency (1–3). Multiple treatments have been used for
HE. However, their efficacy has been assessed infrequently by
well-designed randomized clinical trials. This handicap reflects
the difficulty in evaluating the wide range of neuropsychiatric
symptoms. At the molecular level, HE is associated with a shift in
the balance between inhibitory and excitatory neurotransmission
towards a net increase in inhibitory neurotransmission, which is
generally attributed either to an increased GABAergic tone or to
alterations of the glutamatergic system (2,3).
However, the nature of the respective control mechanisms remains to
be completely resolved.
In the mammalian central nervous system, the
intraneuronal chloride concentration ([Cl]i) determines the
strength and polarity of GABA neurotransmission (4–6). The
[Cl]i is to a large extent determined by the Na-K-2Cl
co-transporter, NKCC1 (mediating Cl entry), and the K-Cl
co-transporter, KCC2 (mediating Cl exit) (4,5).
Changes in the expression of KCC2 and NKCC1 have been shown to be
involved in the generation of brain disorders via the regulation of
chloride homeostasis. NKCC1 activation has been reported to be
involved in astrocyte swelling induced by ammonia and in brain
edema in a thioacet-amide model of acute HE (6). However, the role of KCC2 in HE is
unclear.
Therefore, the present study aimed to compare the
plasma mRNA levels of KCC2 and NKCC1 in cirrhotic patients with
grade III–IV HE with those in cirrhotic patients without HE, using
the real-time RT-PCR technique. Additionally, we analyzed the
associations between the mRNA levels of the two transporters and
the corresponding hepatic functions, as well as the neurological
status of cirrhotic patients with HE.
Materials and methods
Clinical subjects
Data from patients in the Departments of
Hepatobiliary Surgery and Gastroenterology of Xi-Jing Hospital,
Fourth Military Medical University, Xi’an, China, between June 2009
and December 2010 and from healthy controls, were collected and
studied. A total of 85 liver disease patients, comprising 29
patients with HE grade I–II, 36 patients with HE grade III–IV, and
20 patients without HE, were evaluated in the present study. A
total of 15 healthy controls were also included. Clinical
characteristics of studied patients are presented in Table I. Written informed consent was
obtained from the healthy volunteers and from each patient where
this was possible. Clinical investigations were conducted according
to the principles expressed in the Declaration of Helsinki. The
study was approved by the Bioethical Committee of the Fourth
Military Medical University.
 | Table IClinical characteristics of the
studied patients. |
Table I
Clinical characteristics of the
studied patients.
| Liver cirrhosis
|
|---|
| Characteristic | without HE | with grade I–II
HE | with grade III–IV
HE |
|---|
| Age, years; median
(range) | 56 (36–77) | 55 (33–76) | 52 (34–74) |
| Gender,
male/female | 13/7 | 18/11 | 21/15 |
| Liver cirrhosis
etiology (n) | | | |
| PBC | 1 | 6 | 5 |
| HBV | 19 | 22 | 25 |
| HCV | 0 | 1 | 6 |
| GFR, ml/min/1.73
m2; range | 52–90 | 35–90 | 35–90 |
| Child-Pugh score,
median (range) | 6 (5–9) | 6 (5–10) | 8 (5–13) |
| MELD score, median
(range) | 17 (6–35) | 20 (8–35) | 26 (8–40) |
| Ascites (n) | 12 | 13 | 28 |
| ALT, U/l; mean ±
SE | 150.0±36.2 | 190.1±40.3 | 231.5±44.5 |
| Bilirubin, mg/dl;
mean ± SE | 2.04±1.0 | 2.33±1.2 | 2.88±0.8 |
| Albumins, g/dl; mean
± SE | 4.11±1.1 | 3.25±1.3 | 2.95±0.9 |
| Glasgow coma score,
median (range) | 12 (9.0–15.0) | 11 (9.0–15.0) | 6 (4.3–8.5) |
| Ammonia,
μmol/l; mean ± SE | 50.2±33.7 | 134.4±40.2 | 210.1±79.0 |
| MAMC % of standard,
mean ± SE | 97.0±20.6 | 90.0±18.7 | 88.5±15.6 |
| TSF % of standard,
mean ± SE | 96.3±41.8 | 85.1±40.5 | 79.2±43.4 |
A diagnosis of liver cirrhosis had already been made
in all patients by pertinent clinical, laboratory and morphological
procedures performed during previous hospitalization. Diagnosis and
the grade of HE were assessed according to a detailed physical,
neurological and psychometric assessment, with particular note
being made of the mental status, the severity of asterixis and the
performance in the number connection test type A. A complete
neurological examination had been performed on every enrolled
patient. To obtain objective clinical criteria for evaluating the
clinical improvement of HE, we excluded patients with alcoholic
liver cirrhosis to avoid bias by neurological and psychiatric signs
due to chronic or acute ethanol abuse.
Estimation of liver insufficiency and the
neurological status of the subjects
To estimate a possible association between levels of
mRNAs of the two transporters, KCC2 and NKCC1, on the one hand, and
a corresponding degree of liver insufficiency and the neurological
status of the individuals with liver cirrhosis on the other,
Child-Pugh scores, model for end-stage liver disease (MELD) scales
and Glasgow coma scores were evaluated in all included patients.
Moreover, the MELD score, based on serum creatinine, albumin and
bilirubin concentrations, was analyzed. Assessment of the
neurological status of patients was performed according to the
Glasgow coma score (7). The
Glasgow score was recorded in each patient immediately prior to
serum collection by a physician who was unaware of the experimental
processes. Mid-upper arm muscle circumference (MAMC) and triceps
skinfold thickness (TSF) were measured as indices of body fat and
muscle protein compartment, respectively, using standard
techniques, as previously reported. MAMC % (percentage of standard
values) and TSF % (percentage of standard values) were considered
to define the nutritional status. Glomerular filtration rate (GFR,
as calculated from cystatin C) was measured as an index of renal
function (8). Clinical
characteristics of the studied population are presented in Table I. It should be indicated that the
patients in groups A and B were under treatment with lactulose and
diuretics.
Plasma KCC2 and NKCC1 levels were also compared with
those in the 15 healthy volunteers (5 females and 10 males with a
median age of 48 years, range 30–75 years).
Real-time RT-PCR detection
Total RNA was extracted from serum as described by
Vendittelli et al (9). The
quality and integrity of total RNA was evaluated using gel
electrophoresis and spectrophotometric determination. All samples
were treated with RNase-free DNase I (10 U/l) (Boehringer Mannheim,
Mannheim, Germany) and subsequently extracted with
phenol/chloroform/isoamylic alcohol. Total RNA was
reverse-transcribed by MMLV reverse transcriptase (Gibco BRL Life
Technologies, Ltd., Paisley, UK) in the presence of an RNase
inhibitor (Gibco BRL). For a quantitative comparison of mRNA
levels, real-time PCR was performed using SYBR-Green fluorescence
in a LightCycler® System (Roche Diagnostic GmbH, Basel,
Switzerland). The PCR reaction mixture was prepared using the
SYBR-Green PCR Master Mix. Thermal cycling conditions were 10 min
at 95°C followed by 35 cycles of 95°C for 15 sec and at 60°C for 1
min on thermal cycles (DFC-3200, MJ Research Company, Waltham, MA,
USA). Amplification specificity was checked using melting curves.
Both negative and positive controls were included in each PCR
reaction. All assays were carried out three times as independent
PCR runs for each cDNA sample. Gene expression was always related
to the expression of GAPDH as the housekeeping gene, which is known
to be a good choice for normalization of expression levels
(10). Each gene expression was
normalized with respect to GAPDH mRNA content. The sequences of the
human primer for SYBR-Green PCR were as follows: KCC2 (145 bp,
nucleotides 800–817, NM_020708 file in GenBank):
GAAGTGCTCAGAGAGGTGG (sense) and GCAGAAGAG GAAGAAGGC (antisense);
NKCC1 (140 bp, nucleotides 500–523, NM_001046 file in GenBank):
AGGAGCATTCAA GCACAGCTAACA (sense) and CGCTCTGATGATTCC CACGA
(antisense); GAPDH (147 bp): ACTTCAACAGCG ACACCCACT (sense) and
GCCAAATTCGTTGTCATA CCAG (antisense). Calculations of expression
were performed with the 2ΔΔCT method according to a
previous report (11). All
measurements were carried out in the absence of information as
regards the origin of the samples.
Measurement of blood ammonia
To express the association between blood ammonia
levels and KCC2/NKCC1 mRNA expression, the levels of ammonia in the
blood from enrolled subjects with or without HE were measured.
For the determination of plasma ammonia levels,
approximately 10 ml of blood was collected from each patient from a
stasis-free vein in an EDTA container and stored in an ice bath.
Adequate precautions were taken to avoid hemolysis, which
interferes with the assay. The plasma was then separated and an
ammonia assay was carried out within 20 min using a commercially
available Randox kit (Jiancheng Laboratories Ltd., Nanjing, China).
The corresponding increase in absorbance at 340 nm is proportional
to the plasma ammonia concentration.
Statistical analysis
All statistical analyses were performed using the
statistical software package, SSPS 13.0 (SPSS Inc., Chicago, IL,
USA). Data within 95% CI were used for analysis. Results are
expressed as the means ± standard error of the mean (SE). The
significance of differences was calculated by the non-parametric
Mann-Whitney U test. For the correlation analysis, the Spearman
non-parametric correlation was used. A value of P<0.05 was
considered to indicate a statistically significant difference.
Results
Comparison of KCC2 and NKCC1 mRNA levels
in peripheral blood of patients and control subjects
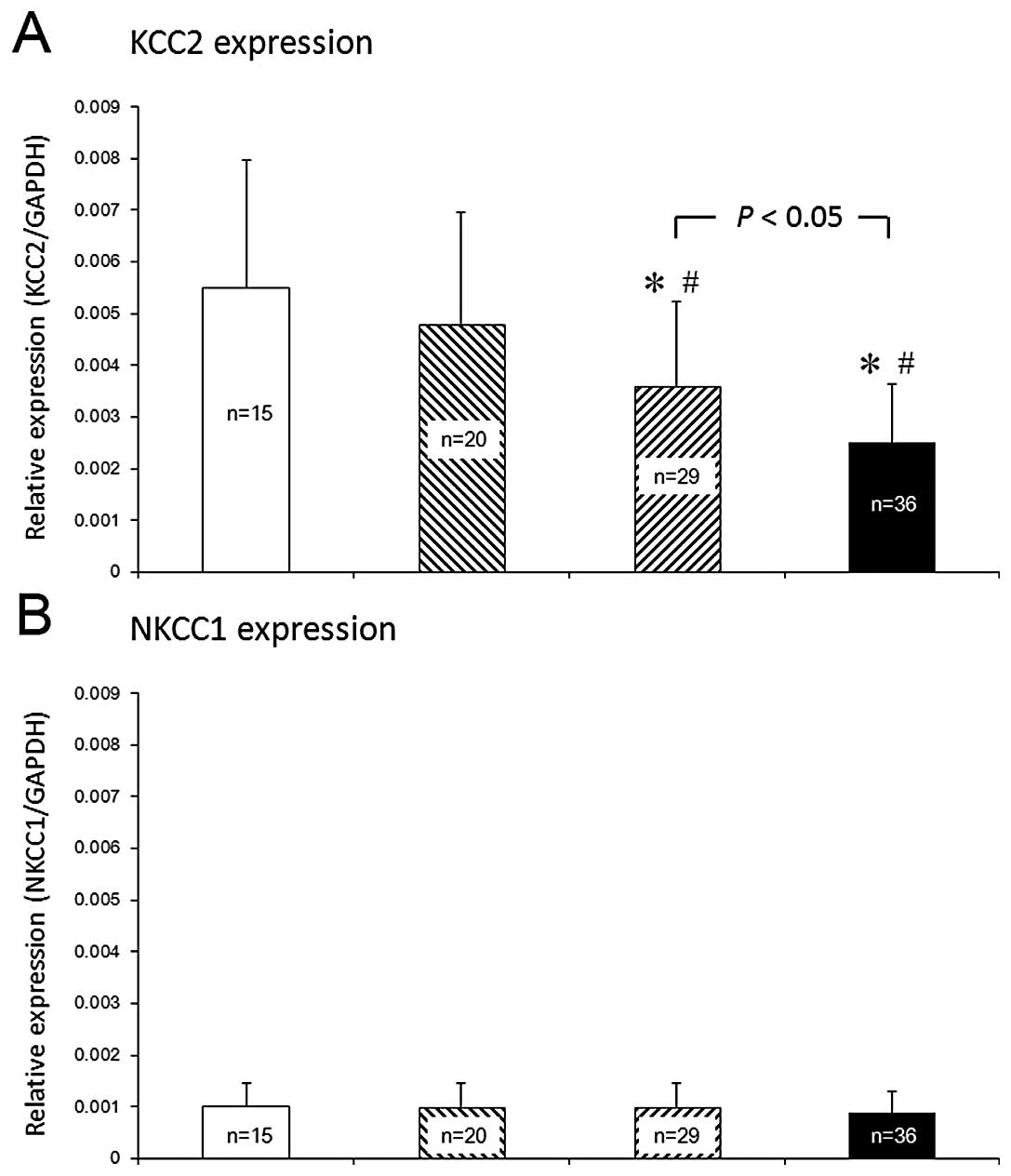
The means ± SEM of the relative KCC2 mRNA levels of
the cirrhotic patients with grade I–II HE (0.0034±0.0011; n=29)
were significantly lower (P<0.05, Fig. 1) than those of the cirrhotic
patients without HE (0.0045±0.0023; n=20) or those of the healthy
individuals (0.0055±0.0023; n=15). Moreover, the relative mRNA
levels of KCC2 of the cirrhotic patients with grade III–IV HE
(0.0021±0.0014; n=36) were much lower (P<0.05, Fig. 1) than those of patients with grade
I–II HE.
The relative mRNA expression levels of KCC2/GAPDH
did not show any significant association with gender (P=0.525), age
(P=0.711), or liver cirrhosis etiology (P=0.114).
In contrast to this, the NKCC1 plasma concentrations
in the cirrhotic patients with HE (0.0011±0.0007, n=36), in the
cirrhotic patients without HE (0.0009±0.0007, n=20) and in the
healthy controls (0.0010±0.0009, n=15) were all similar (Fig. 1B).
In addition, the relative mRNA expression levels of
NKCC1/GAPDH in group A did not show any significant association
with gender (P=0.462), age (P=0.369), or liver cirrhosis etiology
(P=0.128).
Association of KCC2 and NKCC1 mRNA levels
with patient ammonia levels
As shown in Table
I, all cirrhotic patients with HE (grades I–II and III–IV)
showed higher ammonia levels than the upper limit of the range for
the cirrhotic patients without HE (83.9 μmol/l) or the
healthy individuals (57.0 μmol/l). Furthermore, the ammonia
levels of patients with severe HE were higher than those of
patients with mild HE.
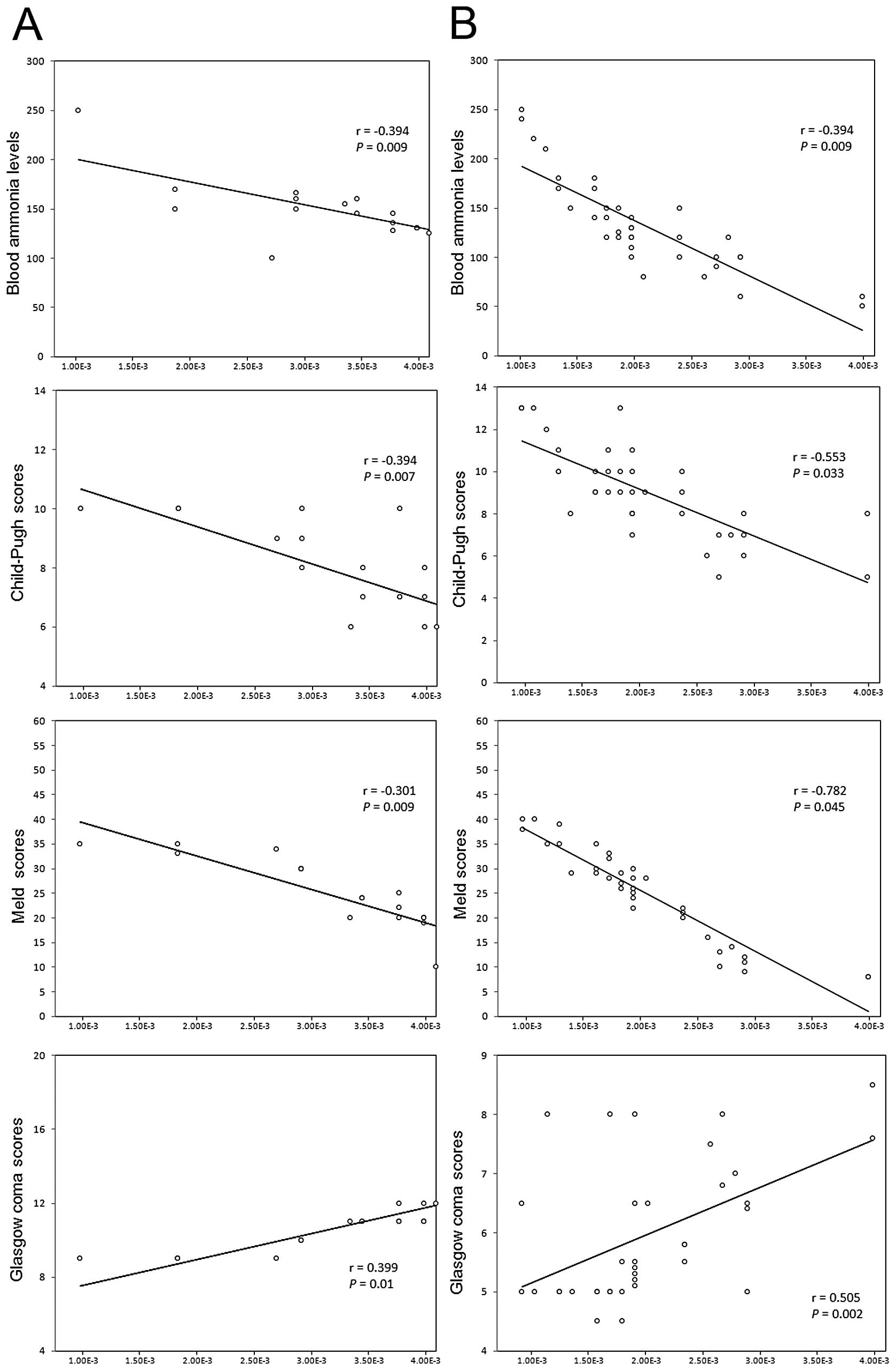
As shown in Fig. 2,
plasma KCC2 mRNA levels in all cirrhotic patients with HE showed a
significant negative correlation with the levels of blood ammonia.
Moreover, the KCC2 levels in patients with grade III–IV HE
(Fig. 2B) were found to have a
stronger correlation with ammonia levels than those in patients
with grade I–II HE (Fig. 2A).
Similarly, plasma KCC2 mRNA levels in cirrhotic patients without HE
showed a significant negative correlation with the levels of blood
ammonia (r=−0.388, P=0.002).
In contrast to this, the relative mRNA expression
levels of NKCC1/GAPDH in all cirrhotic patients with HE or in
cirrhotic patients without HE showed no significant association
with the levels of blood ammonia (P=0.252 and 0.176,
respectively).
Association of KCC2 and NKCC1 mRNA levels
with patient hepatic functions
As shown in Fig. 2,
plasma KCC2 mRNA levels in all cirrhotic patients with HE were
decreased in relation to the degree of liver insufficiency. This
was demonstrated through a significant negative correlation with
Child-Pugh scores and MELD scores. Moreover, the KCC2 levels in
patients with grade III–IV HE (Fig.
2B) were found to have a stronger correlation with Child-Pugh
and MELD scores than those in patients with grade I–II HE (Fig. 2A).
In contrast to this, the relative levels of
NKCC1/GAPDH in the patients without HE showed no significant
association with the Child-Pugh scores (P= 0.556) or the MELD
scales (P= 0.188).
Association of KCC2 and NKCC1 mRNA levels
with patient neurological status
Plasma KCC2 levels showed a significant positive
association with the Glasgow coma scores of patients who presented
with HE (Fig. 2). Patients with
grade III–IV HE (Fig. 2B) were
found to have a stronger correlation with the Glasgow coma scores
than those with grade I–II HE (Fig.
2A). In contrast to this, the relative mRNA expression levels
of NKCC1/GAPDH in all cirrhotic patients with HE showed no
significant association with Glasgow coma scores (P=0.354).
Discussion
The main findings obtained in the present study in
cirrhotic patients with HE are the decrease in the mRNA levels of
KCC2 in the blood, indicating that an impaired GABAergic inhibition
may be present in HE, in contradiction to the established
hypothesis that attributes the pathophysiology of HE to increased
GABAergic tone (12). Altered
GABAergic neurotransmission was first implicated in the
pathophysiology of HE based on the phenomenon of increased
GABAergic tone in a rabbit model of fulminant hepatic failure
(13). However, it should be noted
that the evidence gathered since then is not unequivocal. GABA
concentrations were found to be unaltered (14,15)
or increased (13). Likewise,
GABAA receptor densities have been reported to be
upregulated (16,17) in certain studies; however, the mRNA
expression of the GABA transporter, GAT-2, has been shown to be
increased in the cerebral cortex of the portacaval-shunted rat,
whereas the genes for the GABAB1D receptor and for the
β2 subunit of the GABAA receptor have been shown to be
downregulated (18). Reports
concerning GABAA-associated benzodiazepine (BZ) binding
sites are also controversial. Additionally, attention should be
paid to the fact that the majority of experimental approaches have
made use of animal models (13–18),
and only few studies were based on in vivo investigations of
HE patients (19,20). The present results are based on
comparisons of the mRNA levels of KCC2 and NKCC1 in the plasma of
cirrhotic patients with severe HE (grade III–IV) and those without
HE. This is the first report using blood samples from
encephalopathy patients, thus reflecting the true nature of HE.
Furthermore, our present results are consistent with the previous
finding that there is a significant decrease in the GABAergic tone
in the cerebral cortex of hyperammonemic rats (21). However, we should not focus on a
single neurotransmitter system, which cannot represent the
multifactorial approach required to elucidate the molecular
mechanisms involved in the disease of HE. That is, the balance and
imbalance between excitatory/inhibitory neurotransmissions may be
more important than the change of any one variable.
Although the cause may be explained by a
multifactorial theory, the accumulation of ammonia has been
considered to play an important role in the pathogenesis of HE
(1–3). The present results primarily indicate
a possible association between decreased plasma mRNA levels of KCC2
and increased levels of blood ammonia, the degree of liver
insufficiency, and the deteriorated neurological status of the
patients. The expression of NKCC1 did not change, as is consistent
with results obtained from thioacetamide-induced HE rats (22). The present results indicate that
altered chloride homeostasis was closely related to the severity of
the HE and may be a feature of neurological alterations present in
HE. The imbalance of chloride homeostasis in HE patients suggested
in this study is consistent with previous reports of changes in
Cl− transporters in an animal model of HE in vivo
(21) and a culture model of
hyperammonemia in vitro (6). Accumulating evidence suggests that
abnormal chloride homeostasis, induced by the downregulation of
KCC2, the upregulation of NKCC1, or both, is associated with
neuronal trauma or brain disorders, such as epilepsy (23), neuropathic pain (24), or amyotrophic lateral sclerosis
(25). It is known that diagnostic
methods of modern medicine allow the recognition of functional
abnormalities before the appearance of symptoms. Early recognition
of impairment may allow the avoidance or delay of a disease. For
this reason, the detection of an abnormal balance of chloride
homeostasis or of KCC2/NKCC1 expression levels may be important for
the early diagnosis of HE, and an early identification of patients
at the initial phases of HE may improve the quality of life and the
prognosis for these patients. Further studies are required to fully
elucidate the mechanism and functional significance underlying the
balance of KCC2 and NKCC1 in the manifestation of HE.
Acknowledgements
We thank Professor R.W. Guillery
(Department of Anatomy, MRC Anatomical Neuropharmacology Unit,
Oxford, UK) for his critical reading and constructive suggestions
on the manuscript. This study was supported by grants from the
National Natural Science Foundation of China (Nos. 81070327,
81071895 and 30971174).
References
|
1
|
Jones EA and Mullen KD: Theories of the
pathogenesis of hepatic encephalopathy. Clin Liver Dis. 16:7–26.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Leke R, Bak LK, Iversen P, Sørensen M,
Keiding S, Vilstrup H, Ott P, Portela LV, Schousboe A and
Waagepetersen HS: Synthesis of neurotransmitter GABA via the
neuronal tricarboxylic acid cycle is elevated in rats with liver
cirrhosis consistent with a high GABAergic tone in chronic hepatic
encephalopathy. J Neurochem. 117:824–832. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bismuth M, Funakoshi N, Cadranel JF and
Blanc P: Hepatic encephalopathy: from pathophysiology to
therapeutic management. Eur J Gastroenterol Hepatol. 23:8–22. 2011.
View Article : Google Scholar
|
|
4
|
Chamma I, Chevy Q, Poncer JC and Lévi S:
Role of the neuronal K-Cl co-transporter KCC2 in inhibitory and
excitatory neurotransmission. Front Cell Neurosci. 6:52012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Friauf E, Rust MB, Schulenborg T and Hirtz
JJ: Chloride cotransporters, chloride homeostasis, and synaptic
inhibition in the developing auditory system. Hear Res. 279:96–110.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jayakumar AR and Norenberg MD: The Na-K-Cl
co-transporter in astrocyte swelling. Metab Brain Dis. 25:31–38.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Caregaro L, Alberino F, Amodio P, Merkel
C, Angeli P, Plebani M and Gatta A: Nutritional and prognostic
significance of serum hypothyroxinemia in hospitalized patients
with liver cirrhosis. J Hepatol. 28:115–121. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Myers RP, Shaheen AA, Aspinall AI, Quinn
RR and Burak KW: Gender, renal function, and outcomes on the liver
transplant waiting list: assessment of revised MELD including
estimated glomerular filtration rate. J Hepatol. 54:462–470. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Vendittelli F, Santonocito C, Paradisi A,
Romitelli F, Concolino P, Silveri SL, Sisto T, Capizzi R, Catricalà
C, Mulè A, Di Carlo A, Zuppi C and Capoluongo E: A new standardized
absolute quantitative RT-PCR method for detection of tyrosinase
mRNAs in melanoma patients: technical and operative instructions.
Clin Chim Acta. 409:100–105. 2009. View Article : Google Scholar
|
|
10
|
Biolo G, Amoroso A, Savoldi S, Bosutti A,
Martone M, Pirulli D, Bianco F, Ulivi S, Bertok S, Artero M,
Barazzoni R, Zanetti M, Grassi G, Guarnieri G and Panzetta G:
Association of interferon-gamma +874A polymorphism with reduced
long-term inflammatory response in haemodialysis patients. Nephrol
Dial Transplant. 21:1317–1322. 2006.
|
|
11
|
Bustin SA, Benes V, Nolan T and Pfaffl MW:
Quantitative real-time RT-PCR: a perspective. J Mol Endocrinol.
34:597–601. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Aguilar MA, Miñarro J and Felipo V:
Chronic moderate hyper-ammonemia impairs active and passive
avoidance behavior and conditional discrimination learning in rats.
Exp Neurol. 161:704–713. 2000. View Article : Google Scholar
|
|
13
|
Schafer DF and Jones EA: Hepatic
encephalopathy and the gammaaminobutyric-acid neurotransmitter
system. Lancet. 1:18–20. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Roy S, Pomier-Layrargues G, Butterworth RF
and Huet PM: Hepatic encephalopathy in cirrhotic and portacaval
shunted dogs: lack of changes in brain GABA uptake, brain GABA
levels, brain glutamic acid decarboxylase activity and brain
postsynaptic GABA receptors. Hepatology. 8:845–849. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lavoie J, Giguère J-F, Pomier-Layrargues G
and Butterworth RF: Amino acid changes in autopsied brain tissue
from cirrhotic patients with hepatic encephalopathy. J Neurochem.
49:692–697. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schafer DF, Fowler JM, Munson PJ, Thakur
AK, Waggoner JG and Jones EA: Gamma-aminobutyric acid and
benzodiazepine receptors in an animal model of fulminant hepatic
failure. J Lab Clin Med. 102:870–880. 1983.PubMed/NCBI
|
|
17
|
Dodd PR, Thomas GJ, Harper CG and Kril JJ:
Amino acid neurotransmitter receptor changes in cerebral cortex in
alcoholism: effect of cirrhosis of the liver. J Neurochem.
59:1506–1515. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Song G, Dhodda VK, Blei AT, Dempsey RJ and
Rao VL: GeneChip analysis shows altered mRNA expression of
transcripts of neurotransmitter and signal transduction pathways in
the cerebral cortex of portacaval shunted rats. J Neurosci Res.
68:1072–1086. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Boy C, Meyer PT, Kircheis G, Holschbach
MH, Herzog H, Elmenhorst D, Kaiser HJ, Coenen HH, Haussinger D,
Zilles K and Bauer A: Cerebral A1 adenosine receptors (A1AR) in
liver cirrhosis. Eur J Nucl Med Mol Imaging. 35:589–597. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Iversen P, Hansen DA, Bender D, Rodell A,
Munk OL, Cumming P and Keiding S: Peripheral benzodiazepine
receptors in the brain of cirrhosis patients with manifest hepatic
encephalopathy. Eur J Nucl Med Mol Imaging. 33:810–816. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cauli O, Mansouri MT, Agusti A and Felipo
V: Hyperammonemia increases GABAergic tone in the cerebellum but
decreases it in the rat cortex. Gastroenterology. 136:1359–1367.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Obara-Michlewska M, Pannicke T, Karl A,
Bringmann A, Reichenbach A, Szeliga M, Hilgier W, Wrzosek A,
Szewczyk A and Albrecht J: Down-regulation of Kir 4.1 in the
cerebral cortex of rats with liver failure and in cultured
astrocytes treated with glutamine: implications for astrocytic
dysfunction in hepatic encephalopathy. J Neurosci Res.
89:2018–2027. 2011. View Article : Google Scholar
|
|
23
|
Conti L, Palma E, Roseti C, Lauro C,
Cipriani R, de Groot M, Aronica E and Limatola C: Anomalous levels
of Cl- transporters cause a decrease of GABAergic inhibition in
human peritumoral epileptic cortex. Epilepsia. 52:1635–1644. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hasbargen T, Ahmed MM, Miranpuri G, Li L,
Kahle KT, Resnick D and Sun D: Role of NKCC1 and KCC2 in the
development of chronic neuropathic pain following spinal cord
injury. Ann N Y Acad Sci. 1198:168–172. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fuchs A, Ringer C, Bilkei-Gorzo A, Weihe
E, Roeper J and Schütz B: Downregulation of the potassium chloride
cotransporter KCC2 in vulnerable motoneurons in the SOD1-G93A mouse
model of amyotrophic lateral sclerosis. Metab Brain Dis. 25:31–38.
2010.PubMed/NCBI
|