Introduction
Polo-like kinase 1 (PLK1) is a highly conserved
serine/threonine kinase that plays an important role in mitosis
(1). PLK1 is highly expressed in
numerous tumor tissues or cells and is associated with clinical
stage, metastasis and invasiveness, as well as with the prognosis
of patients with tumors. PLK1 promotes tumor cell proliferation and
cell transformation, thus, it is a new target for antitumor therapy
(2). Thyroid carcinoma is a common
malignancy of the endocrine system. There is no curative regimen
for undifferentiated thyroid carcinoma, which is highly malignant
and progresses rapidly. The PLK1 gene is known to play an important
regulatory role in the cell proliferation of undifferentiated
thyroid carcinoma. PLK1 RNA interference (RNAi) suppresses cell
proliferation and induces apoptosis (3). However, the association between PLK1
and thyroid carcinoma cell invasiveness has yet to be adequately
investigated. In the present study, we first examined the
relationship among PLK1 expression and clinical stage and lymphatic
metastasis in undifferentiated thyroid carcinoma tissue. The effect
of PLK1 RNAi on the invasiveness of undifferentiated thyroid
carcinoma ARO cells was then investigated. Additionally, we
explored the possible mechanism behind CD44v6/matrix
metalloproteinase (MMP)-2/MMP-9.
Materials and methods
Specimens
A total of 36 undifferentiated thyroid carcinoma
specimens were obtained from 36 patients who underwent
thyroidectomy between 2001 and 2007 at The Military General
Hospital of Beijing PLA (Beijing, China). There were 26 males and
10 females, aged 21–72 (47.2±11.3) years. According to the clinical
stage criteria set by the American Joint Commission for Cancer
(AJCC), there were 10 cases at stage I, 11 cases at stage II, 8
cases at stage III and 7 cases at stage IV. Twenty-one cases were
identified with lymphatic metastasis and 15 cases were identified
without lymphatic metastasis. Of the patients included in this
study, 20 patients had succumbed during the follow-up period of 5
years.
Cell strain and antibodies
The undifferentiated human thyroid carcinoma cell
line ARO was maintained in our laboratory. DMEM containing 10%
fetal bovine serum was purchased from Gibco-BRL (Carlsbad, CA,
USA). Goat anti-human PLK1, CD44v6, MMP-2 and MMP-9 antibodies were
purchased from Santa Cruz Biotechnology Inc., (Santa Cruz, CA,
USA). Horseradish peroxidase (HRP) conjugated rabbit anti-goat
antibody was purchased from Boster Biotechnology Ltd. (Wuhan,
China).
Construction of PLK1 siRNA sequence and
transfection of ARO cells
The PLK1 cDNA sequence was retrieved from GenBank,
and three siRNA sequences (S1, S2, S3) (3) as well as a negative control sequence
(Sn) were designed and synthesized (Table I). All sequences were synthesized
by Takara (Dalian, China) and were verified by sequencing. The
sequences (100 nM) were transfected into ARO cells using
Oligofectamine™ (Invitrogen, Carlsbad, CA, USA). ARO cells were
adjusted to 1×105 cells/ml. The cells were divided into
six groups: the blank group (ConB), void vector group (ConA), S1
transfection group (S1), S2 transfection group (S2), S3
transfection group (S3) and the Sn transfection group (Sn). The
blank group was transfected with PBS and the void vector group with
void vectors of the same concentration. The other treatments were
the same among all groups. siRNA with the highest interference
efficiency was used in the subsequent assay.
 | Table IPLK1 siRNA sequences. |
Table I
PLK1 siRNA sequences.
| siRNA | Sequences |
|---|
| S1 | Sense,
5′-GAUUGUGCCUAAGUCUCUGTT-3′ |
| Antisense,
5′-CAGAGACUUAGGCACAAUCTT-3′ |
| S2 | Sense,
5′-UGAAGAUCUGGAGGUGAAATT-3′ |
| Antisense,
5′-CACCUCGAAACUGUGCUCUTT-3′ |
| S3 | Sense,
5′-UUCUCCGAACGUGUCACGUTT-3′ |
| Antisense,
5′-CACCUCGAAACUGUGCUCUTT-3′ |
| Sn | Sense,
5′-UUCUCCGAACGUGUCACGUTT-3′ |
| Antisense,
5′-ACGUGACACGUUCGGGAATTT-3′ |
Immunohistochemical detection of PLK1
protein expression
The surgical specimens were fixed with 10% formalin
and embedded with paraffin. The sections were stained with an LSAB™
kit (Dako, Glostrup, Denmark). In brief, the sections were dewaxed
conventionally to water, immersed in 200 ml citrate buffer, heated
for 15 min and then transferred into a humid box. The sections were
blocked for 10 min with 10% goat serum diluted with PBS at room
temperature, incubated overnight with primary antibody (1:5,000) at
4°C and incubated for 30 min with biotin-conjugated secondary
antibody (1:10,000) at 37°C in the humid box. This was followed by
a 5 min incubation for color development, hematoxylin staining and
mounting. Positive cells had brown- or yellow-stained nuclei or
cytoplasm. Imag-Pro-Plus image analysis software was used to
determine the area (%) of positive cells relative to the reference
system.
Western blot analysis of protein
expression
Total cell protein was extracted by cell lysis with
RIPA lysis buffer and 5-min centrifugation at 4°C and 10,000 rpm
(centrifugation radius, 4 cm). The supernatants were harvested and
protein concentration was determined using the BCA method. Protein
(50 μg) was added with 2X loading buffer. After a 5-min
denaturation at 100°C, proteins were subjected to SDS-PAGE and then
transferred onto a nitrocellulose filter. The filter was incubated
with specific primary and secondary antibodies, followed by
enhanced chemiluminescence (ECL; Boster Biotechnology, Ltd.) and
autoradiography. The resultant autoradiograms were subjected to
grayscale analysis with BandScan software.
Colony formation assay
Cells at the logarithmic phase were digested
conventionally and suspended (1×103 cells/ml). Agarose
(5%) and culture medium were mixed at 1:9 and placed into culture
dishes at room temperature. The cell suspension (1.5 ml) was added
to 1.5 ml of 0.5% agarose, agitated and then added to the
preprepared agarose dishes. Colony formation was observed after a
2-week culture at 37°C in air containing 5% CO2. The
colony formation rate (%) was calculated using the equation:
(number of colonies/number of cells inoculated) ×100%.
In vitro invasion assay
The cell suspension was adjusted to 1×105
cells/ml. The cell suspension (50 μl) was added to the upper
chamber of Transwell (Chemicon, Temecula, CA, USA) and cultured for
24 h. The cells adhering to the interior of chamber were collected
and fixed with 10% formalin, followed by Giemsa staining. Cells
that had penetrated the membrane were counted.
Statistical analysis
Data were expressed as the mean ± SD, and the
two-sided Student’s t-test was performed using SPSS 16.0 software.
P<0.05 was considered to indicate a statistically significant
result.
Results
Correlation between PLK1 expression and
clinical stage, metastasis and prognosis of thyroid carcinoma
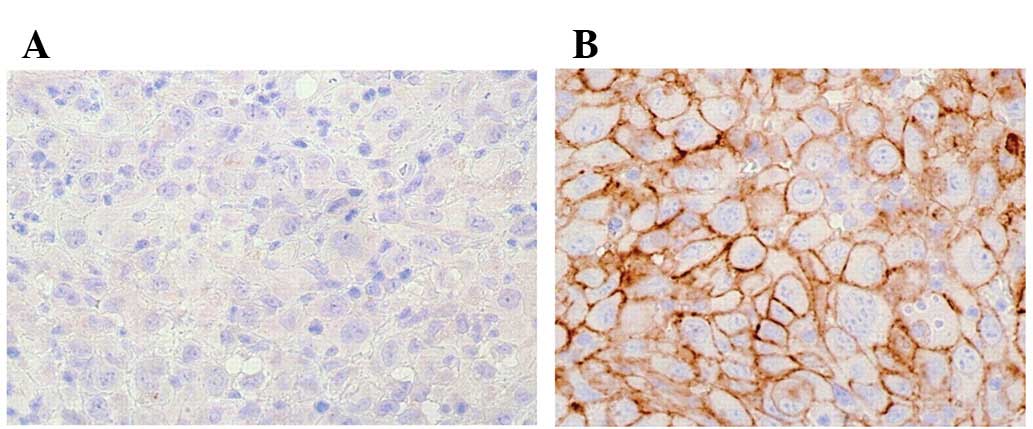
Immunohistochemical investigation demonstrated that
PLK1 protein was expressed weakly in cancer-adjacent tissues
(0.65±0.12%). Moreover, the protein exhibited a significantly
stronger expression in undifferentiated thyroid carcinoma samples
(67.5±10.6%). Immunoreactive sites were mainly located in the
cytoplasm and nuclei of follicular epithelia (Fig. 1). In addition, PLK1 expression
correlated with clinical stage, lymphatic metastasis and prognosis
of undifferentiated thyroid carcinoma (Table II).
 | Table IIPLK1 expression correlated with the
clinical stage, lymphatic metastasis and prognosis of
undifferentiated thyroid carcinoma. |
Table II
PLK1 expression correlated with the
clinical stage, lymphatic metastasis and prognosis of
undifferentiated thyroid carcinoma.
| Clinical
pathology | Case no. | PLK1 expression (mean
± SD%) | t | P-value |
|---|
| Gender | | | | |
| M | 26 | 63.2±11.3 | 0.20 | 0.83 |
| F | 10 | 64.1±12.4 | | |
| Age (years) | | | | |
| <40 | 15 | 59.3±8.9 | 1.63 | 0.11 |
| ≥40 | 21 | 65.2±11.8 | | |
| Lymphatic
metastasis | | | | |
| Yes | 15 | 46.5±7.8 | 6.91 | 0.00 |
| No | 21 | 71.3±13.6 | | |
| Clinical stage | | | | |
| I, II | 21 | 48.9±8.7 | 6.06 | 0.00 |
| III, IV | 15 | 69.2±11.4 | | |
| Prognosis | | | | |
| Survived | 16 | 36.5±4.9 | 9.52 | 0.00 |
| Mortalities | 20 | 71.3±15.4 | | |
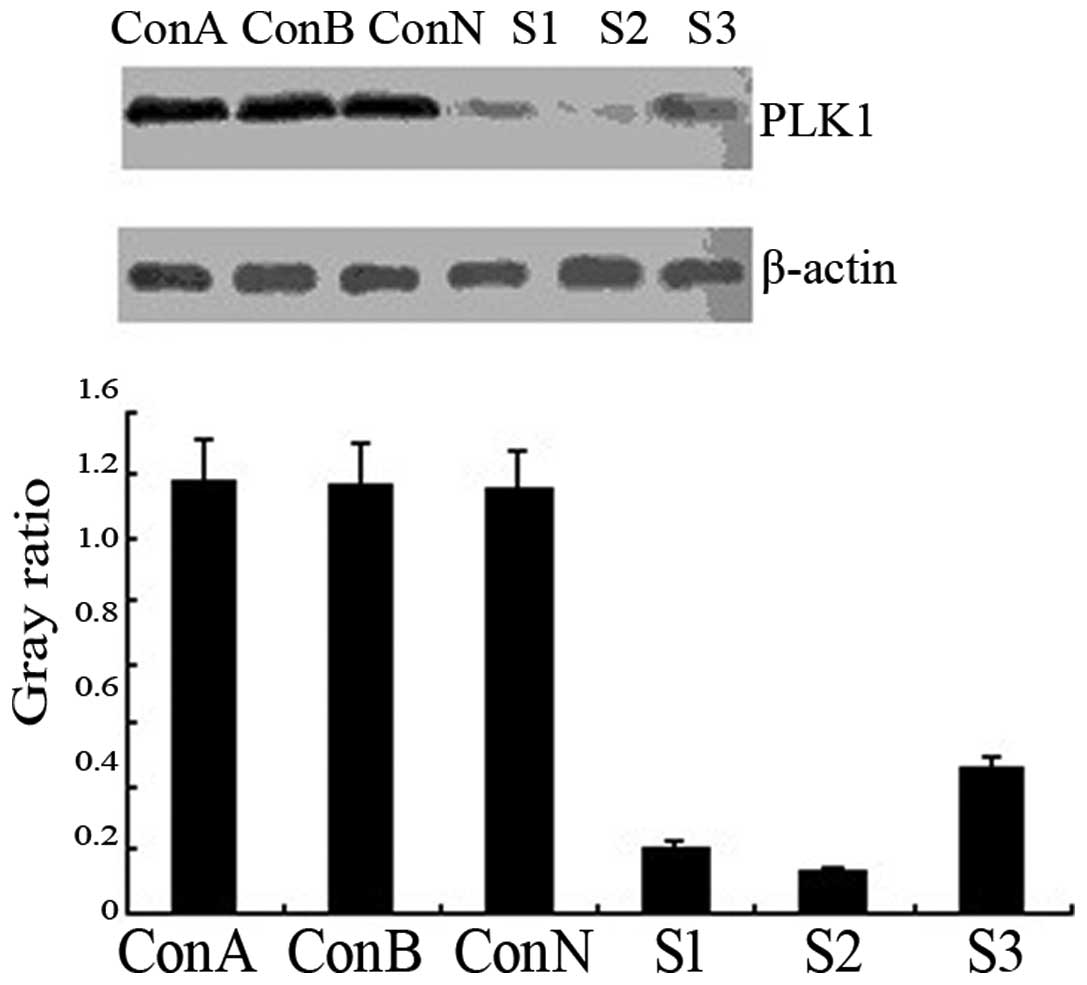
siRNA effect on PLK1 protein
expression
Western blot analysis revealed that the levels of
PLK1 protein expression were similarly high in the blank, negative
control and void vector groups (P>0.05). Following siRNA
transfection, PLK1 protein expression was significantly
downregulated in the transfection groups (P<0.01), particularly
in the S2 group (90.6% reduction vs. the control group; Fig. 2). The results demonstrated that the
siRNA transfection of ARO cells silenced the PLK1 gene specifically
and suppressed PLK1 protein expression.
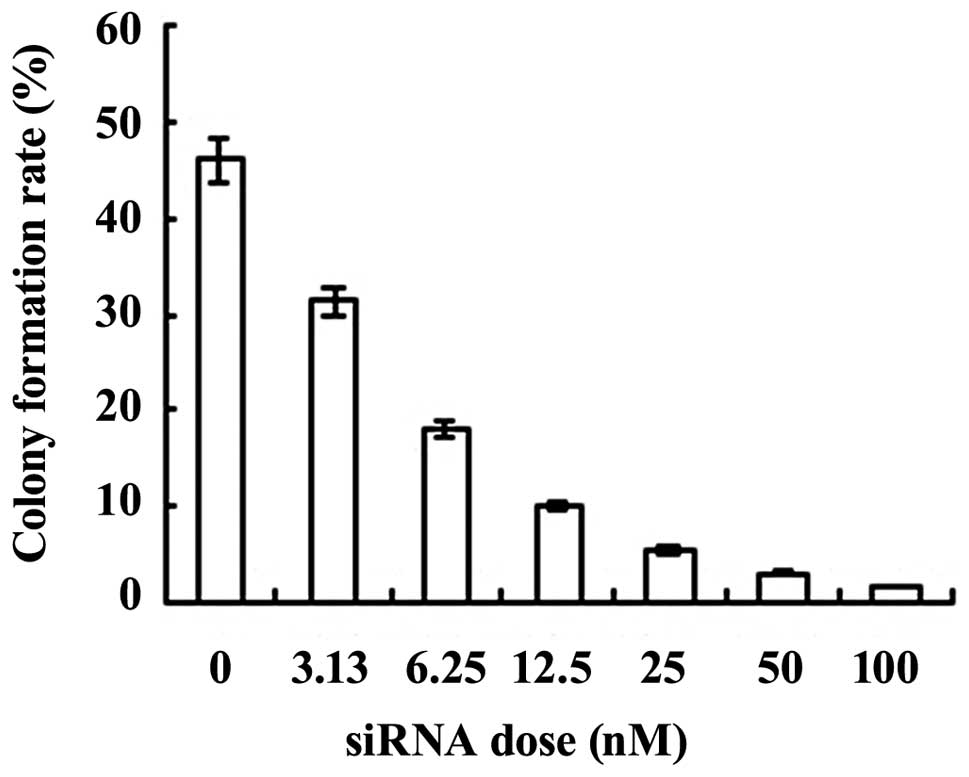
PLK1 siRNA effect on ARO cell
anchorage-dependent growth
S2 exhibited the highest PLK1 interference
efficiency. Thus, S2 was used for specific PLK1 interference. The
colony formation assay showed that ARO cells formed colonies
spontaneously in an in vitro culture system. The colony
formation rate decreased with increasing concentrations of S2 siRNA
(0, 3.125, 6.25, 12.5, 25, 50 and 100 nM; Fig. 3).
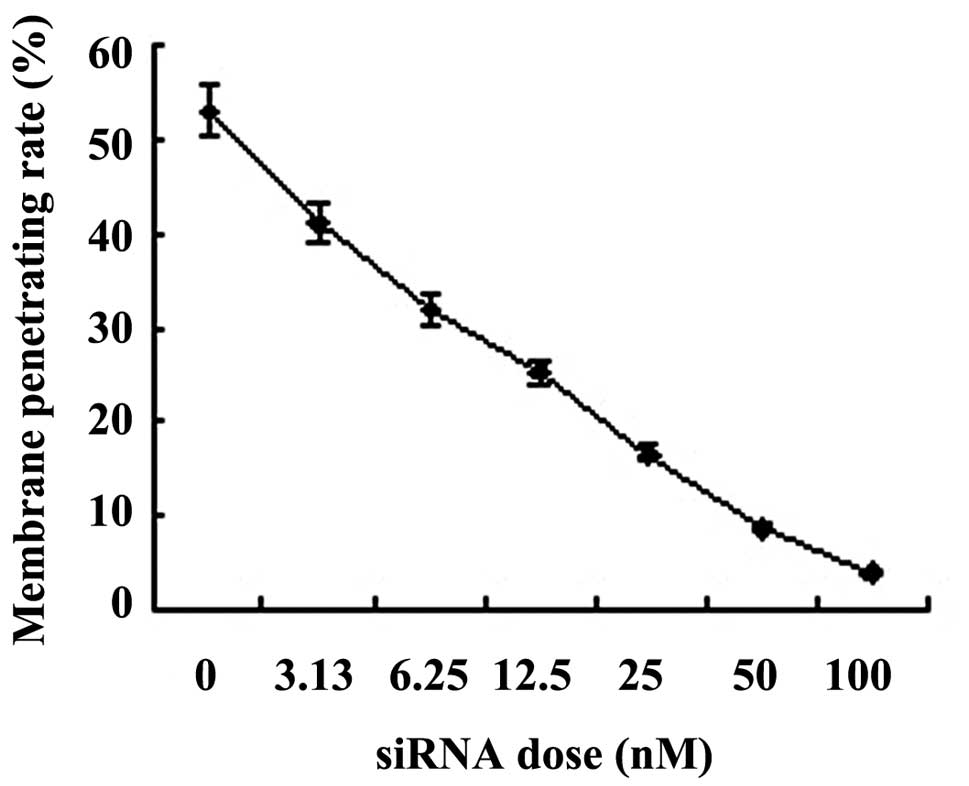
Effect of PLK1 interference on ARO cell
invasion
The results showed that PLK1 interference decreased
membrane- penetrating cells significantly in a siRNA concentration-
dependent manner (P<0.01; Fig.
4).
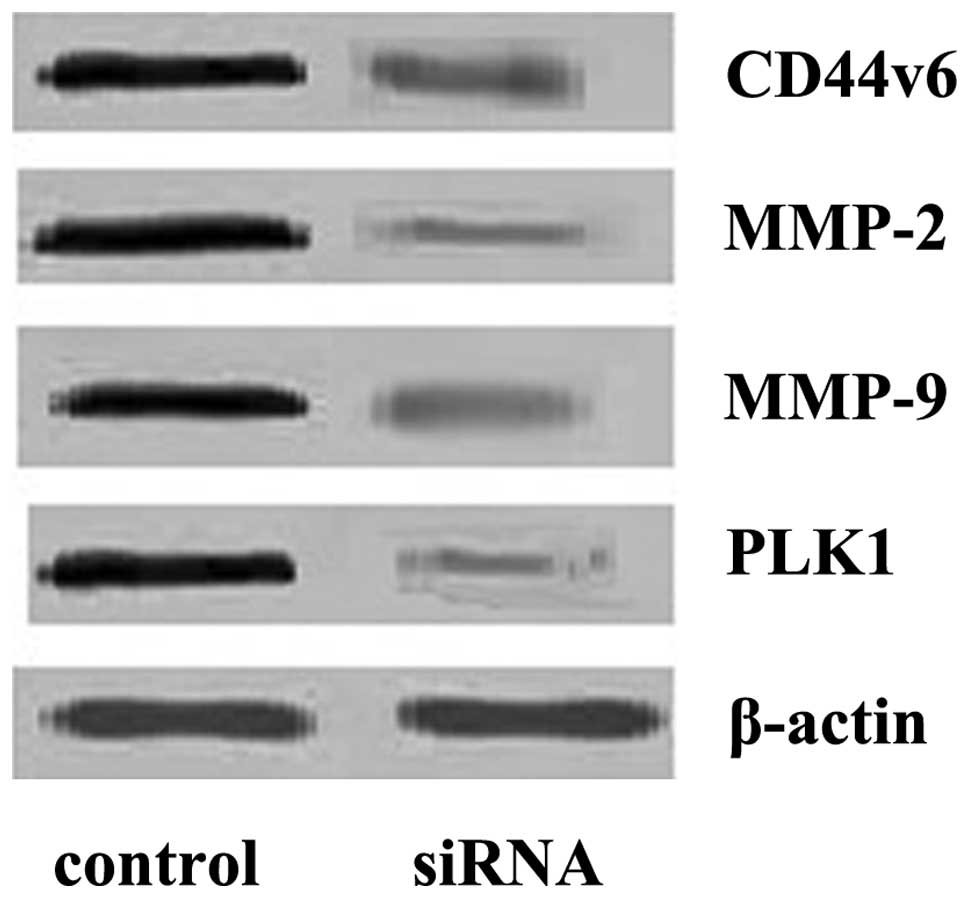
Effect of PLK1 interference on CD44v6,
MMP-2 and MMP-9 proteins
The results showed that PLK1 interference decreased
the level of CD44v6 protein significantly (0.36±0.08) when compared
to the control group (1.15±0.18; P<0.01). MMP-2 and MMP-9
expression in the interference group (0.12±0.03, 0.25±0.06,
respectively) decreased significantly when compared to the control
group (1.21±0.20, 1.25±0.21; P<0.01; Fig. 5).
Discussion
It has been demonstrated that the PLK1 gene plays an
important role in the incidence and development of tumors. PLK1
expression is upregulated in a number of tumors including
esophageal carcinoma (4,5), multiple myeloma (6), gynecological malignant tumors
(7), skin cancer (8), liver cancer (9), gastric carcinoma (10) and cervical cancer (11). PLK1 expression status correlates
with clinical stage, lymphatic metastasis and prognosis. The
present study demonstrated that PLK1 protein expression is
upregulated significantly in cancer tissues when compared with
cancer-adjacent tissues, and that PLK1 expression correlated with
clinical stage and lymphatic metastasis of thyroid carcinoma, as
well as with the prognosis of patients. The higher the PLK1
expression levels, the higher the mortality rate of patients. This
may be associated with the suppression of apoptosis and promotion
of surviving activity by PLK1 (4).
Salvatore et al (12)
demonstrated that the PLK1 gene was highly expressed in
undifferentiated thyroid carcinoma cells and therefore suppressed
P53 and pRB genes. PLK1 RNAi led to cell cycle arrest. Therefore,
PLK1 may become a molecular target for the treatment of
undifferentiated thyroid carcinoma.
Since PLK1 is associated with the incidence,
development and prognosis of tumors, the role of PLK1 activity
suppression in tumor therapy and prognosis has become the focus of
research. In recent years, RNAi technology has been widely used to
silence PLK1 expression and test the role of PLK1 in the incidence,
development and treatment of tumors. It was observed that the
suppression of PLK1 activity decreased the survival rate of
undifferentiated thyroid carcinoma cells significantly (13). Therefore, PLK1 gene therapy may be
a promising treatment for undifferentiated thyroid carcinoma
(14). The present study has shown
that siRNA suppressed PLK1 efficiently.
To investigate the role of PLK1 in the invasion and
metastasis of undifferentiated thyroid carcinoma cells, cell
anchorage dependence and invasiveness were assessed in the present
study via the specific RNAi of PLK1, colony formation assay and
Transwell based in vitro invasion assay. Cell anchorage
dependence refers to the fact that cells may avoid apoptosis and
survive only by adhering to a specific matrix. By contrast, tumor
cells are able to survive without adhering to a specific matrix,
which is known as cell anchorage dependence. A colony formation
assay may be used to assess tumor cell anchorage dependence and
tumor malignancy (15). The
invasiveness of cancer cells correlates positively with the number
of colonies formed. The present study indicates that PLK1 siRNA
suppressed ARO cell colony formation in soft agarose medium in a
dose-dependent manner. This finding suggests that PLK1 interference
suppresses the invasiveness of ARO cells. The metastasis and
invasiveness of tumor cells are associated with the
microenvironment and extracellular matrix (ECM) in which tumor
cells grow. The Transwell model, which simulates the ECM is a
reliable tool for assessing cell invasiveness (16). The present study has demonstrated
that siRNA interference of PLK1 decreased membrane-penetrating
cells significantly in a concentration-dependent manner.
Tumor invasion and metastasis is closely associated
with the ability of tumor cells to induce the production of
proteinases that degrade the ECM and basement membrane (17). A number of molecules are involved
in regulating tumor cell invasion and metastasis. For instance,
CD44v6 is an important member of the cell adhesion molecule CD44
family. CD44v6 regulates the ECM, enhances cell motility and
suppresses tumor apoptosis. Therefore, it is crucial in tumor
invasion and metastasis (18). The
present study has shown that PLK1 interference suppressed ARO cell
invasion and decreased CD44v6 activity, which supports the
involvement of CD44v6 in tumor invasion and metastasis. MMPs are
also associated with tumor invasion. MMP-2 (19,20)
and MMP-9 (19,21–22)
expression was upregulated in thyroid carcinoma and associated with
lymphatic metastasis. Findings of the present study have shown that
PLK1 suppression may inhibit MMP-2 and MMP-9 activity, thus
suppressing cell invasion.
In conclusion, PLK1 expression was upregulated in
undifferentiated thyroid carcinoma and correlated with clinical
stage, lymphatic metastasis and tumor prognosis. PLK1 siRNA
suppressed the cell invasion of undifferentiated thyroid carcinoma,
and CD44v6, MMP-2 and MMP-9 contributed to the regulation of cell
invasion of undifferentiated thyroid carcinoma via PLK1.
Acknowledgements
This study was financially supported
by the Scientific Research Foundation of PLA (no. 2010168).
References
|
1
|
van de Weerdt BC and Medema RH: Polo-like
kinases: a team in control of the division. Cell Cycle. 5:853–864.
2006.PubMed/NCBI
|
|
2
|
Degenhardt Y and Lampkin T: Targeting
Polo-like kinase in cancer therapy. Clin Cancer Res. 16:384–389.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu YZ, Yu L, Gong DD, et al: Construction
of PLK1 siRNA and its effects on proliferation and apoptosis of
undifferentiated human thyroid cancer cells. Chin J Endocr Surg.
5:76–79. 2011.(In Chinese).
|
|
4
|
Feng YB, Lin DC, Shi ZZ, et al:
Overexpression of PLK1 is associated with poor survival by
inhibiting apoptosis via enhancement of survivin level in
esophageal squamous cell carcinoma. Int J Cancer. 124:578–588.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhao CL, Gong L, Li WT and Chen L:
Overexpression of Plk1 promotes malignant progress in human
esophageal squamous cell carcinoma. J Cancer Res Clin Oncol.
136:9–16. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Evans RP, Dueck G, Sidhu R, et al:
Expression, adverse prognostic significance and therapeutic small
molecule inhibition of Polo-like kinase 1 in multiple myeloma. Leuk
Res. 35:1637–1643. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Noriyuki T and Hisashi N: Polo-like
kinases (Plks) are prognostic markers for gynecologic malignancies.
Cur Womens Health Rev. 4:266–269. 2008. View Article : Google Scholar
|
|
8
|
Schmit TL, Zhong WX, Nihal M and Ahmad N:
Polo-like kinase 1 (Plk1) in non-melanoma skin cancers. Cell Cycle.
8:2697–2702. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
He ZL, Zheng H, Lin H, et al:
Overexpression of polo-like kinase1 predicts a poor prognosis in
hepatocellular carcinoma patients. World J Gastroenterol.
15:4177–4182. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lan B, Liu BY, Chen XH, et al: Polo like
kinase 1 expression and prognostic value in gastric carcinomas.
Zhonghua Wei Chang Wai Ke Za Zhi. 10:70–72. 2007.(In Chinese).
|
|
11
|
Zhang Y, Liu Y, Yang YX, et al: The
expression of PLK-1 in cervical carcinoma: a possible target for
enhancing chemosensitivity. J Exp Clin Cancer Res. 28:130–139.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Salvatore G, Nappi TC, Salerno P, et al: A
cell proliferation and chromosomal instability signature in
anaplastic thyroid carcinoma. Cancer Res. 67:10148–10158. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nappi TC, Salerno P, Zitzelsberger H, et
al: Identification of Polo-like kinase 1 as a potential therapeutic
target in anaplastic thyroid carcinoma. Cancer Res. 69:1916–1923.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kojic SL, Strugnell SS and Wiseman SM:
Anaplastic thyroid cancer: a comprehensive review of novel therapy.
Expert Rev Anticancer Ther. 11:387–402. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Thullberg M and Strömblad S:
Anchorage-independent cytokinesis as part of oncogenic
transformation? Cell Cycle. 7:984–988. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Marshall J: Transwell(®) invasion assays.
Methods Mol Biol. 769:97–110. 2011.
|
|
17
|
Duffy MJ, Mc Gowan PM and Gallagher WM:
Cancer invasion and metastasis: changing views. J Pathol.
214:283–293. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jung T, Gross W and Zöller M: CD44v6
coordinates tumor matrix-triggered motility and apoptosis
resistance. J Biol Chem. 286:15862–15874. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cho Mar K, Eimoto T, Tateyama H, et al:
Expression of matrix metalloproteinases in benign and malignant
follicular thyroid lesions. Histopathology. 48:286–294.
2006.PubMed/NCBI
|
|
20
|
Tan H, Ye K, Wang Z and Tang H:
Clinicopathologic evaluation of immunohistochemical CD147 and MMP-2
expression in differentiated thyroid carcinoma. Jpn J Clin Oncol.
38:528–533. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Buergy D, Weber T, Maurer GD, et al:
Urokinase receptor, MMP-1 and MMP-9 are markers to differentiate
prognosis, adenoma and carcinoma in thyroid malignancies. Int J
Cancer. 125:894–901. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rothhut B, Ghoneim C, Antonicelli F and
Soula-Rothhut M: Epidermal growth factor stimulates matrix
metalloproteinase-9 expression and invasion in human follicular
thyroid carcinoma cells through focal adhesion kinase. Biochimie.
89:613–624. 2007. View Article : Google Scholar
|