Introduction
Hepatocellular carcinoma (HCC) is one of the most
common malignant tumors worldwide with an extremely high incidence
and poor survival rate (1,2). The management of patients at risk for
developing HCC remains an intricate process. Despite the large
number of studies devoted to the immunohistochemistry of HCC, at
the present time, the definitive positive and negative markers for
HCC are lacking.
Several key molecules in signaling pathways involved
in cancer development have emerged. TGFβ plays an important role in
the regulation of cell growth and differentiation, angiogenesis,
extracellular matrix formation, immunosuppression and cancer
development (3). It is well known
that signaling by the TGFβ family is most prominent at the
interface between normal tissue development and cancer. The TGFβ
signaling pathway is activated upon ligands binding to type I and
II trans-membrane receptors. The Smad4 protein is the downstream
mediator of TGFβ. Phosphorylation of Smad, by activation of TGFβ
receptors, results in activation of a TGFβ-targeted gene. ELF
associates with SMAD3, presenting it to the cytoplasmic domain of
the TGFβ receptor complex; this has been found to play a pivotal
role in TGFβ signaling (4).
Dysfunction of TGFβ pathway members, including TGFβR2, SMAD3, SMAD4
and ELF, may lead to progenitor/stem cell deregulation and possibly
cancer formation. Previous studies have suggested that Smad3 and
its phosphorylation relatives may be used as biomarkers to identify
patients with a high risk of recurrence (5,6).
Smad3 is exported via XPO4. XPO4 is therefore in control of Smad3
signaling as well as protein synthesis (7,8). A
recent study indicated that XPO4 may be involved in the progression
of human HCC and may serve as a potential target for gene therapy
in its treatment (9). We
consequently selected XPO4 as an indicator in the current
study.
HCC recurrence commonly occurs with an extremely
poor prognosis. Vascular invasion in HCC is one of the key factors
that results in cancer recurrence. To invade, HCC cells must
penetrate the vessel wall, which consists of endothelial cells and
extracellular matrix components, including fibronectin and
fibrinogen. TGFβ specifically phosphorylates integrinβ1 via Smad-2
and Smad-3, causing a conformational change of the extracellular
component with an inside-out mechanism (10). Additionally, a previous study in
breast cancer revealed that the induction of ANGPTL4 by TGFβ via
Smad disrupts vascular endothelial cell-cell junctions, increases
the permeability of lung capillaries and facilitates the
trans-endothelial passage of tumor cells (7,11–14).
Therefore, we employed multiple methods to assess
TGFβ, XPO4, elF5A2 and ANGPTL4 in cancerous and paracancerous liver
tissue samples obtained from 280 patients suffering from liver
cancer. We aimed to determine whether these four indicators may
become biomarkers to evaluate HCC and provide an improved
prognosis.
Patients and methods
Patients and samples
Samples were obtained under informed consent from
280 patients with HCC who underwent surgery to remove liver cancer
between 2005 and 2011 in our hospital (Fudan University, Shanghai,
China). All cases met the criteria set by the University of
California, San Francisco (UCSF) (15). The follow-up of cases was at a mean
of 42 months (range, 3–84 months). In patients with multi-nodular
tumors, tumor samples were obtained from the largest tumor. Ethical
approval was obtained from the Hua Shan Hospital Research Ethics
Committee.
Tissue microarray (TMA)
arrangement
TMA blocks were constructed as described previously
(16). Briefly, all HCC tissues
were reviewed by two histopathologists. Representative tumor areas
free from necrotic and hemorrhagic materials were premarked in the
paraffin blocks. Two cores, 1.5 or 2.0 mm in diameter, were taken
from each representative tumor tissue, and from liver tissue
adjacent to the tumor, and transferred from the recipient paraffin
block at defined array positions. Six TMA blocks were constructed.
Consecutive sections of 4-μm thickness were taken on
3-aminopropyltriethoxysilane-coated slides (Shanghai Outdo Biotech
Co., Ltd., Shanghai, China).
qPCR
RNA isolation and qRT-PCR was used. Total RNA was
extracted from frozen tumor specimens and matched liver tissue
adjacent to the tumor from 16 HCC patients using TRIzol reagent
(Invitrogen Life Technologies, Carlsbad, CA, USA) according to the
manufacturer’s instructions. XPO4, TGFβ1, ANGPTL4 and elF5A2 mRNA
expression in tissues from these patients was measured by qRT-PCR
using an IQ5 instrument (Bio-Rad, Hercules, CA, USA). qRT-PCR was
performed using a SYBR PrimeScript RT-PCR kit (Takara Bio, Inc.,
Shiga, Japan) according to the manufacturer’s instructions. GAPDH
was used as an internal control. The primers were as follows: XPO4
(Genbank NM_022459.4) forward primer 5′-TTGTTCTTGGTGTTTTGTGTTTCC-3′
and reverse primer 5′-CATTCCTTTCCCACTCCTCTT TAG-3′; TGFβ1 (Genbank
NM_000660.3) forward primer 5′-GCAACAATTCCTGGCGATACCT-3′ and
reverse primer 5′-CAGTGTGTTATCCCTGCTGTCACA-3′; ANGPTL4 (Genbank
NM_001039667.1) forward primer 5′-GACCAA GGGGCATGGAGCTT-3′ and
reverse primer 5′-CAGGGG ACC TACACACA ACAG CA-3′; el F5A 2 (G enba
n k NM_020390.5) forward primer 5′-TTGTTCTCAGGG CTATTTGTGCTAA-3′
and reverse primer 5′-GGATGCTAC TGTTTCCATTTTTTTC-3′; and GAPDH
(Genbank NM_002046.3) forward primer 5′-TCCCTCAACATTGTC AGCAA-3′
and reverse primer 5′-AGCTCCACAACGGAT ACATT-3′. Relative mRNA
levels were calculated based on the Ct values and corrected for
GAPDH expression, according to the equation: 2−ΔCt [ΔCt
= Ct (target gene) - Ct (GAPDH)]. All experiments were performed in
triplicate.
Immunostaining
The primary antibodies used for immunohistochemistry
were XPO4 (polyclonal rabbit, diluted 1:100; PAB0297, Abnova,
Taipei, Taiwan), TGFβ1 (monoclonal mouse, diluted 1:3,000; MAB2505,
Abnova), ANGPTL4 (monoclonal mouse, diluted 1:200; L191591e, Enzo,
NY, USA) and elF5A2 (polyclonal rabbit, diluted 1:30; E9781, Sigma,
St. Louis, MO, USA). Immunohistochemistry was carried out using a
two-step method as described previously (16). Following heat-induced antigen
retrieval, tissues were incubated with primary antibodies for 30
min at room temperature. Following a 30-min incubation with a
matched secondary antibody, sections were developed in
3,3′-diaminobenzidine solution (Sigma) under microscopic
observation and counterstained with hematoxylin (Sigma). Negative
control slides in which the primary antibodies had been omitted
were included in all assays.
Statistical analysis
Analysis was performed with SPSS 17.0 for Windows
(SPSS Inc., Chicago, IL, USA); the values are expressed as the mean
± standard error. A two-tailed P-value of <0.05 was considered
to indicate a statistically significant difference. The
differential expression of proteins between carcinoma tissue and
adjacent tissue was determined by a t-test. In statistics,
correlation refers to any of a broad class of statistical
relationships involving dependence. The most common method is the
Pearson correlation coefficient (CC), which is sensitive only to a
linear relationship between two variables. Two variables are more
correlative if the CC value is closer to 1. Relationships between
clinicopathological and molecular parameters were statistically
analyzed using Pearson or Spearman’s rank correlation coefficients.
P<0.05 indicates that the two groups are correlative.
Kaplan-Meier analysis was used to determine survival. The log-rank
test was then used to compare patient survival between
subgroups.
Results
Distribution of indicators in liver
tissues
The four indicators, XPO4, TGFβ1, ANGPTL4 and
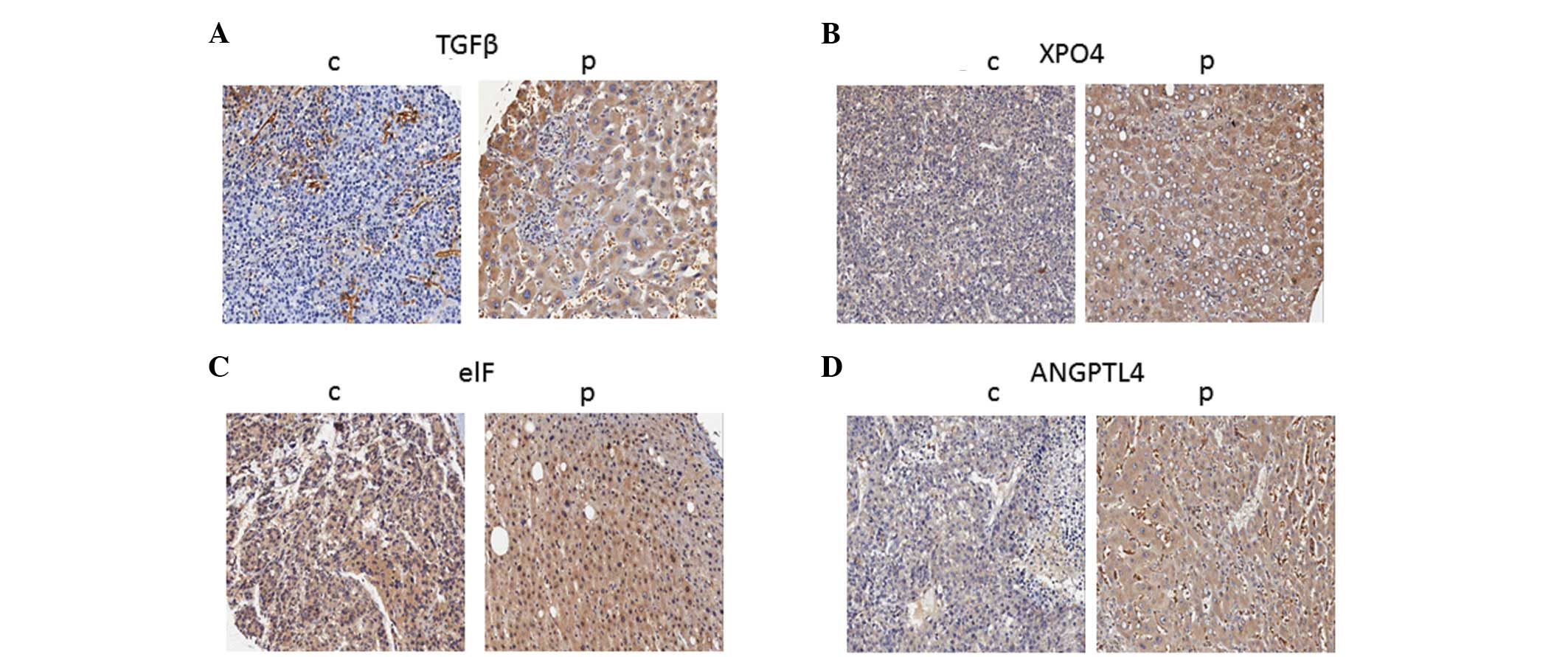
elF5A2, were found to be located within the cellular cytoplasm by
the immunostaining method (Fig.
1). The data shown in Table I
are based on the immunohistochemical staining levels. The values in
the table were obtained from the percentage of positive staining in
the same samples among TMAs. With regard to tissue distribution of
the 4 indicators, they were all expressed in cancerous and
paracancerous liver tissues. They were all found at a significantly
higher density in the paracancerous tissues than in the cancerous
liver tissues (Table I).
 | Table IExpression of XPO4, TGFβ1, ANGPTL4 and
elF5A2. |
Table I
Expression of XPO4, TGFβ1, ANGPTL4 and
elF5A2.
| Indicator | Carcinoma tissue | Adjacent tissue | P-valuea |
|---|
| XPO4 | 0.800±0.194 | 0.855±0.113 | 0.000 |
| TGFβ1 | 0.256±0.284 | 0.502±0.312 | 0.000 |
| ANGPTL4 | 0.723±0.247 | 0.817±0.173 | 0.000 |
| elF5A2 | 0.770±0.176 | 0.814±0.141 | 0.000 |
Correlation in expression among these
indicators
The correlation in expression of XPO4 between the
cancerous and paracancerous liver tissue was positive (CC=0.304,
P<0.001). Expression of XPO4 in the cancerous liver tissue was
positively correlated with expression of TGFβ1 (CC=0.126, P=0.047)
in paracancerous liver tissue, expression of ANGPTL4 (CC=0.506,
P=0.000) in cancerous liver tissue, expression of ANGPTL4
(CC=0.199, P=0.002) in para-cancerous liver tissue and expression
of elF5A2 (CC=0.194, P=0.002) in paracancerous liver tissue
(Table II). The correlation in
expression of ANGPTL4 between the cancerous and paracancerous liver
tissue was positive (CC=0.282, P<0.001). Expression of ANGPTL4
in the cancerous liver tissue was positively correlated with
expression of XPO4 (CC=0.506, P<0.001) in carcinoma liver
tissue, expression of elF5A2 (CC=0.469, P<0.001) in carcinoma
liver tissue and expression of elF5A2 (CC=0.245, P<0.001) in
paracancerous liver tissue. The correlation in expression of elF5A2
between cancerous and paracancerous liver tissues was positive
(CC=0.371, P<0.001). Expression of elF5A2 in the cancerous liver
tissue was positively correlated with expression of XPO4 (CC=0.478,
P<0.001) in carcinoma liver tissue. These results suggest that
the expression of these four indicators is internally connected and
there is modulation between each of them.
 | Table IICorrelation of XPO4, TGFβ1, ANGPTL4
and elF5A2 in carcinoma tissues and adjacent tissues. |
Table II
Correlation of XPO4, TGFβ1, ANGPTL4
and elF5A2 in carcinoma tissues and adjacent tissues.
| Tissue and indicator,
statistical test | Carcinoma tissue
XPO4 | Adjacent tissue
XPO4 | Carcinoma tissue
TGFβ1 | Adjacent tissue
TGFβ1 | Carcinoma tissue
ANGPTL4 | Adjacent tissue
ANGPTL4 | Carcinoma tissue
elF5A2 | Adjacent tissue
elF5A2 | TNM |
|---|
| Carcinoma tissue
XPO4 | | | | | | | | | |
| Correlation | 1 | 0.304b | 0.038 | 0.126a | 0.506b | 0.199b | 0.478b | 0.194b | 0.052 |
| Sig.
(2-tailed) | - | 0.000 | 0.562 | 0.047 | 0.000 | 0.002 | 0.000 | 0.002 | 0.415 |
| n | 261 | 250 | 239 | 251 | 258 | 249 | 257 | 245 | 249 |
| Adjacent tissue
XPO4 | | | | | | | | | |
| Correlation | 0.304b | 1 | 0.029 | 0.142a | 0.118 | 0.224b | 0.106 | 0.215b | 0.042 |
| Sig.
(2-tailed) | 0.000 | - | 0.663 | 0.023 | 0.062 | 0.000 | 0.096 | 0.001 | 0.512 |
| n | 250 | 257 | 234 | 256 | 249 | 254 | 248 | 252 | 247 |
| Carcinoma tissue
TGFβ1 | | | | | | | | | |
| Correlation | 0.038 | 0.029 | 1 | 0.467b | 0.104 | 0.172b | 0.047 | 0.065 | 0.402b |
| Sig.
(2-tailed) | 0.562 | 0.663 | - | 0.000 | 0.109 | 0.008 | 0.468 | 0.329 | 0.000 |
| n | 239 | 234 | 241 | 234 | 238 | 233 | 237 | 230 | 230 |
| Adjacent tissue
TGFβ1 | | | | | | | | | |
| Correlation | 0.126a | 0.142a | 0.467b | 1 | 0.085 | 0.228b | 0.066 | 0.103 | 0.299b |
| Sig.
(2-tailed) | 0.047 | 0.023 | 0.000 | - | 0.181 | 0.000 | 0.299 | 0.104 | 0.000 |
| n | 251 | 256 | 234 | 258 | 250 | 254 | 249 | 251 | 247 |
| Carcinoma tissue
ANGPTL4 | | | | | | | | | |
| Correlation | 0.506b | 0.118 | 0.104 | 0.085 | 1 | 0.282b | 0.469b | 0.245b | 0.125a |
| Sig.
(2-tailed) | 0.000 | 0.062 | 0.109 | 0.181 | - | 0.000 | 0.000 | 0.000 | 0.049 |
| n | 258 | 249 | 238 | 250 | 260 | 248 | 259 | 244 | 248 |
| Adjacent tissue
ANGPTL4 | | | | | | | | | |
| Correlation | 0.199b | 0.224b | 0.172b | 0.228b | 0.282b | 1 | 0.239b | 0.477b | 0.142a |
| Sig.
(2-tailed) | 0.002 | 0.000 | 0.008 | 0.000 | 0.000 | - | 0.000 | 0.000 | 0.025 |
| n | 249 | 254 | 233 | 254 | 248 | 256 | 247 | 252 | 247 |
| Carcinoma tissue
elF5A2 | | | | | | | | | |
| Correlation | 0.478b | 0.106 | 0.047 | 0.066 | 0.469b | 0.239b | 1 | 0.371b | 0.050 |
| Sig.
(2-tailed) | 0.000 | 0.096 | 0.468 | 0.299 | 0.000 | 0.000 | - | 0.000 | 0.437 |
| n | 257 | 248 | 237 | 249 | 259 | 247 | 259 | 244 | 248 |
| Adjacent tissue
elF5A2 | | | | | | | | | |
| Correlation | 0.194b | 0.215b | 0.065 | 0.103 | 0.245b | 0.477b | 0.371b | 1 | 0.127a |
| Sig.
(2-tailed) | 0.002 | 0.001 | 0.329 | 0.104 | 0.000 | 0.000 | 0.000 | | 0.047 |
| n | 245 | 252 | 230 | 251 | 244 | 252 | 244 | 252 | 244 |
| TNM | | | | | | | | | |
| Correlation | 0.052 | 0.042 | 0.402b | 0.299b | 0.125a | 0.142a | 0.050 | 0.127a | 1 |
| Sig.
(2-tailed) | 0.415 | 0.512 | 0.000 | 0.000 | 0.049 | 0.025 | 0.437 | 0.047 | - |
| n | 249 | 247 | 230 | 247 | 248 | 247 | 248 | 244 | 256 |
Correlation between expression of
indicators and pathological information
Indicators and tumor size
In patients with multi-nodular tumors, the tumor
samples were obtained from the largest tumor. The statistical
results revealed that the expression of TGFβ1 in paracancerous
liver tissue was significantly positively correlated with tumor
size (CC=0.147, P=0.021, n=248; Table
III). The other 7 parameters, e.g., TGFβ1 in cancerous liver
tissue, and XPO4 in cancerous liver tissue, had no significant
correlation with tumor size.
 | Table IIICorrelation between indicators and
tumor size. |
Table III
Correlation between indicators and
tumor size.
| Statistical
test | Carcinoma tissue
XPO4 | Adjacent tissue
XPO4 | Carcinoma tissue
TGFβ1 | Adjacent tissue
TGFβ1 | Carcinoma tissue
ANGPTL4 | Adjacent tissue
ANGPTL4 | Carcinoma tissue
elF5A2 | Adjacent tissue
elF5A2 |
|---|
| Tumor size | | | | | | | | |
| Correlation | −0.122 | 0.066 | 0.051 | 0.147a | −0.116 | 0.089 | −0.040 | 0.053 |
| Sig.
(2-tailed) | 0.054 | 0.300 | 0.438 | 0.021 | 0.068 | 0.164 | 0.529 | 0.407 |
| n | 250 | 247 | 232 | 248 | 249 | 247 | 248 | 244 |
Indicators and blood vessel invasion The
statistical results revealed that all indicators in cancerous and
paracancerous liver tissues had no significant correlation with
blood vessel invasion (Table
IV).
 | Table IVCorrelation between XPO4, TGFβ1,
ANGPTL4 and elF5A2 expression and vascular invasion in carcinoma
tissue and adjacent tissue. |
Table IV
Correlation between XPO4, TGFβ1,
ANGPTL4 and elF5A2 expression and vascular invasion in carcinoma
tissue and adjacent tissue.
| Indicator | Tissue type | Vascular invasion
(yes) | Vascular invasion
(no) | P-value |
|---|
| XPO4 | Carcinoma
tissue | 0.689±0.317 | 0.803±0.185 | 0.313 |
| Adjacent
tissue | 0.806±0.174 | 0.855±0.111 | 0.417 |
| TGFβ1 | Carcinoma
tissue | 0.259±0.300 | 0.257±0.281 | 0.984 |
| Adjacent
tissue | 0.539±0.227 | 0.502±0.311 | 0.724 |
| ANGPTL4 | Carcinoma
tissue | 0.780±0.132 | 0.723±0.247 | 0.465 |
| Adjacent
tissue | 0.889±0.042 | 0.816±0.172 | 0.210 |
| elF5A2 | Carcinoma
tissue | 0.760±0.145 | 0.769±0.174 | 0.861 |
| Adjacent
tissue | 0.867±0.070 | 0.812±0.143 | 0.258 |
Indicators and pathological
classification (differentiation)
The patients were divided into two categories
according to the Edmondson classification; high differentiation (I,
II, I–II) and low differentiation (II–III, III, IV). The
statistical results revealed that all indicators exhibited higher
expression levels in the low differentiation group than in the high
differentiation group (Table V).
XPO4 in cancerous liver tissue (CC=0.143, P=0.035) and TGFβ1
(CC=0.195, P=0.004) in paracancerous liver tissue were
significantly correlated with tumor differentiation.
 | Table VAssociation of XPO4, TGFβ1, ANGPTL4
and elF5A2 expression with differentiation. |
Table V
Association of XPO4, TGFβ1, ANGPTL4
and elF5A2 expression with differentiation.
| Indicator | Tissue type | High
differentiation | Low
differentiation | P-value |
|---|
| XPO4 | Carcinoma
tissue | 0.793±0.195 | 0.850±0.150 | 0.035 |
| Adjacent
tissue | 0.849±0.115 | 0.881±0.106 | 0.054 |
| TGFβ1 | Carcinoma
tissue | 0.312±0.290 | 0.303±0.269 | 0.867 |
| Adjacent
tissue | 0.540±0.285 | 0.658±0.245 | 0.003 |
| ANGPTL4 | Carcinoma
tissue | 0.751±0.213 | 0.737±0.211 | 0.643 |
| Adjacent
tissue | 0.833±0.148 | 0.842±0.121 | 0.689 |
| elF5A2 | Carcinoma
tissue | 0.775±0.170 | 0.800±0.106 | 0.253 |
| Adjacent
tissue | 0.816±0.152 | 0.845±0.082 | 0.080 |
Indicators and tumor T classification The
statistical results revealed that expression of TGFβ1 in both
cancerous and paracancerous tissues (CC=0.402, P=0.000; CC=0.299,
P=0.000, respectively) was positively correlated with T
classification; expression of ANGPTL4 in cancerous and
paracancerous liver tissues (CC=0.125, P=0.049; CC=0.142, P=0.025,
respectively) was positively correlated with T classification and
that the expression of elF5A2 in paracancerous liver tissues
(CC=0.127, P=0.047) was positively correlated with T
classification.
Indicators and survival function
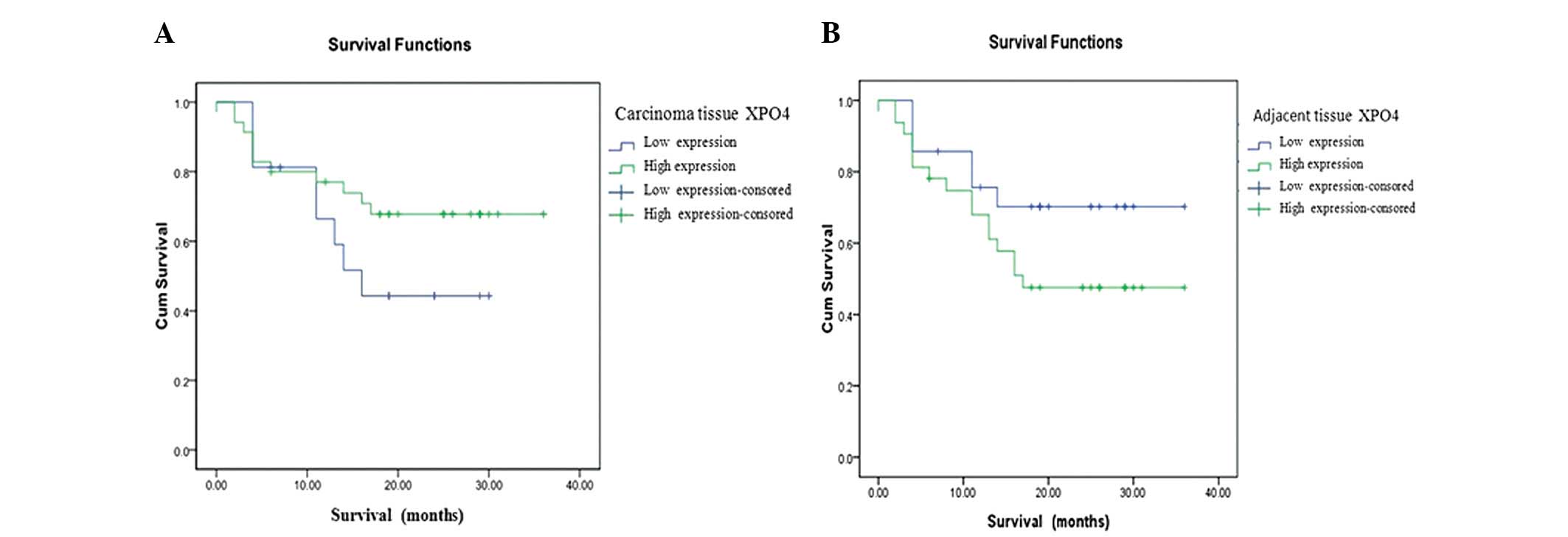
Kaplan-Meier analysis indicated that the expression
of XPO4 in carcinoma tissue did not correlate with survival
function in overexpression and underexpression (P=0.202). The
survival plot indicated that survival rates in patients with XPO4
overexpression were higher than those in patients with XPO4
underexpression (Fig. 2A).
Expression of XPO4 in adjacent tissue did not correlate with
overexpression or underexpression (P=0.139). The survival plot
indicated that survival rates in patients with XPO4 overexpression
in adjacent tissues were lower than those in patients with XPO4
underexpression. These results suggested that higher expression of
XPO4 in cancerous liver tissue was indicative that the patient
would have a better prognosis and increased survival rate. However,
higher concentrations of XPO4 in paracancerous liver tissue
suggested a worse prognosis (Fig.
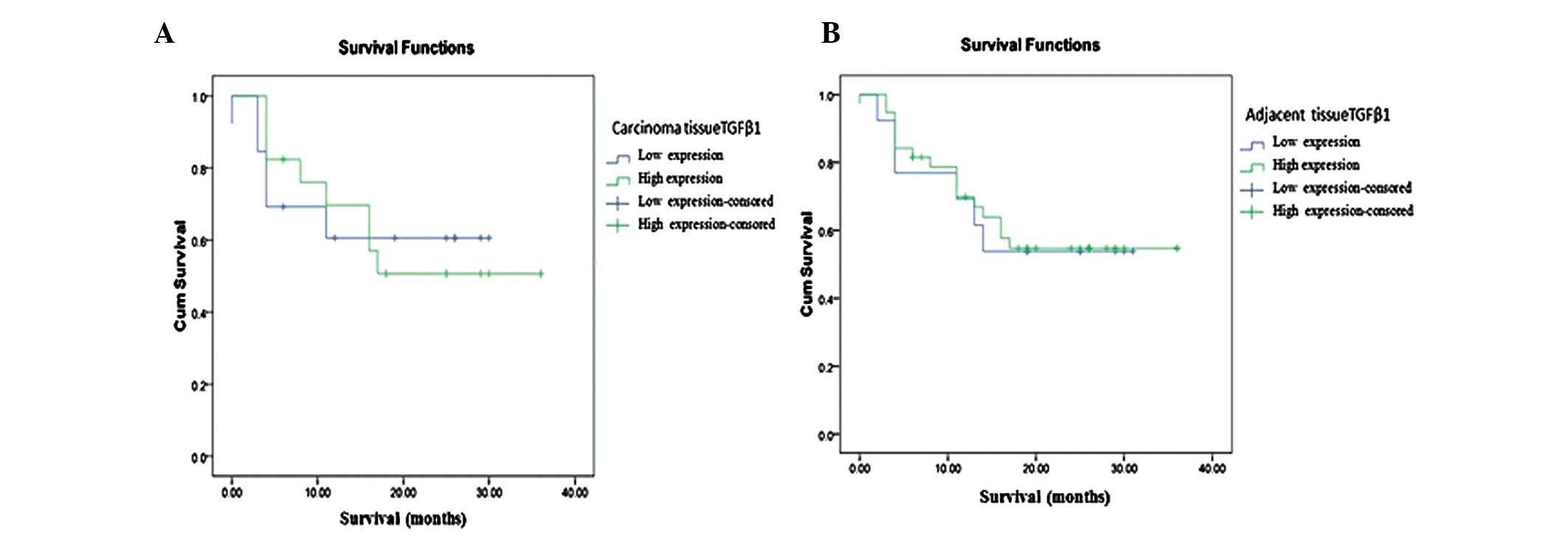
2B). Furthermore, expression of TGFβ1 in carcinoma tissue did
not correlate with overexpression or underexpression (P=0.954). The
survival figure indicated that patients who were positive for TGFβ1
in cancerous liver tissue had a better prognosis than those who
were negative for TGFβ1 in cancerous liver tissue (Fig. 3). Other factors, e.g., ANGPTL4 and
ELF, were not correlated with overexpression or underexpression in
either of the cancerous and adjacent tissues.
Discussion
In the present study, we employed multiple
techniques, including the use of qPCR, immunostaining and TMAs, as
well as histology and pathology analysis, to undertake a study to
evaluate XPO4, TGFβ1, ANGPTL4 and elF5A2 in carcinoma and
paracarcinoma tissues from 280 liver cancer patients. Our results
revealed that all four indicators were located in the cytoplasm and
distributed in both cancerous and paracancerous liver tissues.
Generally, there were higher levels of these indicators in
paracancerous than in cancerous liver tissue. The correlation
analysis results further revealed that expression levels of these
four indicators were correlated and modulated amongst each other.
In connection with patients’ clinical and revisit information,
statistical results revealed that TGFβ1 levels in paracancerous
liver tissue was positively correlated with the tumor size. Higher
levels of TGFβ1 in paracancerous liver tissue were always
associated with bigger liver tumors. XPO4 in cancerous liver tissue
and TGFβ1 in paracancerous liver tissue were positively correlated
with tumor differentiation. TGFβ1, ANGPTL4 and elF5A2 were also
positively correlated with the T classification of tumors.
Additionally, a higher expression of XPO4 in cancerous liver tissue
suggested that the patient would have a better prognosis and
survival rate. However, higher XPO4 levels in paracancerous liver
tissue suggested a worse prognosis. These results suggest that XPO4
may be a potential biomarker to assess HCC prognosis and
differentiation.
In the present study, we performed experimental and
statistical work in cancerous and paracancerous materials from 280
patients suffering from liver cancer. All patient materials were
collected and preserved according to standard protocols (see
Patients and methods). In order to reduce the experimental
error/variance obtained by processing different tissue samples
using immunostaining, we selected the method of TMAs to equalize
the experimental condition among different samples. TMA is an
innovative type of specimen slide with multiple samples on a single
slide, which was first described in 1986 (17,18).
In our study, each slide integrated 200 tissue specimens on a
single slide as 500 dots, which represented multiple tissues,
pathological types, patients and stages, as well as control
samples, aiding in the creation of a low margin for error. This
method allows for high throughput of data of multiple patient cases
and control samples simultaneously. Thus reagent concentrations
were identical for each case, as were incubation times,
temperatures and wash conditions. This greatly improves the
accuracy of the results, leading to more confident conclusions
(19). Each experiment was also
repeated in three slides of TMAs. Data averages were used for
analysis.
We have been aware of the result complicity from
different patients and made numerous attempts to summarize our
results in a realistic way. It is conventional to take the middle
point as a standard to perform analysis in survival function.
However, there is no meaning because the positive probability of
indicators displays skew distribution. The 25th percentile was
therefore eventually taken for correlation analysis in our study.
The suggestion given by this analysis is rational and it is close
to clinic observation by current knowledge.
TGFβ is a multipotent polypeptide which regulates
cell proliferation, differentiation and apoptosis and deters tumor
growth (20). Within the tumor
micro-environment, TGFβ is produced by liver cancer cells. It is
located within the cytoplasm, as shown in our results and
consistently in a number of previous studies (3,5).
However, as a natural response to the hypoxic and inflammatory
conditions that occur during tumor progression, high production
levels of TGFβ1 in our study were unexpectedly found in
paracancerous, but not cancerous, liver tissue. Moreover, TGFβ1 in
cancerous or paracancerous liver tissue had no connection with
either tumor vessel invasion or lymph metastasis. These results
were inconsistent with previous results which demonstrated that the
overexpression of TGFβ1 is associated with a high incidence of
distant metastasis and that TGFβ1 promotes vascular invasion by
activating β1 integrin (10,21).
Additionally, patients positive for TGFβ1 in the cancerous tissue
were suggested to have a better prognosis, as is consistent with a
previous report (6). This result
may partly support the current concept for a dual role of the TGFβ
signaling pathway in hepatocellular cancer suppression and
progression of differentiation (3,6,22,23).
TGFβ receptors phosphorylate Smad3 and induce its
nuclear import, then regulate gene transcription. Smad3 returns to
the cytoplasm to propagate further cycles of signal transduction.
In HCCs, Smad3 and its phosphorylation relatives have been
suggested to be the predictors of prognosis in patients with liver
cancer and also serve as the biomarkers to identify patients with a
high risk of recurrence (5). Smad3
is exported via XPO4. XPO4 is therefore in control of Smad3
signaling as well as protein synthesis (7,8).
Consistent with a previous study (9), our results also demonstrated that
XPO4 was present in cancerous and paracancerous liver tissues, with
a higher density of XPO4 in paracancerous tissue. Our results found
that TGFβ1, but not XPO4 (9), in
paracancerous tissue was significantly positively correlated with
tumor size and histopathological classification. Moreover, our
results also suggested that high XPO4 in cancerous tissue resulted
in a good prognosis. This is consistent with a previous study which
indicated that downregulation of XPO4 resulted in a poor prognosis
(9). Our results in an adequate
sample size emphasized again that XPO4 may be involved in the
progression of human HCC and may serve as a potential biomarker to
evaluate the condition. A further validation study is suggested on
a bigger sample size. Additionally, assessment of tumor behavior in
HCC cell lines with or without rescuing XPO4 may confirm the
therapeutic role of XPO4 in HCC.
elF5A2 is amplified in human tumors, is required for
proliferation of XPO4-deficient tumor cells and promotes HCC in
mice (7). Our results provided
evidence of correlation among these four indicators in HCC.
Production of elF5A2 in paracancerous tissue is significantly
positively associated with cancer histopathological classification.
Previous studies have also revealed that the induction of
angiopoietin-like 4 (ANGPTL4) by TGFβ, via the Smad signaling
pathway, is critical for the trans-endothelial passage of tumor
cells resulting in tumor metastasis (14). Further studies have revealed that
ANGPTL4 regulates endothelial cell junction organization (24) and pericyte coverage (11), resulting in disruption in
endothelial cell-cell junctions (25). We therefore carried out the
rational measurement of ANGPTL4 in the cancerous and paracancerous
liver tissues in a variety of patients. Our results showed that
there was no significant correlation between ANGPTL4 and vessel or
lymphatic invasion. It is inconsistent with previous reports that
expression of ANGPTL4 was statistically correlated with the degree
of differentiation, lymphatic invasion and venous invasion
(12,26). Our results provided evidence that
ANGPTL4 is not a metastasis-inducing factor in HCC.
In summary, the results of the present study
revealed that XPO4, TGFβ1, ANGPTL4 and elF5A2 were present in both
cancerous and paracancerous liver tissues, and that they were
closely correlated with each other. TGFβ1 in paracancerous liver
tissue was positively correlated with the tumor size. XPO4 in
cancerous liver tissue and TGFβ1 in paracancerous liver tissue were
positively associated with tumor differentiation. Meanwhile, TGFβ1,
ANGPTL4 and elF5A2 were significantly correlated with the T
classification of tumors. Of these four indicators, XPO4 appears to
be a potential biomarker to evaluate HCC.
Acknowledgements
This study was supported by a grant
from Scientific Research Projects of Health Bureau of Shanghai
(09411952800) to HZ. This study was approved by the Hua Shan
Hospital, Fudan University Administration Panel of Clinic research,
China.
References
|
1
|
Parkin DM: Global cancer statistics in the
year 2000. Lancet Oncol. 2:533–543. 2001.PubMed/NCBI
|
|
2
|
Fabregat I: Dysregulation of apoptosis in
hepatocellular carcinoma cells. World J Gastroenterol. 15:513–520.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dong ZZ, Yao DF, Yao M, et al: Clinical
impact of plasma TGF-beta1 and circulating TGF-beta1 mRNA in
diagnosis of hepatocellular carcinoma. Hepatobiliary Pancreat Dis
Int. 7:288–295. 2008.PubMed/NCBI
|
|
4
|
Mishra L, Derynck R and Mishra B:
Transforming growth factor-beta signaling in stem cells and cancer.
Science. 310:68–71. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim SH, Ahn S and Park CK: Smad3 and its
phosphoisoforms are prognostic predictors of hepatocellular
carcinoma after curative hepatectomy. Hepatobiliary Pancreat Dis
Int. 11:51–59. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Coulouarn C, Factor VM and Thorgeirsson
SS: Transforming growth factor-beta gene expression signature in
mouse hepatocytes predicts clinical outcome in human cancer.
Hepatology. 47:2059–2067. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zender L, Xue W, Zuber J, et al: An
oncogenomics-based in vivo RNAi screen identifies tumor suppressors
in liver cancer. Cell. 135:852–864. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kurisaki A, Kurisaki K, Kowanetz M, et al:
The mechanism of nuclear export of Smad3 involves exportin 4 and
Ran. Mol Cell Biol. 26:1318–1332. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liang XT, Pan K, Chen MS, et al: Decreased
expression of XPO4 is associated with poor prognosis in
hepatocellular carcinoma. J Gastroenterol Hepatol. 26:544–549.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fransvea E, Mazzocca A, Antonaci S and
Giannelli G: Targeting transforming growth factor (TGF)-betaRI
inhibits activation of beta1 integrin and blocks vascular invasion
in hepatocellular carcinoma. Hepatology. 49:839–850. 2009.
View Article : Google Scholar
|
|
11
|
Perdiguero EG, Galaup A, Durand M, et al:
Alteration of developmental and pathological retinal angiogenesis
in angptl4-deficient mice. J Biol Chem. 286:36841–36851. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li H, Ge C, Zhao F, et al:
Hypoxia-inducible factor 1 alpha-activated angiopoietin-like
protein 4 contributes to tumor metastasis via vascular cell
adhesion molecule-1/integrin β1 signaling in human hepatocellular
carcinoma. Hepatology. 54:910–919. 2011.PubMed/NCBI
|
|
13
|
Huang RL, Teo Z, Chong HC, et al: ANGPTL4
modulates vascular junction integrity by integrin signaling and
disruption of intercellular VE-cadherin and claudin-5 clusters.
Blood. 118:3990–4002. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Padua D, Zhang XH, Wang Q, et al: TGFbeta
primes breast tumors for lung metastasis seeding through
angiopoietin-like 4. Cell. 133:66–77. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yao FY, Ferrell L, Bass NM, et al: Liver
transplantation for hepatocellular carcinoma: expansion of the
tumor size limits does not adversely impact survival. Hepatology.
33:1394–1403. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gao Q, Qiu SJ, Fan J, et al: Intratumoral
balance of regulatory and cytotoxic T cells is associated with
prognosis of hepatocellular carcinoma after resection. J Clin
Oncol. 25:2586–2593. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Battifora H: The multitumor (sausage)
tissue block: novel method for immunohistochemical antibody
testing. Lab Invest. 55:244–248. 1986.PubMed/NCBI
|
|
18
|
Kononen J, Bubendorf L, Kallioniemi A, et
al: Tissue microarrays for high-throughput molecular profiling of
tumor specimens. Nat Med. 4:844–847. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xiang ZL, Zeng ZC, Tang ZY, et al:
Potential prognostic biomarkers for bone metastasis from
hepatocellular carcinoma. Oncologist. 16:1028–1039. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Giannelli G, Mazzocca A, Fransvea E, Lahn
M and Antonaci S: Inhibiting TGFβ signaling in hepatocellular
carcinoma. Biochim Biophys Acta. 1815:214–223. 2011.
|
|
21
|
Picon A, Gold LI, Wang J, Cohen A and
Friedman E: A subset of metastatic human colon cancers expresses
elevated levels of transforming growth factor beta1. Cancer
Epidemiol Biomarkers Prev. 7:497–504. 1998.PubMed/NCBI
|
|
22
|
Mishra L, Banker T, Murray J, et al: Liver
stem cells and hepatocellular carcinoma. Hepatology. 49:318–329.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Baek HJ, Lim SC, Kitisin K, et al:
Hepatocellular cancer arises from loss of transforming growth
factor beta signaling adaptor protein embryonic liver fodrin
through abnormal angiogenesis. Hepatology. 48:1128–1137. 2008.
View Article : Google Scholar
|
|
24
|
Cazes A, Galaup A, Chomel C, et al:
Extracellular matrix-bound angiopoietin-like 4 inhibits endothelial
cell adhesion, migration, and sprouting and alters actin
cytoskeleton. Circ Res. 99:1207–1215. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hasita H, Komohara Y, Okabe H, et al:
Significance of alternatively activated macrophages in patients
with intrahepatic cholangiocarcinoma. Cancer Sci. 101:1913–1919.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shibata K, Nakayama T, Hirakawa H, Hidaka
S and Nagayasu T: Clinicopathological significance of
angiopoietin-like protein 4 expression in oesophageal squamous cell
carcinoma. J Clin Pathol. 63:1054–1058. 2010. View Article : Google Scholar : PubMed/NCBI
|