Introduction
Diabetic nephropathy is a major microvascular
complication of diabetes and one of the leading causes of mortality
in diabetes patients. Due to the increasing incidence of diabetes
and the decreased survival period of patients, diabetes has become
the most common cause of end-stage renal disease (ESRD). Of
patients with type 1 or type 2 diabetes, 20–30% are diagnosed as
having kidney diseases. Globally, approximately 40% of ESRD
patients suffer from diabetic nephropathy as the primary disease
(1). The cost of diabetic ESRD
treatments is increasing. Therefore, effective control of diabetic
nephropathy and prevention of ESRD are essential for improving the
life quality of patients with diabetes, as well as reducing the
economic costs for the patient’s family and society. With the
exception of controlling blood sugar levels, blood pressure and
protein intake, inhibitors of the angiotensin-converting enzyme and
the angiotensin II receptor are the only available therapeutic
approaches for treating diabetic nephropathy. Therefore, novel
drugs for the treatment of diabetic nephropathy are required.
Calcium dobesilate (calcium
dihydroxy-2,5-benzenesulfonate) is a vascular protective compound
which was revealed to be the most effective member of a new family
of efficient fibroblast growth factor (FGF) inhibitors (2). Calcium dobesilate is a small molecule
that has been widely used for treating diabetic retinopathy and
chronic venous insufficiency (3,4).
However, the mechanism of action of this drug has not been
completely elucidated.
It has been demonstrated that calcium dobesilate
binds to the heparin-binding domain of FGF-1, thus reducing its
activity (2), and it has been
demonstrated to be effective for the treatment of rosacea (5) and psoriasis (6), which are clinical manifestations
associated with excessive angiogenesis and the overexpression of
vascular endothelial growth factor (VEGF).
In the present study, using the pathogenic analysis
of diabetic nephropathy and the pharmacological effects of the
compound, it was observed that calcium dobesilate had significant
effects for the treatment of diabetic nephropathy. Alterations to
the associated hematological indicators were observed in patients
with type 2 diabetes, in the presence or absence of calcium
dobesilate. The effects of calcium dobesilate on urinary albumin
levels were also studied. The therapeutic effects were investigated
and the mechanisms of the effect of calcium dobesilate on diabetic
nephropathy were determined.
Materials and methods
Patients
A total of 121 patients were recruited for this
study, including 61 males and 60 females with an average age of
45.3 years (range, 33–68 years). The patients had been diagnosed
with diabetes for between 3 months and 12 years, with an average of
6.8 years. Prior written and informed consent was obtained from
each patient and the study was approved by the ethics review board
of Shandong Provincial Qianfoshan Hospital (Jinan, China).
Treatment
All treated patients received 500 mg of calcium
dobesilate 3 times a day. Blood pressure, urinary albumin excretion
rate (UAER), liver and kidney function and the levels of blood
glucose, blood lipids, glycated hemoglobin (HbA1c), fasting
C-peptide, blood viscosity, endothelin (ET) and plasminogen
activator inhibitor-1 (PAI-1) were all measured prior to and
following treatment. UAER was measured once a month during the
treatment. Urinary albumin (ALB) levels were determined using
radioimmunoassays. UAER was calculated using the urine volumes and
ALB concentrations.
Venous blood was taken between 7 and 8 am after
fasting for 10 h and 2 ml of the venous blood serum was separated
for the detection of fasting blood glucose (FBG), HbA1c, total
cholesterol (CH), triglyceride (TG), high-density
lipoproteinprotein cholesterol (HDL-C), low-density lipoprotein
cholesterol (LDL-C), alanine aminotransferase (ALT), γ-Valley
glutamyl transferase (γ-GT), urea nitrogen (BUN), creatinine (Cr)
and fasting C-peptide.
Detection of blood specimens
FBG was detected using the glucose oxidase method.
HbA1c, CH, TG, HDL-C, LDL-C, ALT, γ-GT, BUN and Cr were determined
using biochemical methods with a Beckman Coulter (Miami, FL, USA)
automatic biochemical analyzer. Fasting C-peptide was detected
using a chemiluminescence method with an ACS 180 SE instrument
(Bayer, Leverkusen, Germany). Plasma viscosity, high, medium and
low shear rate whole blood viscosity were determined using the
capillary method with an MVIS-2030 automatic blood rheology
analyzer (Chongqing Tianhai, Chongqing, China).
ELISA
An ELISA kit containing the anti-human PAI-1
antibody and PAI-1 test plasma were purchased from Shanghai Sun
Biotechnology Company (Shanghai, China). The presence of the target
complex was determined by the color reaction of horseradish
peroxidase and the value measured at 492 nm was proportional to the
PAI-1 content of the test plasma.
RNA preparation
Peripheral blood mononuclear cells were isolated
from the blood samples of patients by centrifugation, washed with
buffers twice and then stored at −80°C for further analysis.
Isolation of total RNA was performed using guanidine isothiocyanate
(GIT) methods. The purity of the total RNA was assessed using a
UV240 spectrophotometer (Daojin company, Shimanishiki, Japan).
Quantitative reverse transcription
(RT)-PCR
Quantitative RT-PCR analysis of the PAI-1 mRNA
levels in tissues and cells was performed. The total RNA was
harvested from peripheral blood mononuclear cells using the RNeasy
kit (Qiagen, Hilden, Germany) according to the manufacturer’s
instructions. The RT-PCR experiments were repeated at least 3
times. RNA was reverse transcribed into cDNA using random primers
in a Reverse Transcription II system (Promega, Madison, WI, USA)
according to the manufacturer’s instructions. Expression of PAI-1
mRNA was quantified by quantitative PCR using an ABI Prism Sequence
Detection System (Applied Biosystems, Carlsbad, CA, USA). The
primers used in the present study are shown in Table I. An assay reagent containing
premixed primers and a VIC™ labeled probe (Applied Biosystems; cat.
no. 4310884E) was used to quantify the expression of endogenous
GAPDH mRNA. Template-negative and RT-negative conditions were used
as controls. Amplification of the endogenous GAPDH cDNA was
monitored. The levels (mean values) of the PAI-1 transcripts in
patients were calculated.
 | Table IPrimers used for RT-PCR. |
Table I
Primers used for RT-PCR.
| Primer | Sequence of
primer |
|---|
| β-GAPDH F |
5′-GTGGGGCGCCCCAGGCACCA-3′ |
| β-GAPDH R |
5′-CTCCTTAATGTCACGCACGATTT-3′ |
| PAI-1 F |
5′AACATATCTACTAGAAATCTGTT3′ |
| PAI-1 R |
5′GCTACTCTACGTCGGCGAGAC3′ |
Immunoblot assays
Total protein was harvested from the peripheral
blood mononuclear cells of the patients. The proteins were
separated on 10% SDS-PAGE gels and subjected to immunoblot
analysis. The primary antibodies against PAI-1 (∼50 kDa) and
β-actin were purchased from Santa Cruz Biotechnology, Inc. (Santa
Cruz, CA, USA; anti-PAI-1, cat. no. sc-5297, 1:200 dilution;
anti-β-actin, cat. no. sc-130301, 1:10,000 dilution). The secondary
antibody used in the present study was goat anti-mouse IgG-HRP
(cat. no. sc-2005, 1:10,000 dilution, Santa Cruz Biotechnology,
Inc.). Bound antibodies were detected using an ECL system (Pierce
Biotechnology, Rockford, IL, USA). The immunoblot experiments were
repeated at least 3 times. The mean normalized optical density (OD)
of the PAI-1 protein bands relative to the OD of the β-actin band
from the same individual was calculated.
Statistical analysis
Continuous variables were summarized as mean values
(mean ± standard error) and compared using the independent sample
t-test. P<0.05 was considered to indicate statistically
significant differences. All statistical calculations were
performed using the SPSS 10.0 software.
Results
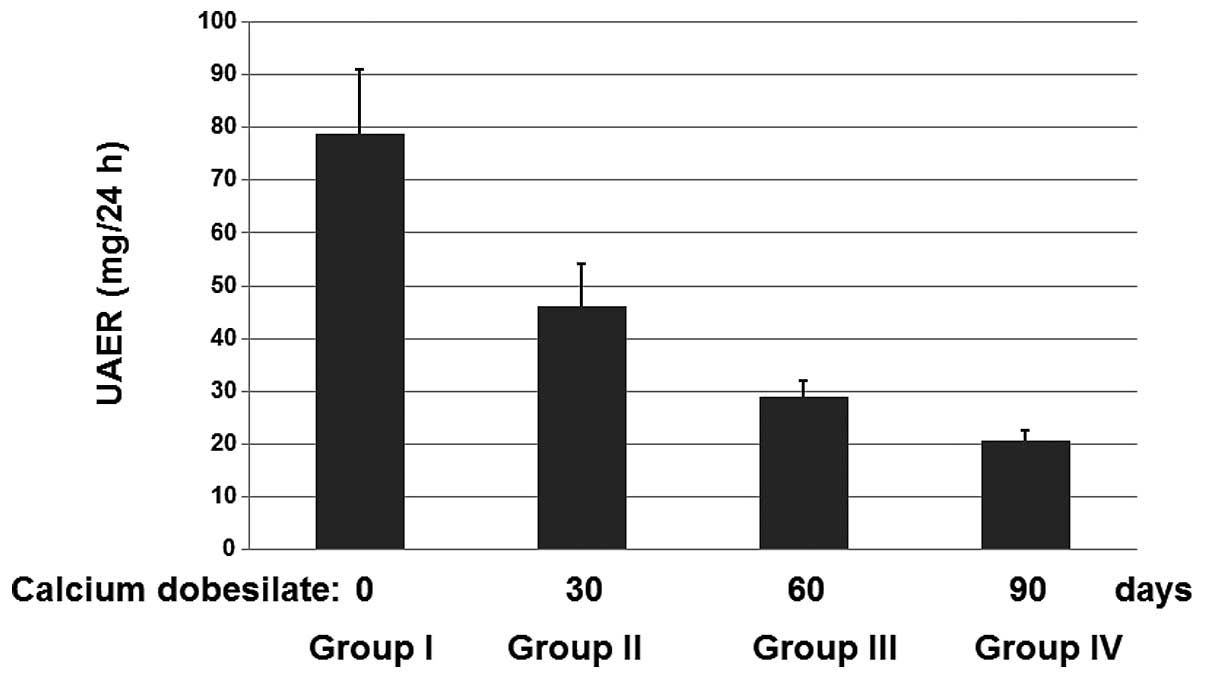
Calcium dobesilate reduces levels of UAER
in patients
The diagnosis and staging standard of diabetic
nephropathy is based on the UAER. The 121 patients were divided
into 4 groups. The patients in group I did not receive calcium
dobesilate and served as the control group. The patients in groups
II, III and IV received calcium dobesilate for 30, 60 and 90 days,
respectively. No adverse drug responses were identified. After 90
days, the UAERs were determined for the patients in each of the 4
groups (Fig. 1). It was observed
that the mean UAERs decreased significantly upon treatment with
calcium dobesilate compared with the control group. These results
suggest that calcium dobesilate is effective for treating diabetic
nephropathy.
Calcium dobesilate significantly reduces
the shear rates of whole blood viscosity in patients
As shown in Table
II, for the patients receiving calcium dobesilate, PAI-1, ET,
plasma viscosity, whole blood reduced viscosity, high, medium shear
rate and low shear rate whole blood viscosity and fibrinogen (Fbg)
were determined. The medium shear rate and low shear rate whole
blood viscosity decreased by a statistically significant amount
(Table II).
 | Table IIIndicators of patients who were
untreated or treated with calcium dobesilate. |
Table II
Indicators of patients who were
untreated or treated with calcium dobesilate.
| Indicator | Untreated (mean ±
SE) | Treated (mean ±
SE) | P-value |
|---|
| Plasma viscosity
(mPas) | 1.920±0.962 | 1.474±0.067 | 0.081 |
| Whole blood reduced
viscosity (mPas) | 6.896±0.790 | 6.467±0.562 | 0.473 |
| High shear rate whole
blood viscosity (mPas) | 4.620±0.288 | 4.407±0.318 | 0.090 |
| Medium shear rate
whole blood viscosity (mPas) | 5.474±0.274 | 5.096±0.374 | 0.040 |
| Low shear rate whole
blood viscosity (mPas) | 12.835±0.650 | 12.101±0.969 | 0.020 |
| Fbg (g/l) | 3.611±0.637 | 3.064±0.940 | 0.070 |
| PAI-1 (pg/ml) | 69.10±10.600 | 10.50±11.520 | 0.062 |
| ET (pg/ml) | 68.390±9.562 | 51.498±8.570 | 0.060 |
Calcium dobesilate did not affect general
indicators in patients
The general indicators were also determined for
patients treated with or without calcium dobesilate. As shown in
Table III, there were no
statistically significant effects on the detected levels of FBG,
HbA1c, CH, TG, HDL-C, LDL-C, ALT, γ-GT, BUN, Cr, fasting C-peptide,
post-prandial 2 h blood glucose (p2hBG), HbA1c, Hct, systolic and
diastolic blood pressure (Table
III).
 | Table IIIGeneral indicators in patients who
were untreated or treated with calcium dobesilate. |
Table III
General indicators in patients who
were untreated or treated with calcium dobesilate.
| General
indicator | Untreated (mean ±
SE) | Treated (mean ±
SE) | P-value |
|---|
| FBG (mmol/l) | 6.048±0.167 | 6.183±0.251 | 0.508 |
| p2hBG (mmol/l) | 8.260±0.192 | 8.330±0.842 | 0.899 |
| HbA1c (%) | 6.520±0.233 | 6.315±0.322 | 0.400 |
| Fasting C-peptide
(ng/ml) | 2.562±1.023 | 2.130±1.724 | 0.682 |
| TG (mmol/l) | 1.198±0.805 | 1.394±1.003 | 0.062 |
| CH (mmol/l) | 5.023±1.087 | 4.807±0.992 | 0.482 |
| LDL-C (mmol/l) | 2.576±0.516 | 2.502±0.519 | 0.746 |
| HDL-C (mmol/l) | 1.370±0.305 | 1.385±0.338 | 0.936 |
| ALT (mmol/l) | 25.900±14.449 | 25.700±14.712 | 0.963 |
| γ-GT (mmol/l) | 20.730±20.195 | 21.3±15.159 | 0.876 |
| BUN (mmol/l) | 4.546±1.425 | 4.575±1.932 | 0.956 |
| Cr (μmol/l) | 74.590±19.523 | 77.300±18.439 | 0.537 |
| Hct (%) | 0.433±0.038 | 0.413±0.042 | 0.189 |
| Systolic blood
pressure (mmHg) | 121.000±6.990 | 122.500±6.350 | 0.193 |
| Diastolic blood
pressure (mmHg) | 74.000±10.490 | 76.000±2.560 | 0.309 |
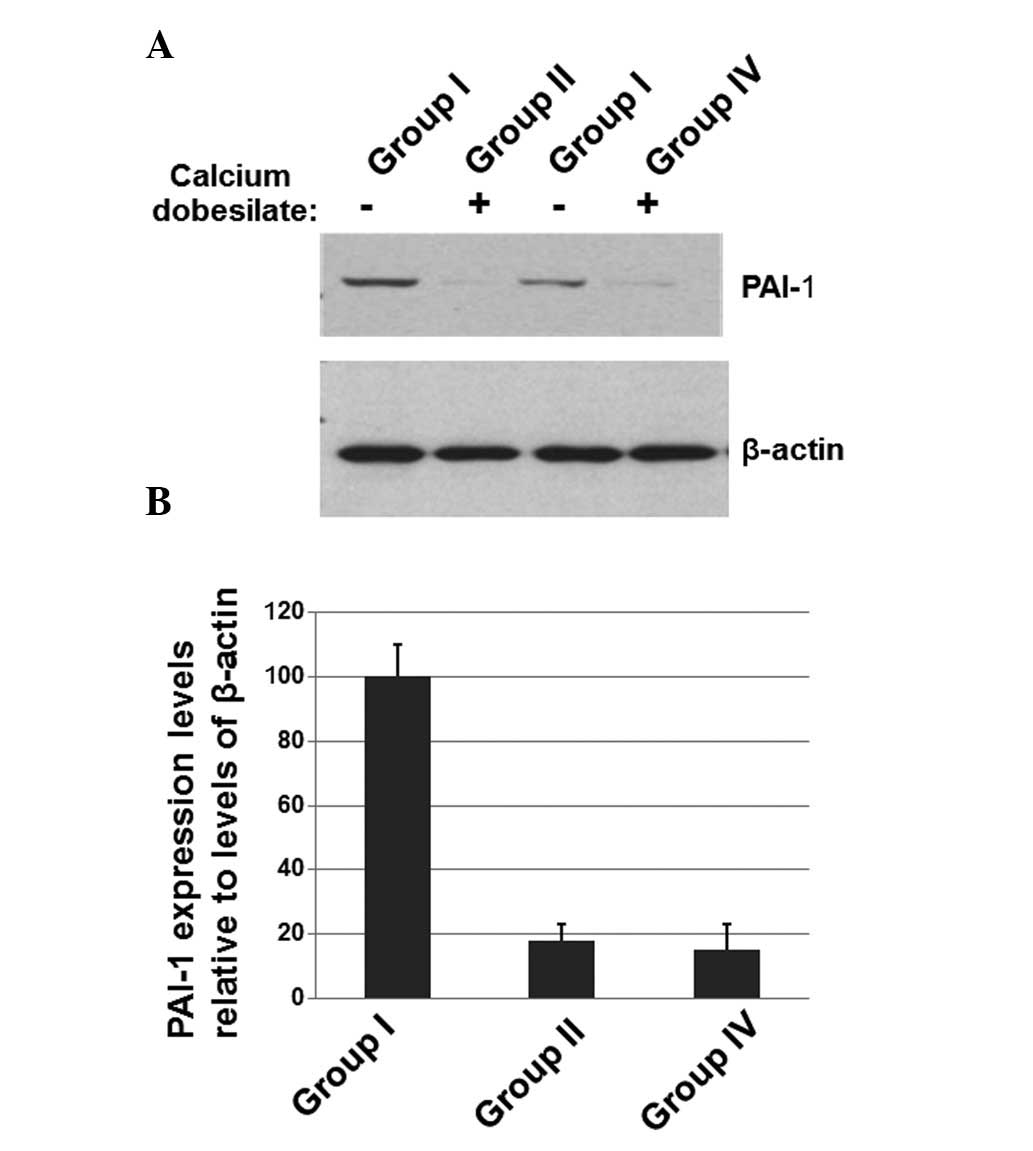
Calcium dobesilate decreases the levels
of PAI-1 in patients
To determine whether the effects of calcium
dobesilate on diabetic nephropathy were associated with the
expression of PAI-1, the peripheral blood mononuclear cells were
isolated from the blood samples of patients and western blotting
was performed. As shown in Fig.
2A, the expression levels of PAI-1 were decreased in patients
receiving calcium dobesilate compared with patients who had not
received calcium dobesilate. The levels of β-actin were used as a
loading control. The mean normalized OD of the PAI-1 protein bands
relative to the OD of the β-actin bands was calculated for each of
the 4 groups (Fig. 2B). The data
for group III are not shown in Fig.
2 since they were similar to those of group II. The results
shown in Fig. 2 suggest that
calcium dobesilate significantly reduces the expression of PAI-1
which may be associated with the effect of calcium dobesilate on
diabetic nephropathy patients.
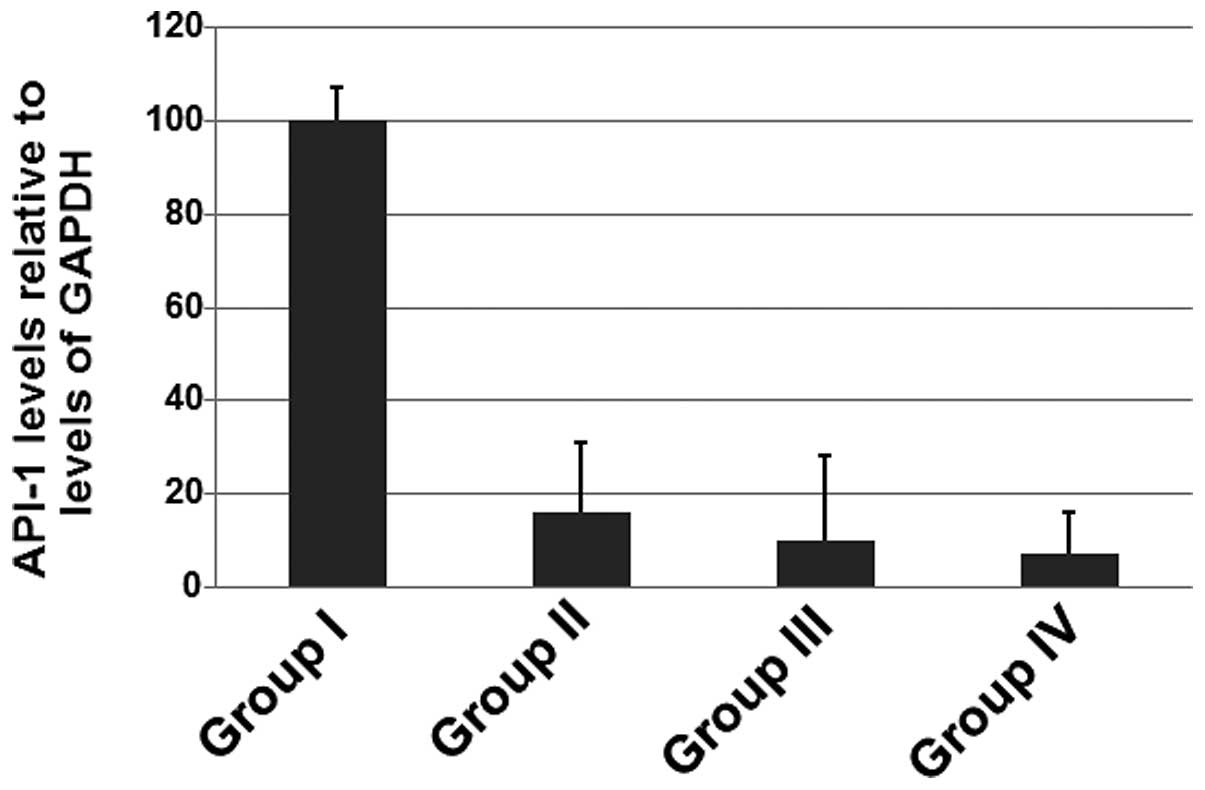
Calcium dobesilate decreases the levels
of PAI-1 mRNA in patients
To determine whether calcium dobesilate affects
PAI-1 mRNA levels, RT-PCR was performed. As shown in Fig. 3, the expression levels of PAI-1
mRNA were decreased in patients receiving calcium dobesilate
compared with those in patients who did not receive calcium
dobesilate. The results shown in Fig.
3 suggest that calcium dobesilate significantly reduces the
level of PAI-1 mRNA.
Discussion
The severity of nephropathy is usually defined by
the proteinuria level which is closely correlated with kidney
damage. It is generally considered that proteinuria increases the
glomerular protein filtration channel load, eventually leading to
ESRD (7). It was observed in the
present study that, following treatment with calcium dobesilate,
the UAERs decreased significantly in patients with diabetic
nephropathy. The decrease in UAERs was positively correlated with
the treatment time, suggesting a therapeutic benefit of this
treatment.
In the present study, PAI-1 and Fbg levels were
decreased in patients following the administration of calcium
dobesilate. There are no previous studies on the effects of calcium
dobesilate on PAI-1. However, there are numerous studies concerning
the effects of calcium dobesilate on plasma Fbg (8–12)
which all reveal decreases in plasma Fbg levels. PAI-1 reduces the
inhibition of urokinase and tissue plasminogen activator which
activate plasminogen and thus plasmin and eventually alleviates
glomerulosclerosis. The decrease in Fbg levels was positively
correlated with the reduction of PAI-1 levels.
In the present study, plasma ET levels were
decreased following treatment with calcium dobesilate compared with
the baseline plasma ET levels. There is a limited number of studies
concerning the effect of calcium dobesilate on ET. Zhong and Guo
(13) reported that ET levels were
significantly decreased in 20 patients with diabetic retinopathy
following calcium dobesilate treatment. The reduction effect may be
due to the following: i) a reduction in thromboxane A2 (TXA2)
levels (14); ii) the removal of
oxygen-derived free radicals (15); and iii) a reduction in blood
viscosity, thus increasing tissue ischemia, hypoxia and vascular
shear stress. Changes in TXA2, free radicals, tissue ischemia and
hypoxia and vascular shear stress induce the synthesis and release
of ET. This causes the contraction of kidney blood vessels,
promotion of cell growth, proliferation and synthesis of
extracellular matrix, induction of local inflammation, platelet
aggregation and microthrombosis of ET so that a reduction in ET
levels may reverse kidney disease. It has also been reported that
blood viscosity increases significantly in patients with diabetic
nephropathy (16–18).
In the present study, medium shear rate whole blood
viscosity and low shear rate whole blood viscosity decreased
significantly. Plasma viscosity, whole blood viscosity and high
shear rate whole blood viscosity decreased slightly. This
demonstrated that the treatment affected aggregation more than the
deformability of red blood cells. This effect on blood viscosity
was previously only measured in the whole blood or plasma (19–21).
In the present study, these indicators were evaluated under various
whole blood viscosity shear rates. The effect of calcium dobesilate
on diabetic nephropathy may be mediated through a reduction in the
expression of PAI-1.
Acknowledgements
This study was funded by a grant
(grant No: 2001BB1DBA1) from the Department of Science and
Technology of Shandong province.
References
|
1
|
Menon R, Mohd Noor FS, Draman CR, Seman MR
and Ghani AS: A retrospective review of diabetic nephropathy
patients during referral to the sub-urban nephrology clinic. Saudi
J Kidney Dis Transpl. 23:1109–1114. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fernández IS, Cuevas P, Angulo J, et al:
Gentisic acid, a compound associated with plant defense and a
metabolite of aspirin, heads a new class of in vivo fibroblast
growth factor inhibitors. J Biol Chem. 285:11714–11729.
2010.PubMed/NCBI
|
|
3
|
Allain H, Ramelet AA, Polard E and
Bentué-Ferrer D: Safety of calcium dobesilate in chronic venous
disease, diabetic retinopathy and haemorrhoids. Drug Saf.
27:649–660. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ribeiro ML, Seres AI, Carneiro AM, Stur M,
Zourdani A, Caillon P and Cunha-Vaz JG; DX-Retinopathy Study Group:
Effect of calcium dobesilate on progression of early diabetic
retinopathy: a randomised double-blind study. Graefes Arch Clin Exp
Ophthalmol. 244:1591–1600. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cuevas P and Arrazola JM: Therapeutic
response of rosacea to dobesilate. Eur J Med Res. 10:454–456.
2005.PubMed/NCBI
|
|
6
|
Cuevas P and Arrazola JM: Dobesilate in
the treatment of plaque psoriasis. Eur J Med Res. 10:373–376.
2005.PubMed/NCBI
|
|
7
|
Reutens AT and Atkins RC: Epidemiology of
diabetic nephropathy. Contrib Nephrol. 170:1–7. 2011. View Article : Google Scholar
|
|
8
|
Liu HX and Han XY: The clinical
observation of the conductivity of the treatment of diabetic
nephropathy. J Hunan Med Univ. 19:423–425. 1994.(In Chinese).
|
|
9
|
Androulakis G and Panoysis PA:
Plethysmographic confirmation of the beneficial effect of calcium
dobesilate in primary varicose veins. Angiology. 40:1–4. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sinzinger H, Rauscha F and Vinazzer H:
Platelet function and prostaglandins in patients with peripheral
vascular disease treated with calcium dobesilate. Prostaglandins
Leukot Med. 29:1–9. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Benarroch IS, Brodsky M, Rubinstein A, et
al: Treatment of blood hyperviscosity with calcium dobesilate in
patients with diabetic retinopathy. Ophthalmic Res. 17:131–138.
1985. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sun SX, Wang H and Sun XQ: Calcium
dobesilate in the pharmacology and clinical application. J Hos
Pharma. 23:100–101. 2003.(In Chinese).
|
|
13
|
Zhong H and Guo L: The plasma levels of
endothelin in diabetic retinopathy and their changes after
treatment with doxium. Hunan Yi Ke Da Xue Xue Bao. 22:56–58.
1997.(In Chinese).
|
|
14
|
Yang JX: Clinical application of Calcium
dobesilate. Foreign medicine. 25:9–11. 1998.(In Chinese).
|
|
15
|
Tejerina T and Ruiz E: Calcium dobesilate:
pharmacology and future approaches. Gen Pharmacol. 31:357–360.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Simpson LO, Shand BI and Olds RJ: A
reappraisal of the influence of blood rheology on glomerular
filtration and its role in the pathogenesis of diabetic
nephropathy. J Diabet Complications. 1:137–144. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Simpson LO: Intrinsic stiffening of red
blood cells as the fundamental cause of diabetic nephropathy and
microangiopathy: a new hypothesis. Nephron. 39:344–351. 1985.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gordge MP, Patel A, Faint RW, et al: Blood
hyperviscosity and its relationship to progressive renal failure in
patients with diabetic nephropathy. Diabet Med. 7:880–886. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Költringer P, Eber O, Rothlauer W, Klima
G, et al: Calcium dobesilate and its effects on hemorheology and
microcirculation. Int J Clin Pharmacol Ther Toxicol. 26:500–502.
1988.
|
|
20
|
Vojnikovic B: Doxium (calcium dobesilate)
reduces blood hyperviscosity and lowers elevated intraocular
pressure in patients with diabetic retinopathy and glaucoma.
Ophthalmic Res. 23:12–20. 1991. View Article : Google Scholar
|
|
21
|
Yang JK, Yuan SY and Feng LZ: Calcium
dobesilate on the blood rheology and thrombosis formation in rats.
J Chin Microcirculation. 7:20–23. 2003.(In Chinese).
|