Introduction
HCC is the third leading cause of cancer mortality
in the world, and the second in China (1,2).
Even with improvement in surgical procedures and other adjuvant
therapies, rapid growth, high recurrence and metastasis are still
the main cause of treatment failure and cancer mortalities.
Angiogenesis is essential for the growth, invasion and metastasis
of solid tumors. Therefore, anti-angiogenesis therapy may inhibit
the development of HCC and prolong the lives of patients.
NRP-1 was first identified as a 120–130 kDa membrane
protein from the optic tract of Xenopus laevis(3). It functions as a semaphorin (SEMA)
receptor in the developing nervous system (4–6).
Subsequently, NRP-1 was found to be a co-receptor for vascular
endothelial growth factor 165 (VEGF 165) and is expressed in
endothelial cells (EC), where it is involved in the regulation of
angiogenesis and endothelial cell migration (7–9).
Overexpression of NRP-1 in a transgenic mouse model increased
capillary and blood vessel formation and resulted in hemorrhage
(10), whereas functional
inactivation of NRP-1 in mice led to embryonic lethality with
multiple vascular abnormalities, including of avascular regions,
heterogeneous blood vessel size and abnormally formed dorsal aorta
(11). These results indicated
that NRP-1 was a key regulator of developmental angiogenesis.
NRP-1 is also expressed in several tumors, including
glioma, acute myeloid leukemia, pancreatic, lung, breast, prostate,
colon and gastric cancers, where it regulates the growth, invasion
and metastasis of malignant tumors (12–20).
However, the role of NRP-1 in HCC progression remains unknown. In
this study, the endogenous NRP-1 expression in HCCLM6 cells was
knocked down using lentivirus-mediated RNA interference (RNAi), to
investigate the role of NRP-1 in regulating HCC progression.
Materials and methods
Cell line and laboratory animals
The human hepatoma-derived cell line HCCLM6, with a
high metastatic ability as evaluated by xenograft models, was
established by the Liver Cancer Institute (Fudan University,
Shanghai, China) and cultured in Dulbecco’s modified Eagle’s medium
(DMEM) (Gibco BRL, Grand Island, NY, USA) supplemented with 10%
fetal bovine serum (FBS). All cells were maintained at 37°C in a
humidified incubator containing 5% CO2. Five-week-old
male nude mice with an average weight of 20±5 g were purchased from
Institute of Materia Medica (CAS, Shanghai, China). The nude mice
were kept in specific pathogen-free conditions with lamina flow
devices in accordance with related regulations. The study was
approved by the ethics committee of Zhongshan Hospital (Fudan
University, China).
Construction and transfection of
lentiviral vectors with specific shRNA for NRP-1
The RNAi candidate sequences for human NRP-1 were
designed according to a previous study (13) and the detailed sequences were as
follows: sense, 5′-GAGAGGTCCTGAATGTTCC-3′; anti-sense,
5′-GGAACATTCAGGACCTCTC-3′. Stem-loop DNA oligonucleotides were
synthesized and cloned into the lentivirus-based RNAi vector
pLVTHM. The negative control plasmid was formed by an empty vector
pLVTHM. Lentiviral particles were prepared as described previously
(21). HCCLM6 cells were seeded in
six-well plates at a concentration of 5×105 per well.
Lentivirus transfection was conducted when the cells reached 70–80%
confluence. Cells were divided into three groups as follows: the
knockdown (KD) cells were transfected with NRP-1 shRNA lentivirus
(MOI 30); the negative control (NC) cells were transfected with
empty lentivirus (MOI 30) and the blank control (BC) cells were not
transfected. After 48 h, transfection efficiency was detected using
flow cytometry (BD Pharmingen, San Diego, CA, USA).
Real-time PCR assay
Total RNA extraction was carried out using the
TRIzol Reagent (Invitrogen, Carlsbad, CA, USA) according to the
manufacturer’s instructions. RNA quality was assured by the
A260/280 absorbance ratio and 0.5 μg total RNA was reverse
transcribed into single-strand cDNA using PrimeScript™ RT Enzyme
Mix I (Takara, Kyoto, Japan). RT-PCR was carried out for 15 min at
37°C and 5 sec at 85°C in a thermocycler. For the NRP-1 gene, 2.0
μl cDNA template was used for routine PCR in a final volume
of 20 μl. The forward primer 5′-TCCCGCCTGAACTACCCTTGAGA-3′
and the reverse primer 5′-TTTGAAATGGCGCCCTGTGTCC-3′ were used and
amplified in one cycle at 95°C for 10 sec, then 40 cycles at 95°C
for 5 sec and 60°C for 30 sec. To measure the relative amount of
the NRP-1 gene in the different samples, the glyceraldehyde
3-phosphate dehydrogenase (GAPDH) level was determined and employed
as a control. The PCR was performed in a DNA Engine Opticon system
(MJ Research, Reno, NV, USA) by using SYBR®-Green PCR
Master mix (Takara, Kyoto, Japan). The ΔΔCt method was used for
relative quantitative comparison among samples (22).
Western blot analysis
Approximately 20 mg total protein extracted from
groups of cells were separated on 6% sodium dodecyl sulphate
polyacrylamide gel (SDS-PAGE) and transferred onto polyvinylidene
fluoride membranes (Millipore, MA, USA). After blocking the
membranes, the diluted primary antibodies against NRP-1 and GAPDH
(Santa Cruz Biotechnology, CA, USA) were incubated for 24 h at 4°C.
After extensive washing in Tris-buffered saline (TBS) buffer, the
membranes were incubated with horseradish peroxidase-conjugated
goat anti-mouse secondary antibody for 1 h at room temperature.
Labeled proteins on western blots were visualized using the
Chemiluminescence Reagent Plus detection system (New England
Nuclear, Boston, MA, USA). Quantification of bands was carried out
using an Alpha Imager (Alpha Innotech, San Leandro, CA, USA).
MTT analysis
Three groups of cells were individually seeded into
96-well plates at a concentration of 7×103 per well
filled with DMEM supplemented with 10% FBS. After 24 h, the medium
was removed and replaced with fresh medium. One plate was examined
immediately after the medium change and other plates were analyzed
every 24 h for 3 days. Assays were initiated by adding 20 μl
MTT (5 mg/ml) to each well and incubating the cells for an
additional 4 h. Finally, the medium was removed and 150 μl
dimethylsulphoxide (DMSO) was added to each well. Plates were read
at a wavelength of 570 nm.
In vivo tumor model
Eighteen nude mice were randomly divided into 3
groups and each was subcutaneously injected with 1×107
KD cells, NC cells or BC cells, respectively, in the right upper
flank region, for the establishment of subcutaneous xenograft
models. Every 5 days after the injection, tumors in the mice were
observed visually. Mice were sacrificed at 50 days and tumor mass
and volume were recorded. Volume was calculated as (length/2) ×
(width2). Then NRP-1 protein expression in tumors was
detected by western blot analysis as described above.
Immunohistochemistry
One xenograft from each group was formalin-fixed,
paraffin-embedded and sectioned into 4-μm-thick sections.
Then, sections were deparaffinized in xylene and treated with a
graded series of alcohol [100, 95 and 80% (V/V) ethanol in
double-distilled water] and rehydrated in phosphate-buffered saline
(pH 7.5). For MVD assessment, the slides were microwaved for 5 min
for antigen retrieval. Then rat monoclonal anti-CD34 antibody
(Abcam, Cambridgeshire, UK) was added and incubated at room
temperature for 2 h. Afterwards, horseradish peroxidase-conjugated
goat anti-rat secondary antibody (Beyotime, Shanghai, China) was
added and incubated for a further 1 h at room temperature. Slides
were incubated with stable 3,3′-diaminobenzidine (DAB) for 10–15
min, and then rinsed with distilled water and counter-stained with
Gill’s hematoxylin (Sigma, St. Louis, MO, USA) for 1 min. Then
slides were observed under a Leica CTR 5000 microscope system
(Leica Microsystems, Hong Kong, China) to count MVD as described
previously (23).
Statistical analysis
Statistical analysis was performed using SPSS 13.0
software (SPSS Inc., Chicago, IL, USA) using a Student’s t-test
throughout the present study. P<0.05 was considered to indicate
a statistically significant difference.
Results
NRP-1 shRNA lentivirus significantly
suppressed mRNA and protein expression of NRP-1
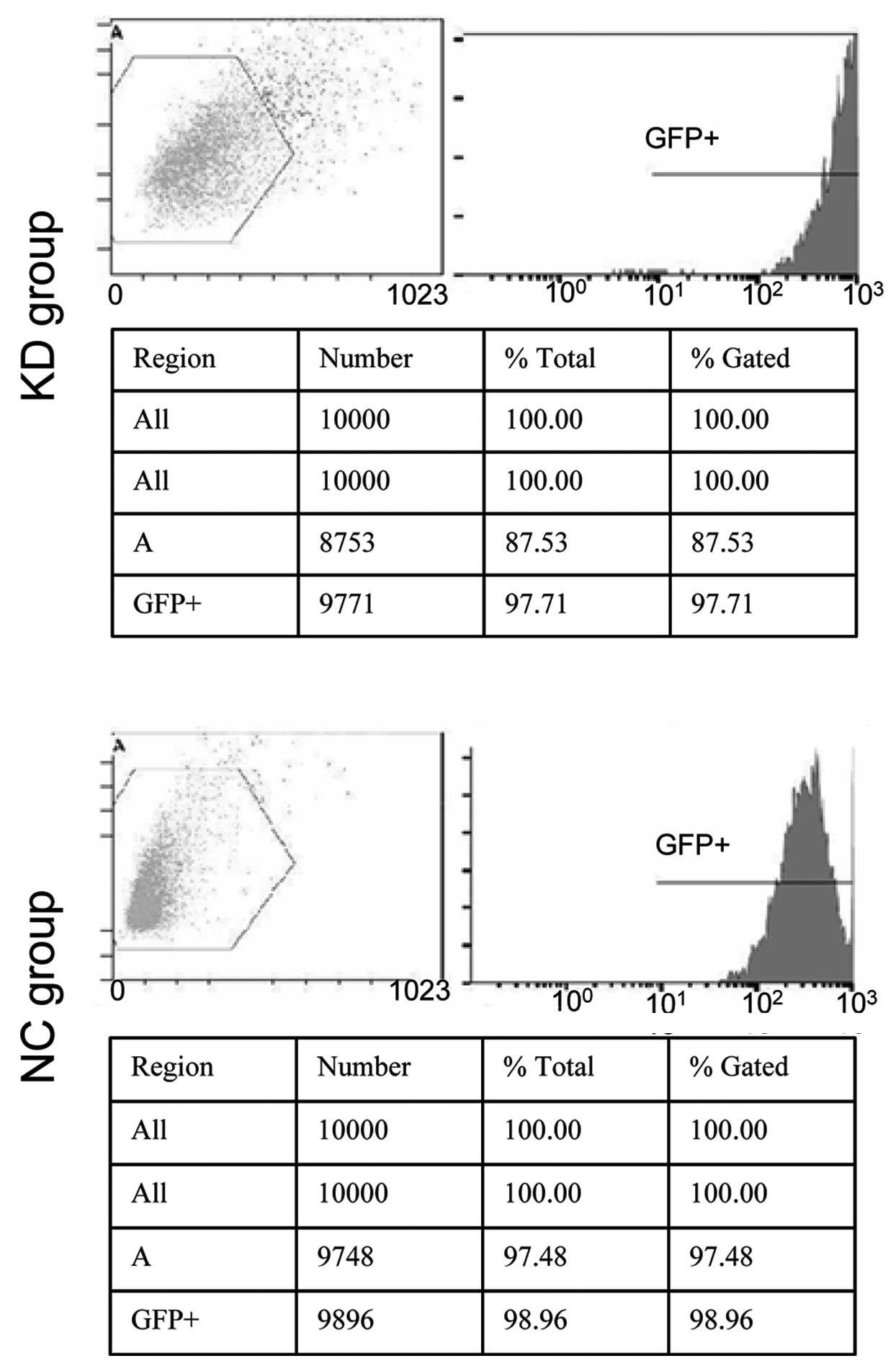
After 48 h transfection, green fluorescent protein
(GFP) expression rates of the KD and NC cells were 97.71 and
98.96%, respectively (Fig. 1). The
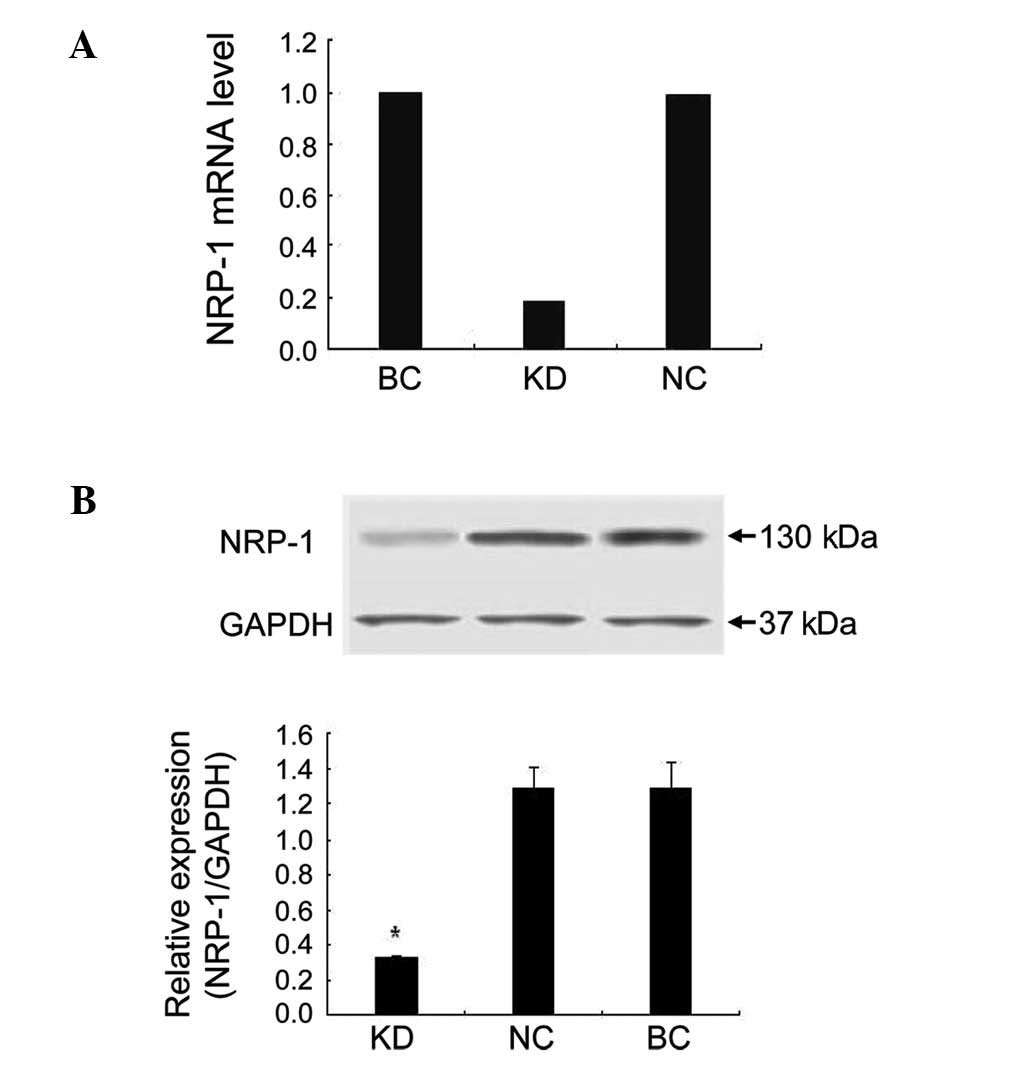
real-time PCR results demonstrated that NRP-1 shRNA had a
significant suppressive effect. The NRP-1 mRNA level of the KD
group decreased by 81.5% compared to the BC group. In the NC group
cells transfected with empty lentivirus, the expression of NRP-1
mRNA was barely affected (Fig.
2A). In the western blot assay, NRP-1 protein expression was
inhibited by 74.9% in the KD group compared to the BC group
(P<0.05). No obvious variance was found between the NC and BC
group (P>0.05; Fig. 2B).
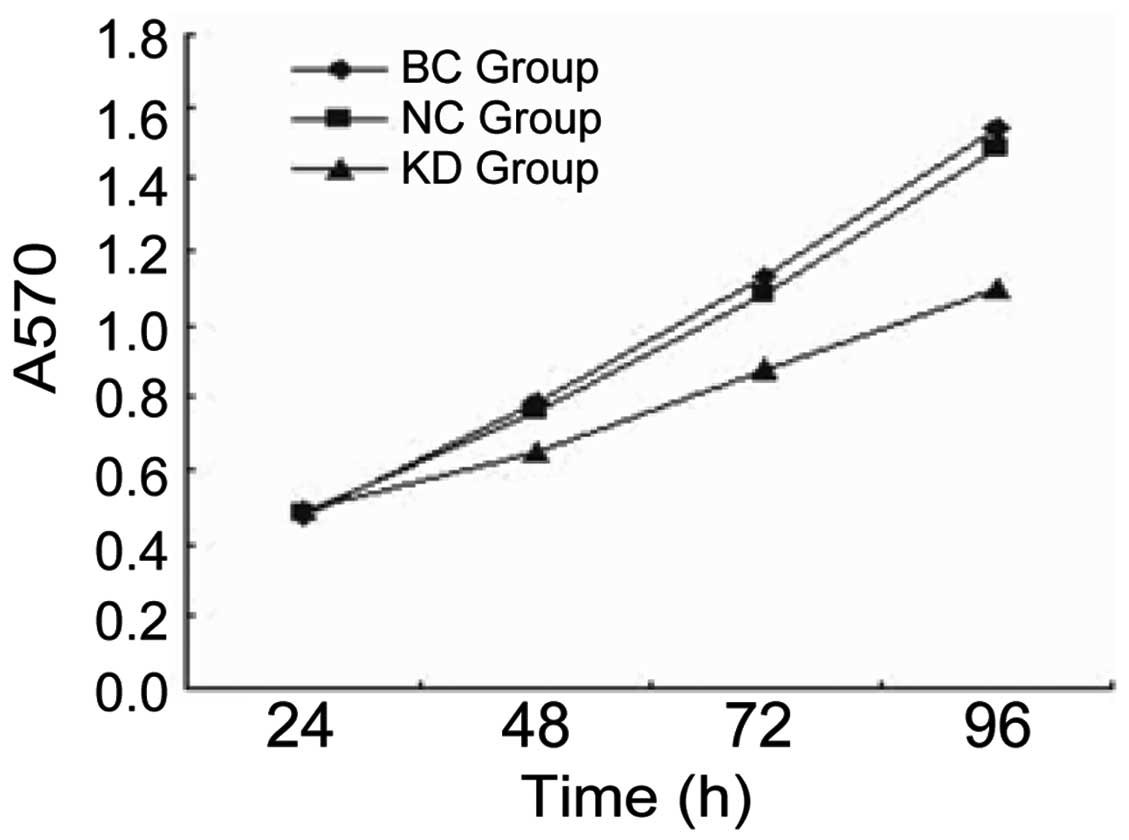
NRP-1 shRNA lentivirus led to inhibition
of cell growth in vitro
For the MTT assay, the growth rate of the KD group
was significantly lower than that of the BC and NC groups
(P<0.05). There was no significant difference between the NC and
BC group (P>0.05; Fig. 3).
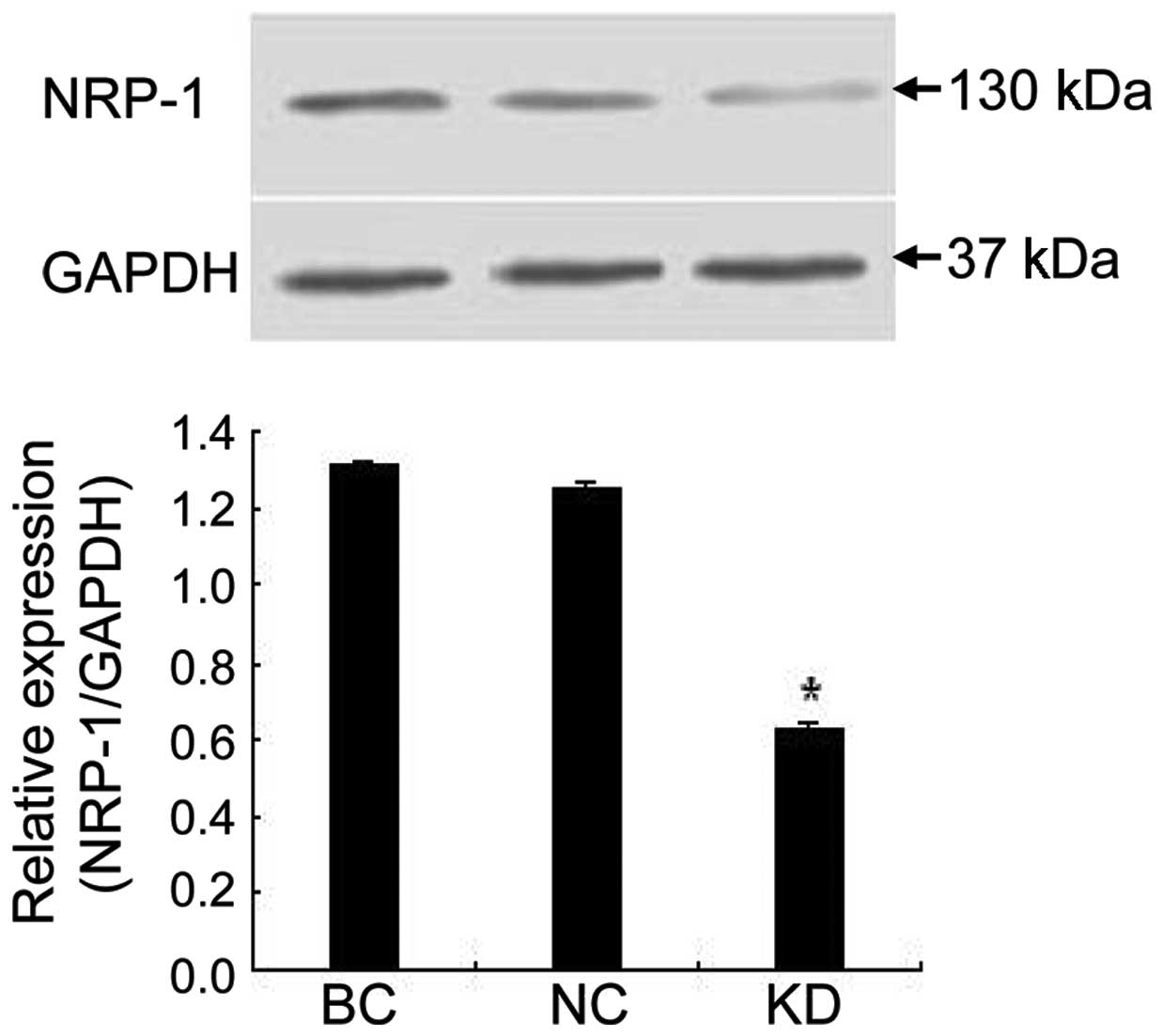
NRP-1 shRNA lentivirus suppressed NRP-1
protein expression and tumor growth in xenografts
As shown in Fig. 4,
NRP-1 shRNA also caused significant inhibition of NRP-1 protein
expression in xenograft tumor tissue. NRP-1 protein expression was
inhibited by 52.2% in the KD group compared to the BC group
(P<0.05) while little variance was found between the NC and BC
group (P>0.05). Additionally, we found that tumors in the KD
group were smaller in size and lighter in weight than those in the
BC group (P<0.05), while no obvious difference was found between
the NC and BC group (P>0.05; Table
I).
 | Table IXenograft tumors in the KD, NC and BC
group. |
Table I
Xenograft tumors in the KD, NC and BC
group.
| Group | Volume
(cm3) | P-value | Weight (g) | P-value |
|---|
| KD | 1.34±0.12 | 0.000 | 1.39±0.18 | 0.000 |
| NC | 1.46±0.14 | 0.11 | 1.98±0.16 | 0.089 |
| BC | 1.43±0.12 | - | 1.96±0.13 | - |
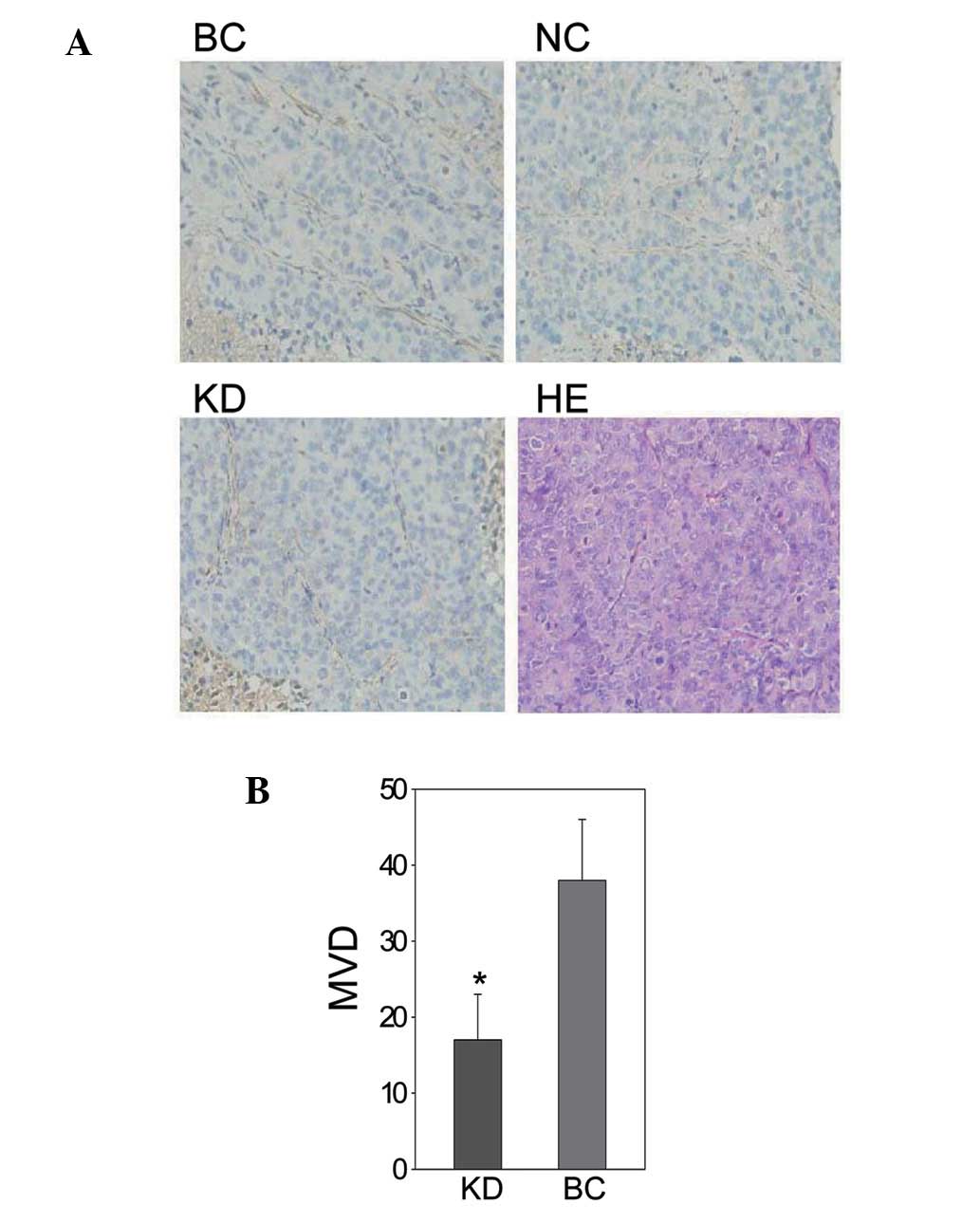
NRP-1 shRNA lentivirus led to decreased
angiogenesis in vivo
As the results of the immunohistochemical analysis
showed, NRP-1 shRNA had a suppressive effect on the
neovascularization and angiogenesis of tumors. The MVD of the KD
group was 17±6, lower than that of the BC group (38±8; P<0.05)
while no significant difference was found between the NC group
(34±7) and the BC group (P>0.05; Fig. 5).
Discussion
In this study, NRP-1 shRNA lentivirus caused
inhibition of NRP-1 mRNA and protein expression, thus suppressing
HCC cell growth in vitro. In vivo, it also downregulated
NRP-1 protein expression in xenograft tumors, whose growth and
angiogenesis were significantly suppressed. These findings
demonstrated that NRP-1 played an important role in HCC growth by
promoting neovascularization and angiogenesis.
Considerable experimental evidence has shown that
NRP-1 plays an essential role in the tumor growth and metastasis by
regulating angiogenesis. The majority of studies supported that
NRP-1 functioned as a co-receptor of VEGF 165 and enhanced vascular
endothelial growth factor receptor-2 (VEGFR-2) activity in the
presence of VEGF (9,17,19,24,25).
However, several papers studying tumor cells lacking VEGFR-2
expression have suggested that NRP-1 may transduce VEGF-mediated
signals either alone or in concert with other receptors (13,15,26,27).
Recently, NRP-1 was found to promote tumor progression by
interacting with other proteins, such as integrin beta-1 and
hepatocyte growth factor/scatter factor (18,20).
However, the exact pathway mediated by NRP-1 in angiogenesis of HCC
needed further investigation.
The NRP-1 shRNA lentivirus achieved an effective
gene silence in vitro, as NRP-1 protein expression was
decreased by 74.9% in transfected cells. However, in the xenograft
tumors, NRP-1 shRNA only led to a 52.2% decrease of NRP-1 protein.
Theoretically, NRP-1 shRNA should have the same gene silencing
effect in vivo as in vitro. Many reasons may be
responsible for the difference. One is that the tumor tissue
removed from mice for protein extraction not only included HCC
cells, but also vascular endothelial cells (ECs), mononuclear cells
and so on, which may have normal NRP-1 protein expression.
HCC is a hypervascular tumor and a rich vascular bed
providing the necessary nutrients for tumor growth and providing a
channel for tumor invasion and metastasis. Knockdown of NRP-1 by
the NRP-1 shRNA lentivirus led to a significant reduction of tumor
MVD, significantly inhibiting the growth of HCC. In conclusion,
NRP-1 is a potential target for anti-angiogenetic therapy for
HCC.
Acknowledgements
This study was supported by the
Natural Science Foundation of Shanghai Municipality (Grant No.
07ZR14021).
References
|
1
|
Tang ZY: Hepatocellular carcinoma-cause,
treatment and metastasis. World J Gastroenterol. 7:445–454.
2001.PubMed/NCBI
|
|
2
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar
|
|
3
|
Fujisawa H, Takagi S and Hirata T:
Growth-associated expression of a membrane protein, neuropilin, in
Xenopus optic nerve fibers. Dev Neurosci. 17:343–349. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kolodkin AL, Levengood DV, Rowe EG, Tai
YT, Giger RJ and Ginty DD: Neuropilin is a semaphorin III receptor.
Cell. 90:753–762. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
He Z and Tessier-Lavigne M: Neuropilin is
a receptor for the axonal chemorepellent Semaphorin III. Cell.
90:739–751. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rossignol M, Gagnon ML and Klagsbrun M:
Genomic organization of human neuropilin-1 and neuropilin-2 genes:
identification and distribution of splice variants and soluble
isoforms. Genomics. 70:211–222. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bernatchez PN, Rollin S, Soker S and
Sirois MG: Relative effects of VEGF-A and VEGF-C on endothelial
cell proliferation, migration and PAF synthesis: role of
neuropilin-1. J Cell Biochem. 85:629–639. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lee P, Goishi K, Davidson AJ, Mannix R,
Zon L and Klagsbrun M: Neuropilin-1 is required for vascular
development and is a mediator of VEGF-dependent angiogenesis in
zebrafish. Proc Natl Acad Sci U S A. 99:10470–10475. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Soker S, Takashima S, Miao HQ, Neufeld G
and Klagsbrun M: Neuropilin-1 is expressed by endothelial and tumor
cells as an isoform-specific receptor for vascular endothelial
growth factor. Cell. 92:735–745. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kitsukawa T, Shimono A, Kawakami A, Kondoh
H and Fujisawa H: Overexpression of a membrane protein, neuropilin,
in chimeric mice causes anomalies in the cardiovascular system,
nervous system and limbs. Development. 121:4309–4318.
1995.PubMed/NCBI
|
|
11
|
Takashima S, Kitakaze M, Asakura M, et al:
Targeting of both mouse neuropilin-1 and neuropilin-2 genes
severely impairs developmental yolk sac and embryonic angiogenesis.
Proc Natl Acad Sci U S A. 99:3657–3662. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hansel DE, Wilentz RE, Yeo CJ, Schulick
RD, Montgomery E and Maitra A: Expression of neuropilin-1 in
high-grade dysplasia, invasive cancer, and metastases of the human
gastrointestinal tract. Am J Surg Pathol. 28:347–356. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bachelder RE, Lipscomb EA, Lin X, et al:
Competing autocrine pathways involving alternative neuropilin-1
ligands regulate chemotaxis of carcinoma cells. Cancer Res.
63:5230–5233. 2003.PubMed/NCBI
|
|
14
|
Stephenson JM, Banerjee S, Saxena NK,
Cherian R and Banerjee SK: Neuropilin-1 is differentially expressed
in myoepithelial cells and vascular smooth muscle cells in
preneoplastic and neoplastic human breast: a possible marker for
the progression of breast cancer. Int J Cancer. 101:409–414. 2002.
View Article : Google Scholar
|
|
15
|
Ochiumi T, Kitadai Y, Tanaka S, Akagi M,
Yoshihara M and Chayama K: Neuropilin-1 is involved in regulation
of apoptosis and migration of human colon cancer. Int J Oncol.
29:105–116. 2006.PubMed/NCBI
|
|
16
|
Kreuter M, Woelke K, Bieker R, et al:
Correlation of neuropilin-1 overexpression to survival in acute
myeloid leukemia. Leukemia. 20:1950–1954. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lu L, Zhang L, Xiao Z, Lu S, Yang R and
Han ZC: Neuropilin-1 in acute myeloid leukemia: expression and role
in proliferation and migration of leukemia cells. Leuk lymphoma.
49:331–338. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hu B, Guo P, Bar-Joseph I, et al:
Neuropilin-1 promotes human glioma progression through potentiating
the activity of the HGF/SF autocrine pathway. Oncogene.
26:5577–5586. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hong TM, Chen YL, Wu YY, et al: Targeting
neuropilin 1 as an antitumor strategy in lung cancer. Clin Cancer
Res. 13:4759–4768. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fukasawa M, Matsushita A and Korc M:
Neuropilin-1 interacts with integrin beta1 and modulates pancreatic
cancer cell growth, survival and invasion. Cancer Biol Ther.
6:1173–1180. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lois C, Hong EJ, Pease S, Brown EJ and
Baltimore D: Germline transmission and tissue-specific expression
of transgenes delivered by lentiviral vectors. Science.
295:868–872. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-DeltaDeltaC(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Weidner N, Semple JP, Welch WR and Folkman
J: Tumor angiogenesis and metastasis-correlation in invasive breast
carcinoma. New Engl J Med. 324:1–8. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Miao HQ and Klagsbrun M: Neuropilin is a
mediator of angiogenesis. Cancer Metastasis Rev. 19:29–37. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Miao HQ, Lee P, Lin H, Soker S and
Klagsbrun M: Neuropilin-1 expression by tumor cells promotes tumor
angiogenesis and progression. Faseb J. 14:2532–2539. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bachelder RE, Crago A, Chung J, et al:
Vascular endothelial growth factor is an autocrine survival factor
for neuropilin-expressing breast carcinoma cells. Cancer Res.
61:5736–5740. 2001.PubMed/NCBI
|
|
27
|
Barr MP, Byrne AM, Duffy AM, et al: A
peptide corresponding to the neuropilin-1-binding site on VEGF(165)
induces apoptosis of neuropilin-1-expressing breast tumour cells.
Br J Cancer. 92:328–333. 2005.PubMed/NCBI
|