Introduction
Previous experimental evidence suggests that nerve
root inflammation, with or without concomitant nerve compression,
is an important contributing factor to sciatica or radicular pain
(1). Human herniated discs have
been shown to express a number of pro-inflammatory mediators,
including tumor necrosis factor-α (TNF-α), interleukin-1 (IL-1),
IL-6 and IL-8 (1). TNF-α and its
receptor are reportedly upregulated in dorsal root ganglion (DRG)
neurons following lumbar injury in rats (2,3). The
application of recombinant TNF-α to the lumbar nerve roots of
rodents induces mechanical allodynia and hyperalgesia (1). Etanercept, a recombinant TNF receptor
(p75)-Fc fusion protein, competitively inhibits TNF-α (4). Sommer et al(4) reported that etanercept reduced pain
and hyperalgesia in a rat model of painful neuropathy induced by
the chronic constriction injury (CCI) of the sciatic nerve.
High mobility group box 1 (HMGB1) is a part of the
nucleic acid-sensing system and binds to immunogenic nucleotides in
order to activate innate immune responses during microbial
infection and tissue damage (5).
Biologically active HMGB1 is expressed on the plasma membrane or is
secreted into the extracellular milieu where it acts as a cytokine
and interacts with the receptor for advanced glycation end products
(RAGE) and toll-like receptor (TLR)-2, TLR4 and TLR9 (6,7). It
has been reported that the induction of HMGB1 in DRGs contributes
to pain hypersensitivity following peripheral nerve injury
(8) and an anti-HMGB1
neutralization antibody improves pain-related behavior induced by
the application of autologous nucleus pulposus onto nerve roots in
rats (9). It has been suggested
that HMGB1 plays an important role in the pathophysiology of CCI
and sciatica-related nociception.
In the present study, we for the first time examined
the effect of etanercept on HMGB1 expression in DRG neuron cells in
a rat CCI model, with the aim of exploring the molecular mechanism
underlying the therapeutic effect of etanercept on sciatica-related
nociception and the potential interaction between TNF-α and HMGB1
in DRG neuron cells.
Materials and methods
Animals
Male inbred Sprague-Dawley rats (weight 250–300 g)
were purchased from Central South University and were housed at the
Xiangya Hospital BioResources Centre. Animals were placed in a
quiet, temperature (22±2°C) and humidity (60±6%) controlled room
with a 12:12 h light-dark cycle (light beginning at 8 a.m.) and all
tests were performed during the light phase of the cycle.
Pharmaceutical-grade etanercept was purchased from Amgen (Thousand
Oaks, CA, USA). Anti-HMGB1 (sc-12523) antibody was purchased from
Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). Anti-p38
mitogen-activated protein kinase (p38 MAPK; #8690),
anti-phospho-p38-MAPK (Thr180/Tyr182; #9211) and
anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH; #2118)
antibodies were purchased from Cell Signaling Technology (Beverly,
MA, USA). All secondary antibodies were from Jackson ImmunoResearch
Laboratories (West Grove, PA, USA). All chemicals of reagent grade
were purchased from Sigma (St. Louis, MO, USA).
Establishment of rat CCI model and
treatment groups
CCI was induced according to the method of Bennett
and Xie (10). Briefly, each
animal was anesthetized by an intraperitoneal injection of sodium
pentobarbital at a dose of 60 mg/kg. The common sciatic nerve was
exposed and freed from adherent tissue at the mid-thigh by
separating the biceps femoris muscles by blunt dissection. Four
loose ligatures were placed 1 mm apart using chromic gut suture
(4-0 absorbable suture; Jorgensen Laboratories, Inc., Loveland, CO,
USA). The animals were randomly assigned to seven groups
(n=20/group): untreated, sham only (animals subjected to sham
surgery only), sham/saline (animals subjected to sham surgery plus
intrathecal injection of 20 μl saline every two days from
two days before surgery), sham/etanercept [animals subjected to
sham surgery plus intrathecal injection of 20 μl (100
μg) etanercept every two days from two days before surgery],
CCI only (animals subjected to CCI surgery only), CCI/saline
(animals subjected to CCI surgery plus intrathecal injection of 20
μl saline every two days from two days before surgery) and
CCI/etanercept group [animals subjected to CCI surgery plus
intrathecal injection of 20 μl (100 μg) etanercept
every two days from two days before surgery]. This study was
conducted in accordance with our institutional guidelines on the
use of live animals for research and the experimental protocol was
approved by the Laboratory Animal Users Committee at Xiangya
Hospital, Central South University.
Behavior examination
Thermal hyperalgesia was measured according to the
Hargreaves test (1) using a
plantar analgesia instrument (Stoelting, Wood Dale, IL, USA) every
day from one day before surgery to 13 days after surgery. The
radiant infrared heat source stimulus intensity was set to IR50 and
the cut-off time was set at 15 sec. The rats were placed on a glass
platform and allowed to habituate to the testing chambers for a
minimum of 15 min prior to each testing session. The thermal
stimulus was applied to the plantar surface of the paw. Thermal
thresholds were defined as the latency in seconds at the first pain
behavior, which includes paw withdrawal, flinching, biting and/or
licking of the stimulated paw. The readings for all animals were
averaged and the mean and standard error of the mean were
determined for each treatment group. Mechanical allodynia was
measured using von Frey monofilaments (Stoelting) with varying
stiffness (2.0–15.0 g) every day from one day before surgery to 13
days after surgery. The rats were placed on a perforated metallic
platform and allowed to habituate to their surroundings for a
minimum of 15 min before testing. The paw withdrawal threshold
response was determined by a sequential increasing and/or
decreasing of the stimulus strength.
DRG neuron cell isolation and real-time
quantitative reverse transcription-polymerase chain reaction
(RT-PCR)
On days 3, 7 and 13 after surgery, four randomly
selected rats were sacrificed at each time point and DRG neuron
cells were isolated from the enlarged part of the lumbar spinal
cord as previously described (11). Cells were used for experiments
24–48 h after isolation. DRG neurons used for mRNA extraction were
stored at −80°C immediately after isolation. RNA samples were
prepared using TRIzol reagent followed by purification with Turbo
DNA-free system (Ambion, Austin, TX, USA). The cDNAs were
synthesized using SuperScript II reverse transcriptase (Invitrogen,
Carlsbad, CA, USA). Real-time quantitative PCR was performed on the
LightCycler thermal cycler system (Roche Diagnostics, Indianapolis,
IN, USA) using a SYBR-Green I kit (Roche Diagnostics) as described
by the manufacturer. Each result was normalized against that of the
housekeeping gene GAPDH in the same sample. The primers used were
as follows: for rat HMGB1, 5′-GTACGGTACCAAGTGCATTT TGGAGGAATT-3′
(forward) and 5′-GTACAAGCTTGTACT GCAATGGCTGTGAGA-3′ (reverse) and
for rat GAPDH, 5′-AAGCCCATCACCATCTTCCA-3′ (forward) and
5′-CCTGCTTCACCACCTTCTTG -3′ (reverse). Each experiment was repeated
twice in triplicate.
Western blot analysis
Immunoblotting was performed as described previously
with respective antibodies (12).
Briefly, DRG neuron cells were lysed in 0.1% Nonidet P-40 lysis
buffer [(0.1% Nonidet P-40, 50 mM Tris-HCl (pH 7.4), 150 mM NaCl
and 1 mM ethylenediamine tetraacetic acid (EDTA)]. Equal amounts of
lysates were loaded onto 10% sodium dodecyl sulphate
(SDS)-polyacrylamide gels and the proteins were blotted onto a
polyvinylidene difluoride microporous membrane (Millipore,
Billerica, MA, USA). The membranes were incubated for 1 h with a
1/1,000 dilution of anti-HMGB1, anti-p38 MAPK, anti-phospho-p38
MAPK (Thr180/Tyr182) or anti-GAPDH antibodies and then washed and
revealed using secondary antibodies with a horseradish peroxidase
conjugate (1/5,000, 1 h). Peroxidase was revealed with an ECL kit
(GE Healthcare Life Sciences, Piscataway, NJ, USA). The proteins
were quantified before being loaded onto the gel and equal loading
of extracts was verified by Ponceau coloration.
Statistical analysis
Statistical analyses were performed with SPSS (SPSS
Inc., Chicago, IL, USA) for Windows 10.0. Data values were
expressed as the mean ± standard deviation. Comparisons of means
among multiple groups were performed with one-way analysis of
variance (ANOVA) followed by post hoc pairwise comparisons using
the least significant difference method. P<0.05 was considered
to indicate a statistically significant difference.
Results
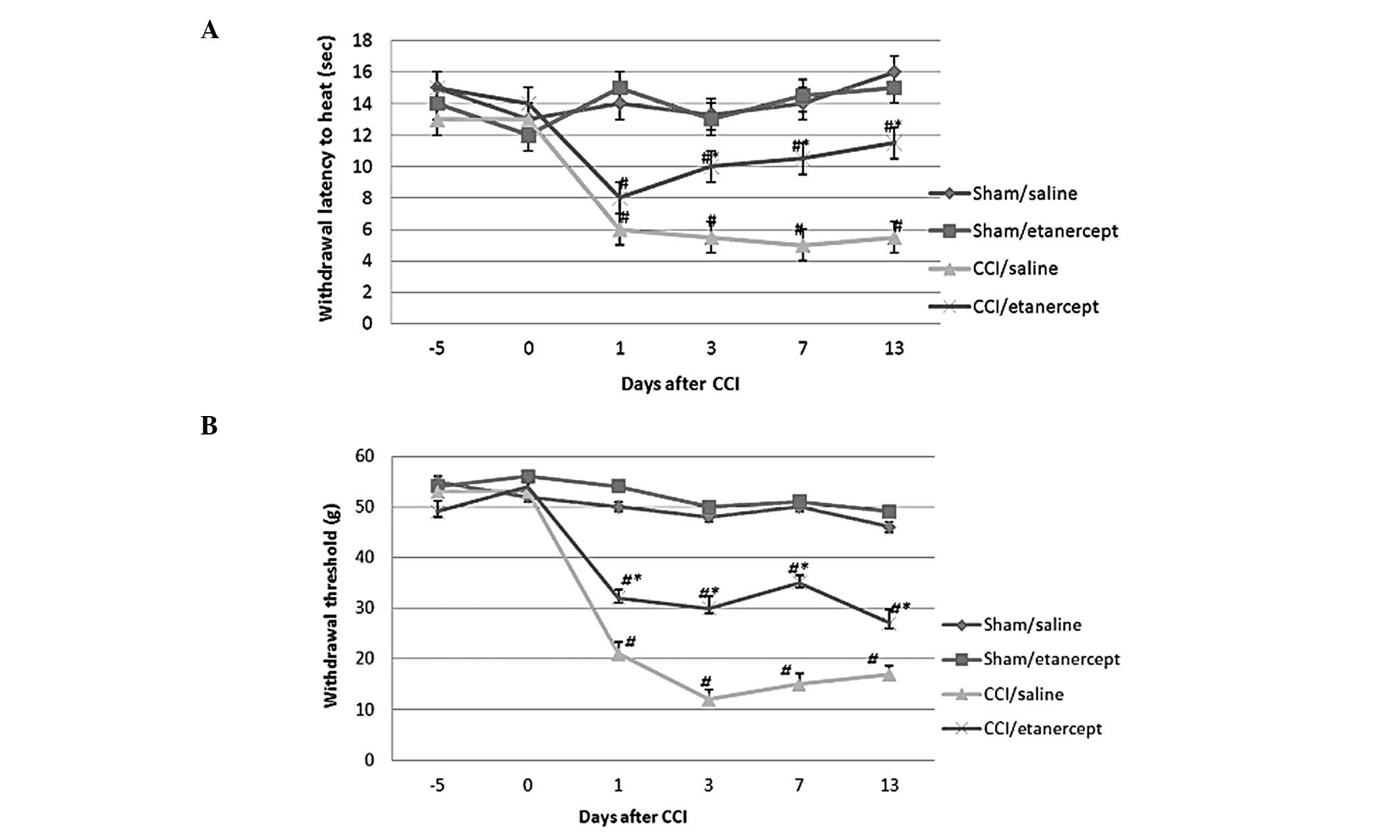
As shown in Fig. 1,
compared with the sham/saline and sham/etanercept groups, thermal
and mechanical hyperalgesia were induced by CCI on all testing
days. Although etanercept showed no significant effect on paw
withdrawal latency and threshold in the sham group, it
significantly inhibited the thermal and mechanical hyperalgesia
induced by CCI. The untreated and sham only groups showed no
significant difference from the sham/saline and the sham/etanercept
groups. The CCI only group showed no significant difference from
the CCI/saline group (data not shown).
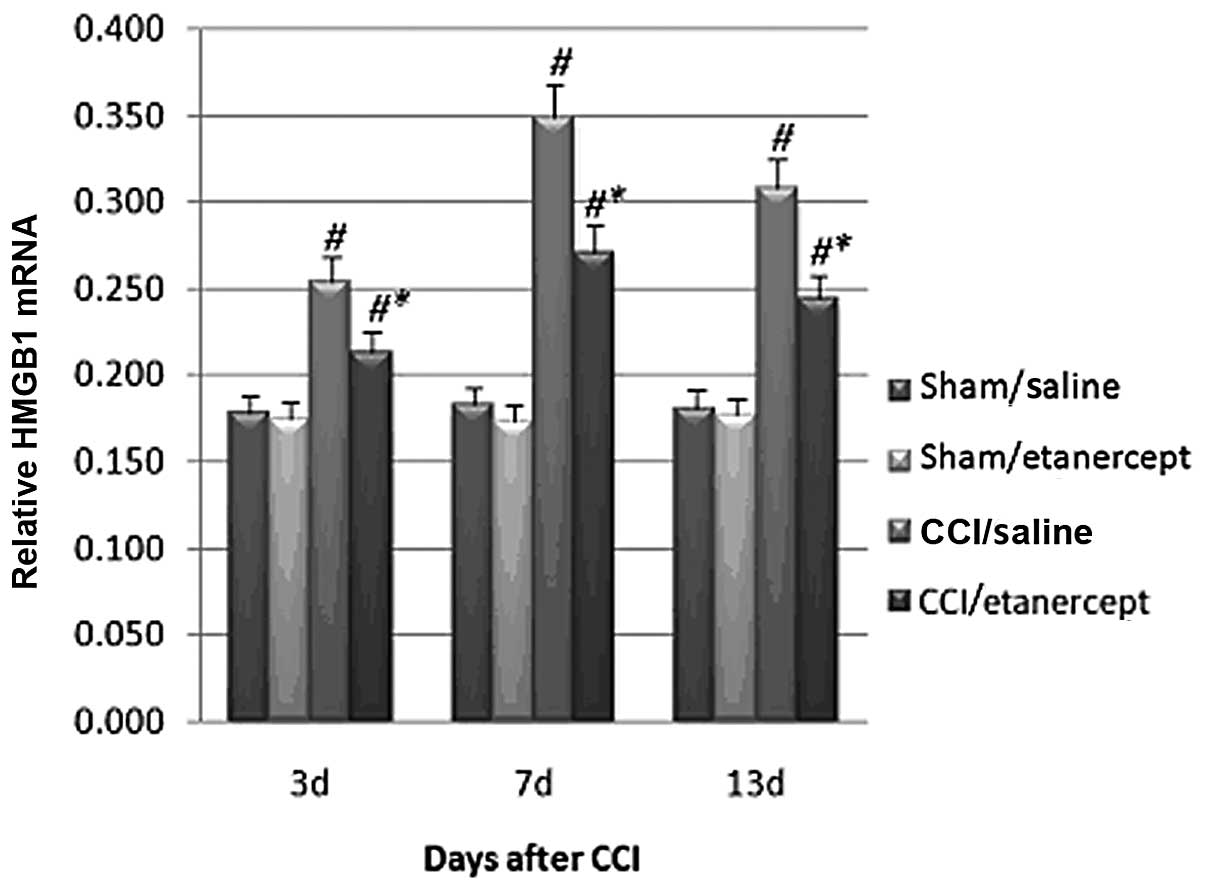
Real-time RT-PCR revealed that compared with those
in the sham/saline and sham/etanercept groups, the HMGB1 mRNA
levels in the DRG neuron cells were significantly increased by CCI
on all testing days (Fig. 2).
Treatment with etanercept showed no significant effect on the sham
group. By contrast, it significantly reduced CCI-induced HMGB1 mRNA
level (Fig. 2).
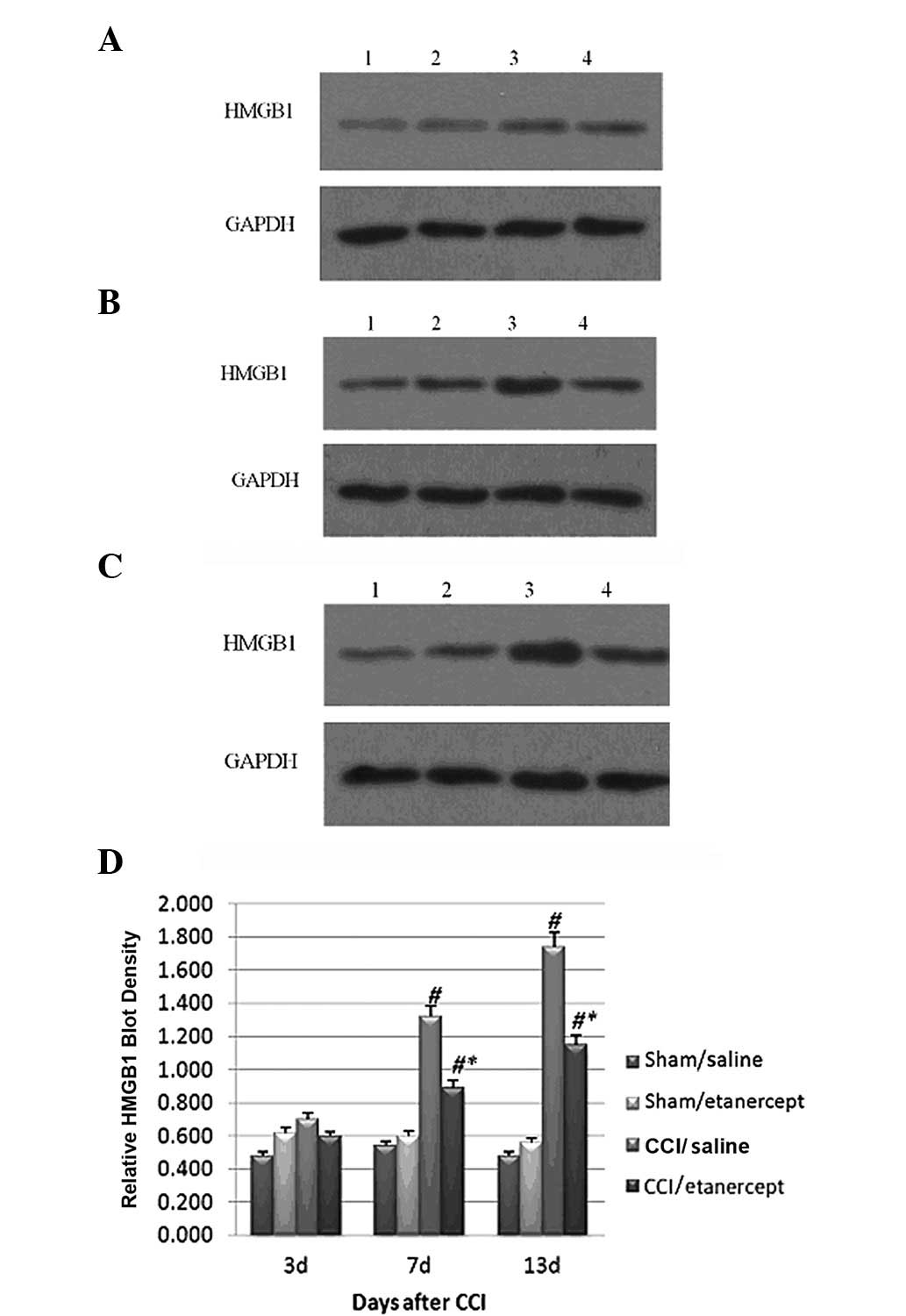
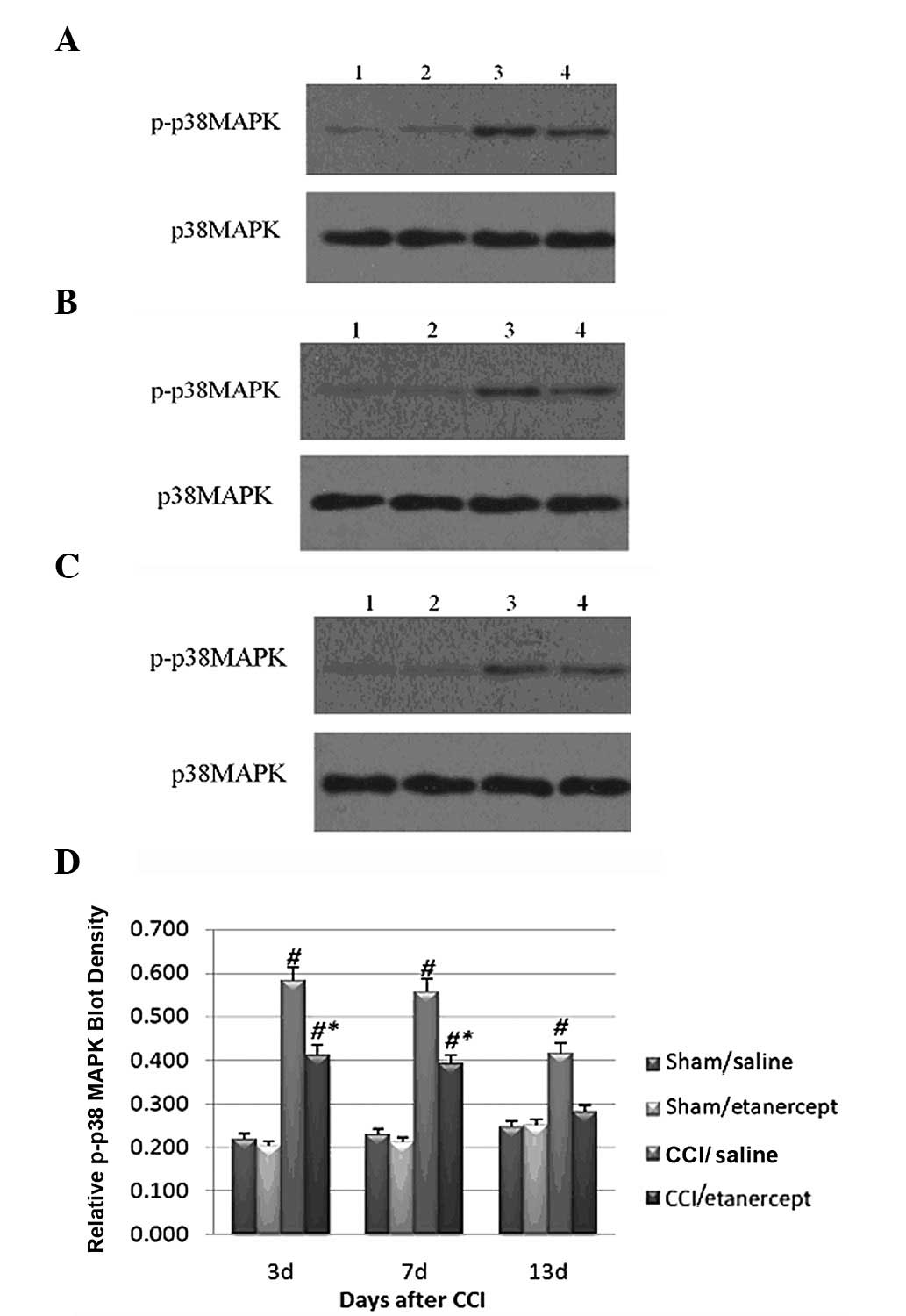
Western blot analysis showed that the HMGB1 protein
expression (Fig. 3) and
phosphorylated p38 MAPK levels (Fig.
4) were significantly induced by CCI on days 7 and 13 after
surgery compared with those of the sham/saline and sham/etanercept
groups,. Although etanercept showed no significant effect on the
sham group, it significantly decreased the HMGB1 protein expression
(Fig. 3) and phosphorylated p38
MAPK levels induced by CCI (Fig.
4).
Discussion
Etanercept, a TNF-α inhibitor, reportedly exerts
therapeutic effects on neuropathic pain in a rat CCI model
(4). In the present study, we
report for the first time that etanercept significantly decreased
HMGB1 expression in DRG neuron cells in a rat CCI model, which
provides fresh insights into the molecular mechanism underlying the
therapeutic effect of etanercept on sciatica-related
nociception.
HMGB1, which is abundantly expressed and presented
in virtually all human cell types (13), functions as a cytokine (5). The induction of HMGB1 in DRGs
reportedly contributes to pain hypersensitivity following
peripheral nerve injury (8). In
line with these reports, our study demonstrated that CCI
significantly induced HMGB1 expression in DRG neuron cells, as well
as thermal hyperalgesia and mechanical hyperalgesia.
TNF-α plays a pivotal role in CCI and
sciatica-related nociception (1)
and is upregulated in DRG neurons following spinal cord injury
(2). It has been reported that
HMGB1 is released from activated macrophages partly in a
TNF-dependent manner (14). Our
study demonstrated that etanercept, a TNF-α inhibitor,
significantly decreased HMGB1 expression in DRG neuron cells,
providing indirect evidence that TNF-α regulates HMGB1 expression
in DRG neurons.
p38 MAPK is activated by phosphorylation at Thr180
and Tyr182 in response to inflammatory cytokines and stress, making
it an important enzyme in diseases such as asthma and autoimmune
disorders, as well as in the stress response of the nervous system
(15). Kawahara et
al(16) revealed that p38 MAPK
is required for the active release of HMGB1 in macrophage cells.
Kikuchi et al(17) reported
that HMGB1 release from PC12 neuronal cells was blocked by a p38
MAPK inhibitor. In the current study, etanercept significantly
decreased the phosphorylated p38 MAPK (Thr180 and Tyr182) level and
HMGB1 expression induced by CCI, suggesting that etanercept
inhibited HMGB1 expression by suppressing the activation of p38
MAPK. As TNF-α reportedly induces activation of p38 MAPK in DRG
neurons (18), our findings
provide indirect evidence that TNF-α regulates HMGB1 expression in
DRG neurons through the p38 MAPK signaling pathway.
In conclusion, etanercept significantly reduced the
HMGB1 expression induced by CCI in DRG neuron cells. This study not
only explored the molecular mechanism underlying the therapeutic
effect of etanercept on sciatica-related nociception, but also
provided indirect evidence for the interaction between TNF-α and
HMGB1 in DRG neuron cells.
Acknowledgements
This study was supported by the
Planned Science and Technology Project of Hunan Province, China
(2010SK3096).
References
|
1.
|
Zanella JM, Burright EN, Hildebrand K,
Hobot C, Cox M, Christoferson L and McKay WF: Effect of etanercept,
a tumor necrosis factor-alpha inhibitor, on neuropathic pain in the
rat chronic constriction injury model. Spine (Phila Pa 1976).
33:227–234. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Niu YL, Guo Z and Zhou RH: Upregulation of
TNF-alpha in neurons of dorsal root ganglia and spinal cord during
coronary artery occlusion in rats. Cytokine. 47:23–29. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Schafers M, Sorkin LS, Geis C and Shubayev
VI: Spinal nerve ligation induces transient upregulation of tumor
necrosis factor receptors 1 and 2 in injured and adjacent uninjured
dorsal root ganglia in the rat. Neurosci Lett. 347:179–182. 2003.
View Article : Google Scholar
|
|
4.
|
Sommer C, Schafers M, Marziniak M and
Toyka KV: Etanercept reduces hyperalgesia in experimental painful
neuropathy. J Peripher Nerv Syst. 6:67–72. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
de Souza AW, Westra J, Limburg PC, Bijl M
and Kallenberg CG: HMGB1 in vascular diseases: Its role in vascular
inflammation and atherosclerosis. Autoimmun Rev. 11:909–917.
2012.PubMed/NCBI
|
|
6.
|
Abdulahad DA, Westra J, Limburg PC,
Kallenberg CG and Bijl M: HMGB1 in systemic lupus erythematosus:
Its role in cutaneous lesions development. Autoimmun Rev.
9:661–665. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Andersson U and Tracey KJ: HMGB1 is a
therapeutic target for sterile inflammation and infection. Annu Rev
Immunol. 29:139–162. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Shibasaki M, Sasaki M, Miura M, et al:
Induction of high mobility group box-1 in dorsal root ganglion
contributes to pain hypersensitivity after peripheral nerve injury.
Pain. 149:514–521. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Otoshi K, Kikuchi S, Kato K, et al:
Anti-HMGB1 neutralization antibody improves pain-related behavior
induced by application of autologous nucleus pulposus onto nerve
roots in rats. Spine (Phila Pa 1976). 36:E692–E698. 2011.
View Article : Google Scholar
|
|
10.
|
Bennett G and Xie Y: A peripheral
mononeuropathy in rat that produces disorders of pain sensation
like those seen in man. Pain. 33:87–107. 1988. View Article : Google Scholar
|
|
11.
|
Verdru P, De Greef C, Mertens L, Carmeliet
E and Callewaert G: Na+-Ca2+ exchange in rat
dorsal root ganglion neurons. J Neurophysiol. 77:484–490. 1997.
|
|
12.
|
Sun P, Xiong H, Kim TH, Ren B and Zhang Z:
Positive inter-regulation between beta-catenin/T cell factor-4
signaling and endothelin-1 signaling potentiates proliferation and
survival of prostate cancer cells. Mol Pharmacol. 69:520–531. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Stoetzer OJ, Wittwer C, Lehner J, et al:
Circulating nucleosomes and biomarkers of immunogenic cell death as
predictive and prognostic markers in cancer patients undergoing
cytotoxic therapy. Expert Opin Biol Ther. 12(Suppl 1): S217–S224.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Rendon-Mitchell B, Ochani M, Li J, et al:
IFN-gamma induces high mobility group box 1 protein release partly
through a TNF-dependent mechanism. J Immunol. 170:3890–3897. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Roux PP and Blenis J: ERK and p38
MAPK-activated protein kinases: A family of protein kinases with
diverse biological functions. Microbiol Mol Biol Rev. 68:320–344.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Kawahara K, Biswas KK, Unoshima M, et al:
C-reactive protein induces high-mobility group box-1 protein
release through activation of p38MAPK in macrophage RAW264.7 cells.
Cardiovasc Pathol. 17:129–138. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Kikuchi K, Kawahara K, Biswas KK, et al:
Minocycline attenuates both OGD-induced HMGB1 release and
HMGB1-induced cell death in ischemic neuronal injury in PC12 cells.
Biochem Biophys Res Commun. 385:132–136. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Xu JT, Xin WJ, Wei XH, et al: p38
activation in uninjured primary afferent neurons and in spinal
microglia contributes to the development of neuropathic pain
induced by selective motor fiber injury. Exp Neurol. 204:355–365.
2007. View Article : Google Scholar : PubMed/NCBI
|