Introduction
The global increase in the prevalence and incidence
of thyroiditis has been attributed to the introduction of table
salt, water or oil iodation to prevent goitre in iodine-deficient
areas and is also associated with the elevated urinary iodine
observed in non-defficient iodine areas (1–5),
which has encouraged the development of a number of experimental
studies (6,7). Several sources of iodine induced
thyroiditis were correlated with the development of thyroid
autoantibodies (8), including
iodinated radiological contrasts (9–12)
and medical drugs (13). However,
the role of iodine in these processes remains controversial and not
fully understood.
The initial steps of the autoimmune process in the
thyroid are poorly understood. Necrosis of follicular cells is
considered to be the crucial event that triggers the autoimmune
process (14) and its induction by
iodine is well documented (7,15).
Thyroid epithelial cells are constantly exposed to reactive oxygen
species (ROS) which are physiologically necessary for thyroid
hormone synthesis. However, when ROS are produced excessively, they
become toxic and induce cellular destruction and inflammation in
various models of iodine-induced thyroid involution (7)
The inflammatory autoimmune condition of the thyroid
gland depends on numerous factors, presumably including the patient
genetic profile which is proposed to be responsible for at least
50% of all autoimmune thyroiditis, although no conclusive genetic
factors have been described (16).
Environmental factors, such as thiocyanates from tobacco and
stress, and endogenous, including pregnancy, have been implicated
in its etiopathogeny (16). There
is also evidence to support the theory that non-inflammatory
processes, such as apoptosis, are important in the destruction of
thyroid follicles (7,17–20).
Notably, a significant reduction of caspase-3 expression by
peripheral T cells in thyroiditis has been demonstrated (21). Certain animals spontaneously
develop auto-immune thyroiditis (SAT), such as NOD.H-2h4 mice, BB/W
rats, Cornell C and obese strain chickens (OSCs) (6,22,23),
and for this reason, the majority have been used in various studies
aiming to demonstrate the effects of iodine in induced
thyroiditis.
The present study aimed to investigate the effects
of oral iodine consumption in non-obese diabetic (NOD) mice in an
experimental model tailored to observe the histopathological
alterations of the thyroid under light microscopy (LM) and electron
microscopy (EM).
Materials and methods
Animals and treatment
Matrices of wild-type NOD mice were acquired from
the Center for Development of Experimental Models for medicine and
Biology (CEDEME) from the University of São Paulo (Brazil) and were
maintained at the Animal Care Facility of Faculty of Medicine of
São Paulo University (FMUSP). A total of 64 female NOD mice aged
between 4 and 6 weeks were selected and divided into 4 groups of
equal size. Of these groups, EG60 and EG90 received ∼0.2
mg/animal/day of oral potassium iodine in drinking water while the
remaining groups (CG60 and CG90) had no oral supplement of iodine.
Mice assigned to the first treatment group (EG60) were supplemented
with iodine for 60 days. The second group (EG90) received treatment
with iodine for 90 days. The control groups were labeled either
CG60 or CG90 depending on whether they were sacrificed after 60 or
90 days, respectively. The animals were fed ad libitum with
standard commercial food. All experimental procedures were approved
by the Ethics Commission for Research Project Analysis (CAPPesq) of
FMUSP.
Specimen preparation
The animals were weighed and anesthetized with
intraperitoneal injections of 100 mg/kg ketamine and 10 mg/kg
xylazine prior to the thyroidectomy. Euthanasia was performed by
cervical dislocation. The thyroid tissues were fixed in buffered
formalin (10%) and embedded in paraffin. Sections (3 μm) were
stained with hematoxylin and eosin and the slides were mounted with
Entellan medium (Merck, Darmstadt, Germany) and analyzed using LM.
For EM, the sections of thyroid material were fixed in 2%
glutaraldehyde phosphate buffer 0.1 M (pH 7.4) and dissected while
immersed in this glutaraldehyde solution at x4 magnification. The
first post-fixation step was performed in 1% osmium tetroxide and
the second step with 1% uranila overnight. Material dehydration was
performed using acetone in a graduated series of 30–100% before
being embedded in Araldite resin. Sections (70 nm) were examined
using a Jeol 1010 transmission electron mircroscope (TEM) (Tokyo,
Japan).
Thyroiditis was defined as mononuclear interstitial
infiltration regardless of its intensity.
Statistical analysis
Pearson’s Chi-squared (χ2) test and
Fisher’s exact test (when n<5) were used to study the
association between iodine treatment and frequency of thyroiditis.
P≤0.05 was considered to indicate a statistically significant
difference.
Results
Body weight
The average weight of the animals in the
experimental and control groups was ∼25 g and no evidence of
catabolic processes was observed in these animals.
Development of thyroiditis
Treatment with oral iodine for 60 days was
associated with the development of thyroiditis in NOD mice. Of 16
mice in the EG60 group, 8 exhibited thyroiditis with oral iodine
ingestion. By contrast, no thyroiditis was observed in the CG60
mice (Table I). These differences
in the frequency of mice with thyroiditis were statistically
significant (P=0.0012) according to Fisher’s exact test. Oral
iodine ingestion for an additional 30 days did not increase the
frequency of mice with thyroiditis and 3 animals in the CG90 group
exhibited thyroiditis (Table I).
The difference in the frequency of thyroiditis between the EG90 and
CG90 groups, however, was not statistically significant (P=0.0812).
Of the mice assigned to EG90, 1 died of unknown causes.
 | Table I.Summary of histological alterations
during the time. |
Table I.
Summary of histological alterations
during the time.
| Group | Exposure period | n | Thyroiditis | Necrosis | Relative risk |
|---|
| EG60 | 60 days | 16 | 8 | 5 | 17.00
(1.06–271.78) |
| EG90 | 90 days | 15a | 7 | 1 | 2.49 (0.78–7.89) |
| CG60 | 60 days | 16 | 0 | 0 | - |
| CG90 | 90 days | 16 | 3 | 0 | - |
Light microscopy findings
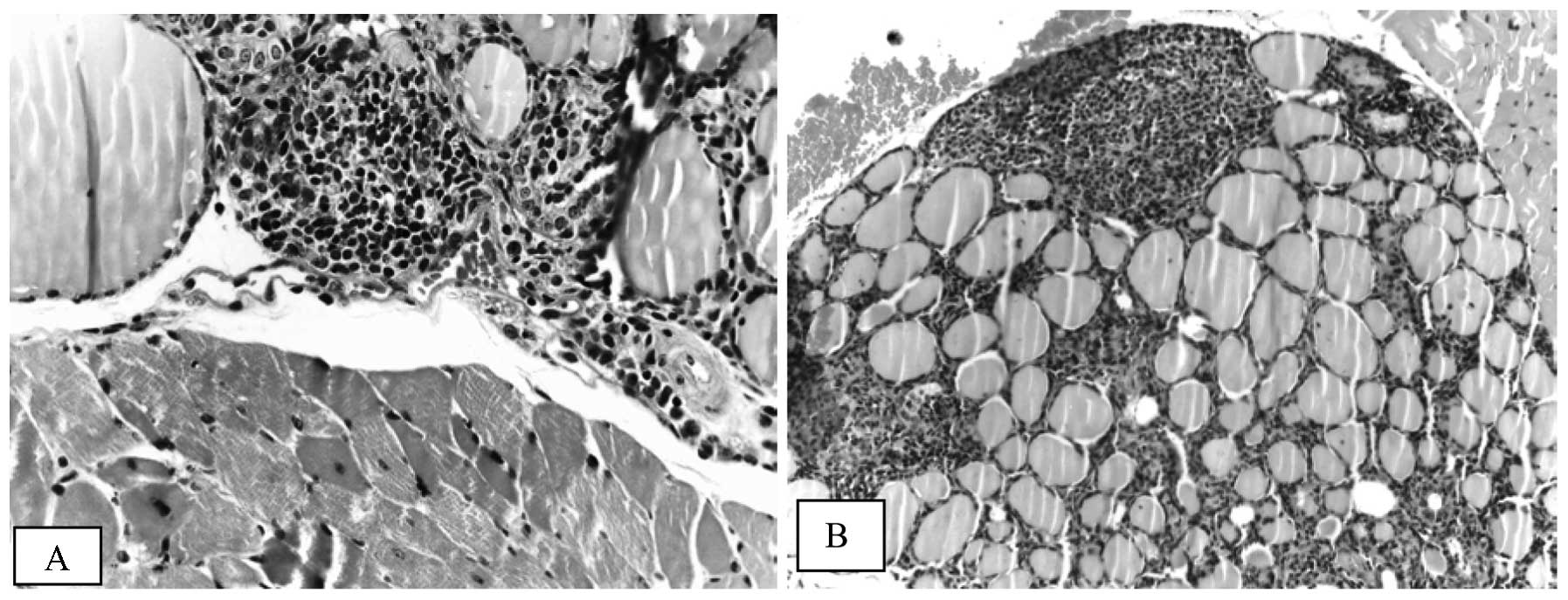
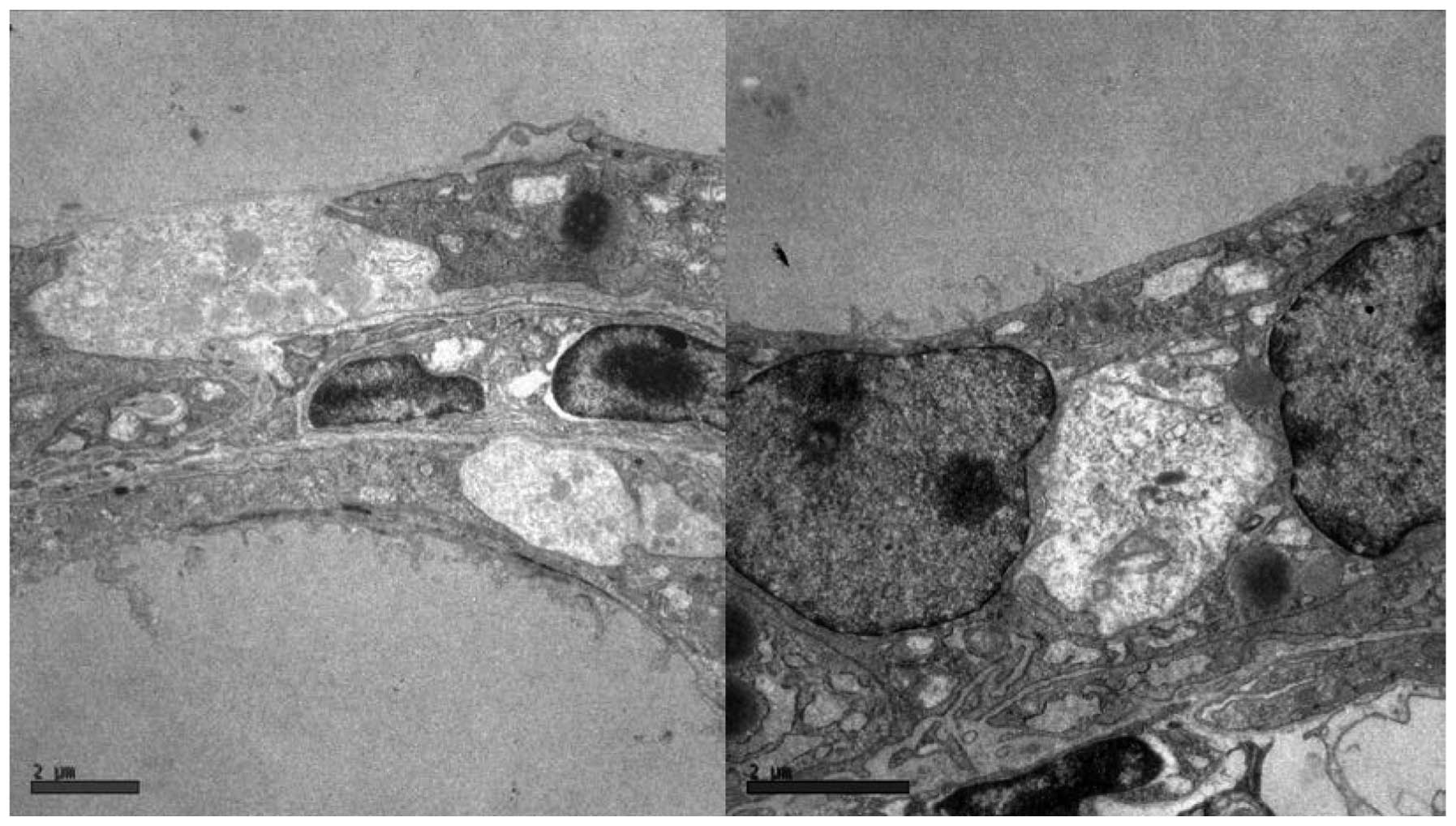
The most prominent morphological feature
characterizing iodine-induced thyroiditis in NOD mice was
lymphocyte infiltration which varied from few small foci of
invading lymphocytes located in the interstitia of 3 or 4 follicles
(Fig. 1A) to the extensive
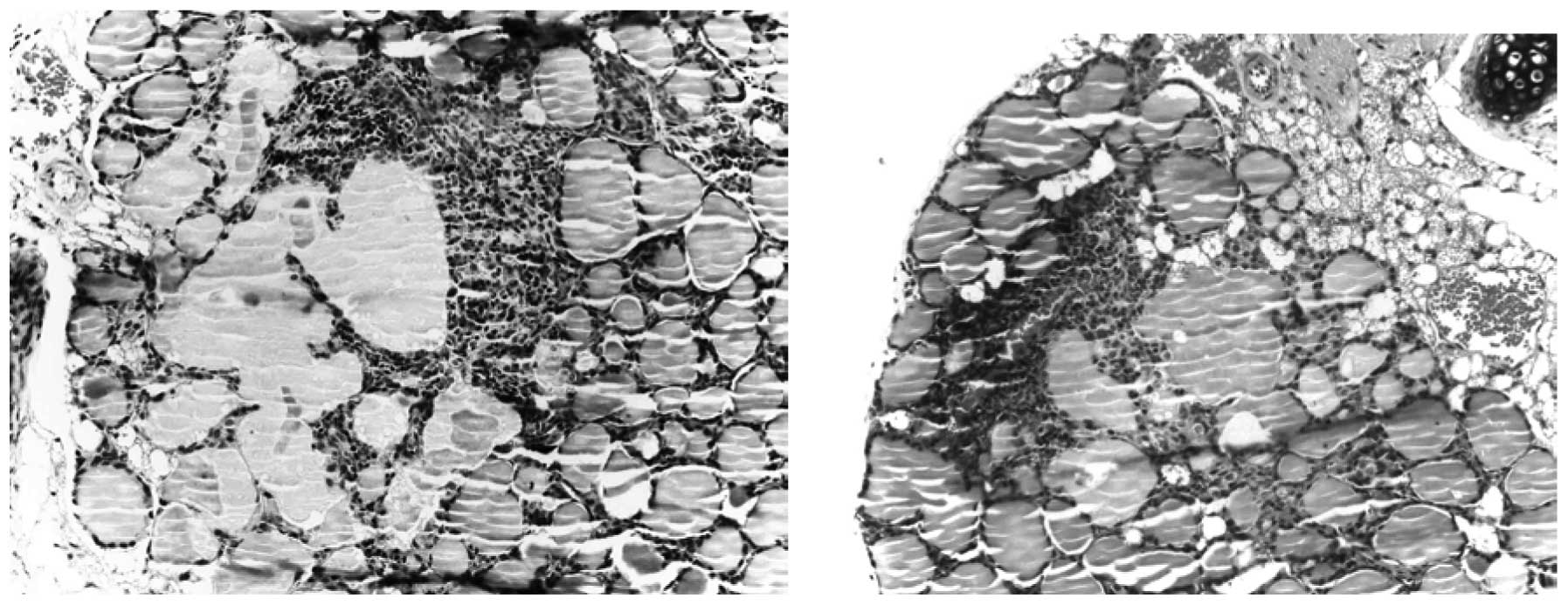
infiltration of a substantial portion of the thyroid lobe (Fig. 1B). The lymphocytic infiltration was
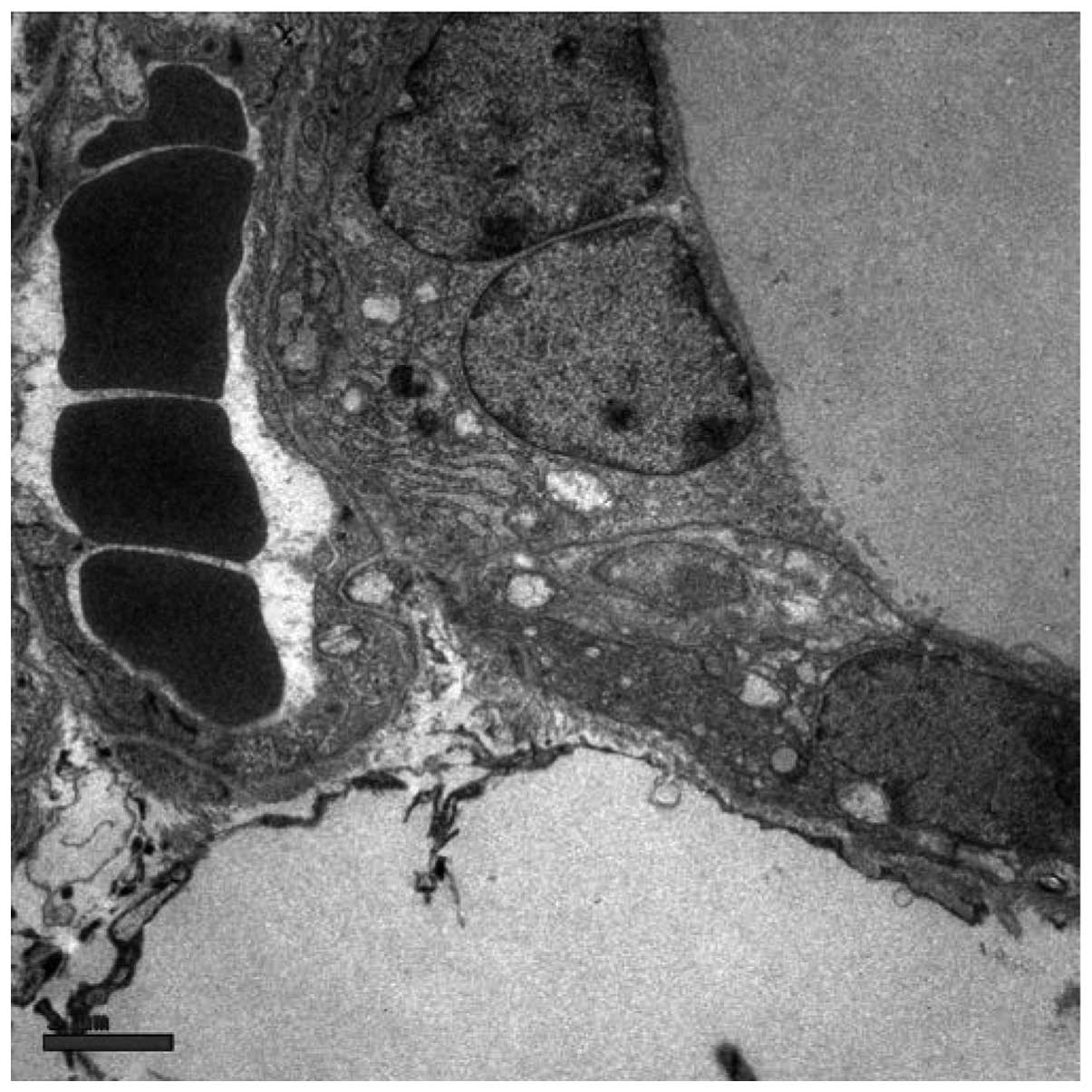
repeatedly observed in the vicinity of large follicles or cysts
formed by coalescing follicles (Fig.
2) and sometimes coexisted with the necrosis of follicle cells
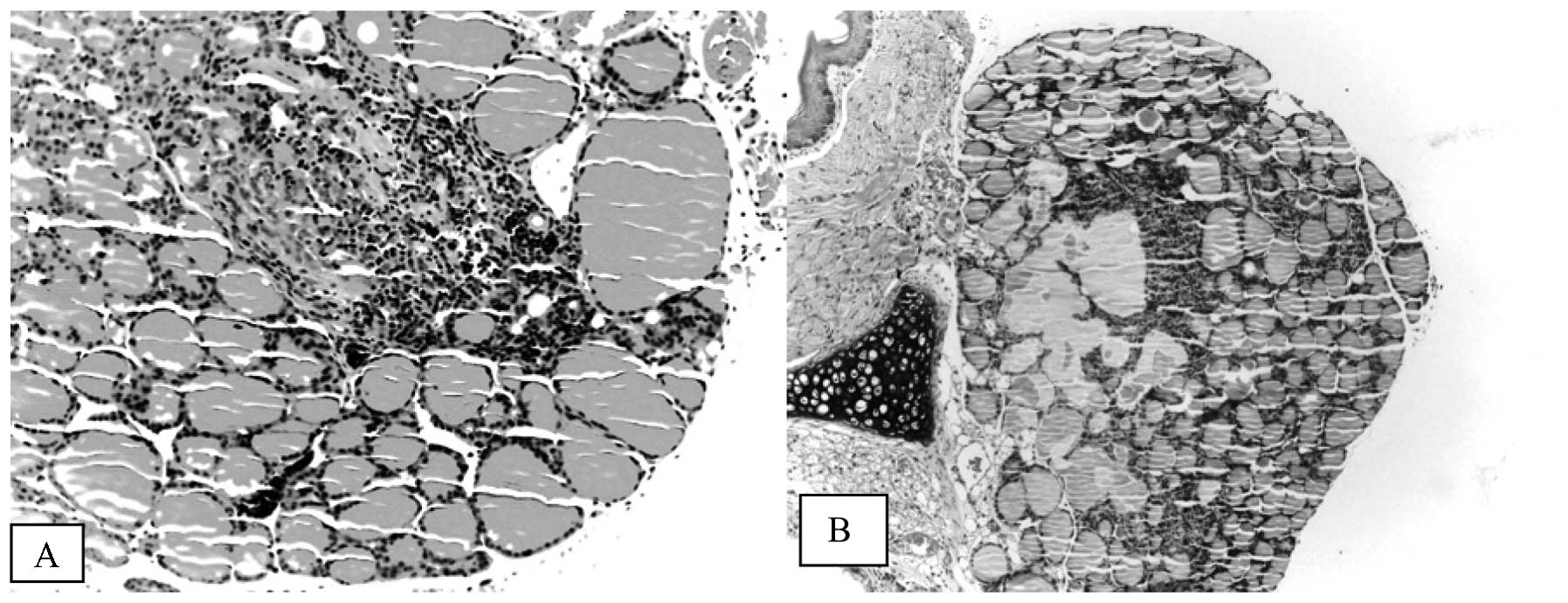
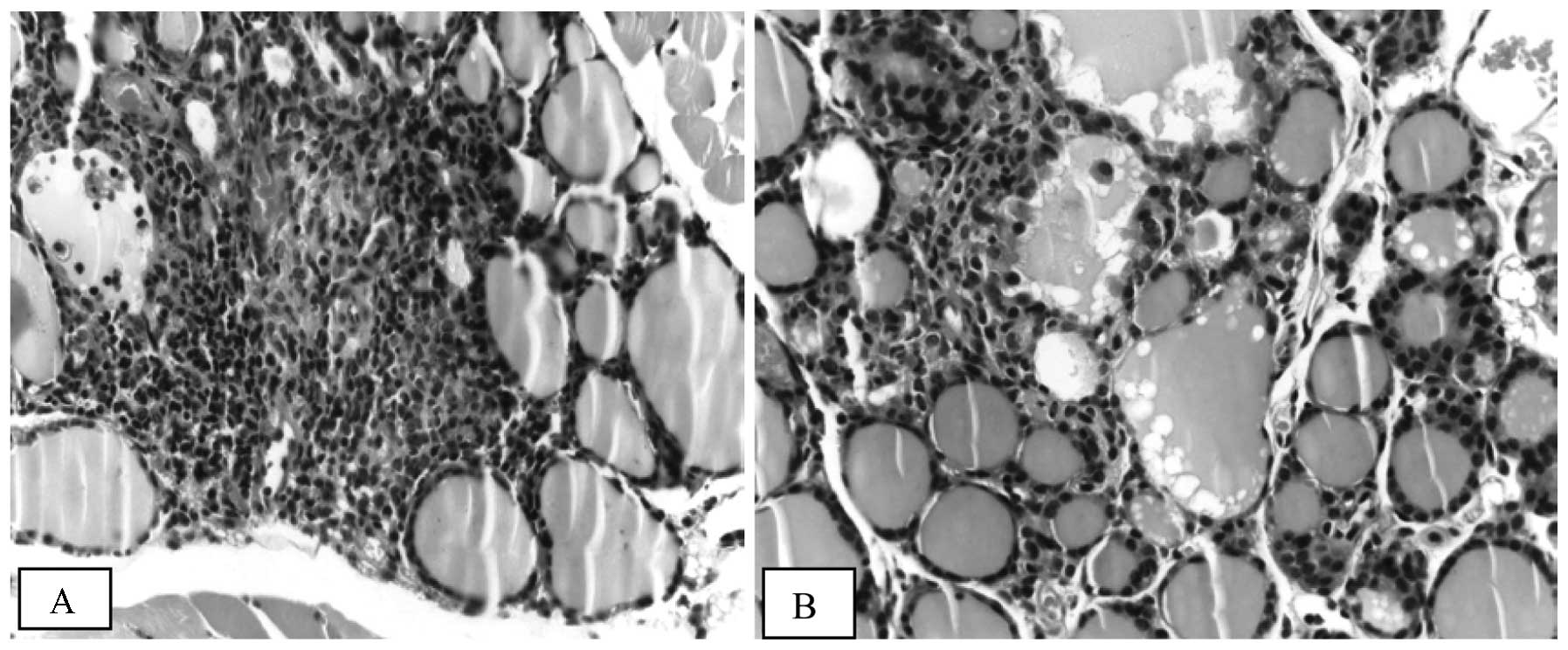
and disruption of thyroid architecture (Fig. 3). Atrophic follicles with reduced
diameters were observed surrounded by or in the periphery of a
lymphocytic infiltration (Fig. 4).
Longer treatment with iodine was associated with a more diffuse and
abundant lymphocyte infiltration pattern (data not shown). Similar
lymphocyte infiltration was observed in the control mice (3 animals
in CG90) that exhibited spontaneous thyroiditis (data not
shown).
Focal areas of necrosis were observed in 5 mice of
the EG60 group and these were observed concomitantly with
thyroiditis in 3 of these animals. In the EG90 group, 1 mouse
developed thyroid necrosis without thyroiditis. None of control
animals in either the CG60 or the CG90 exhibited necrosis (Table I).
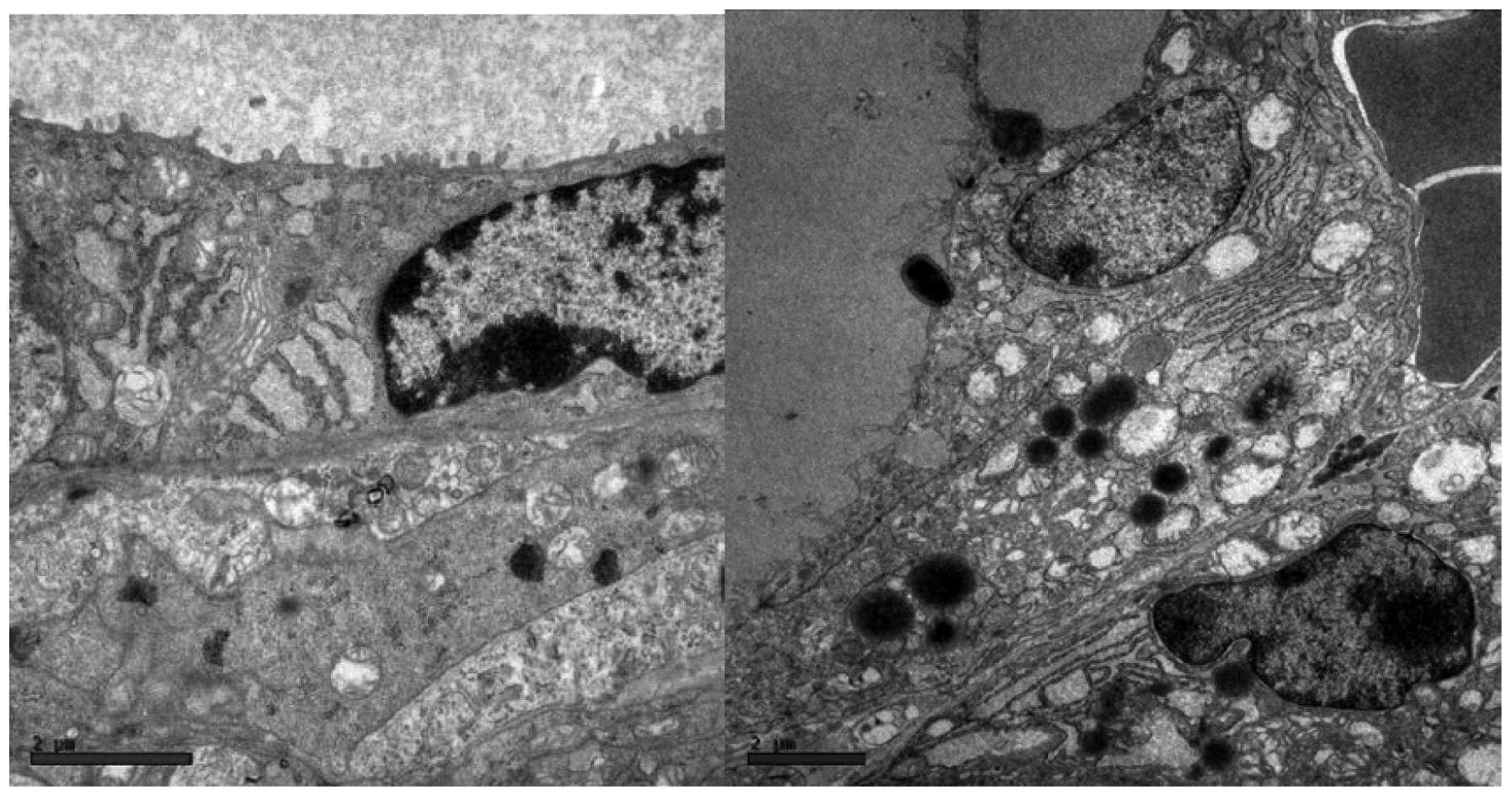
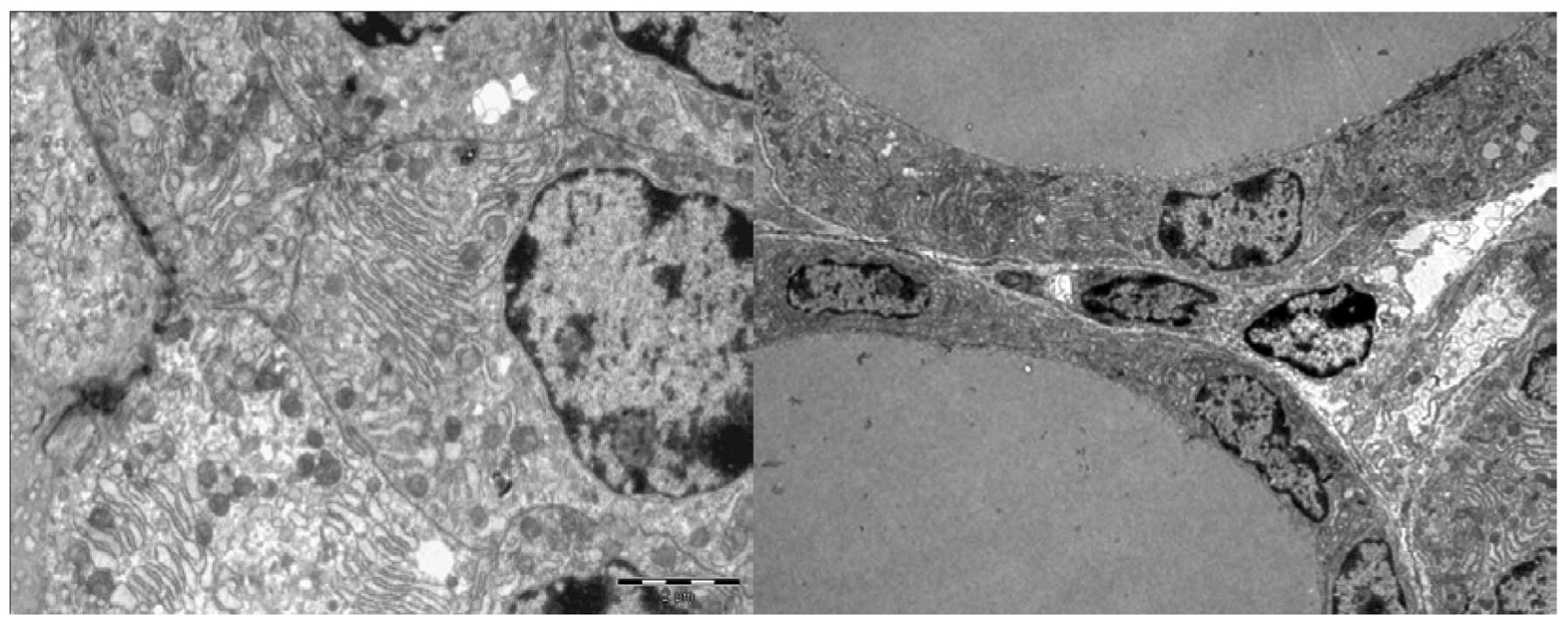
The follicle cells in iodine-treated mice (EG60)
exhibited distended rough endoplasmic reticulum and swollen,
degenerated mitochondria with a loss of cristae (Fig. 5). Other ultrastructural
abnormalities included subcellular debris (Fig. 6) and clear, ill-defined spaces
where no nuclei or organelles could be identified with certainty
(Fig. 7). We named this structure
an ‘amorphous space’ due to its unknown nature. Examination of
semi-thin sections of the thyroid specimens did not reveal
lymphocytic infiltration above the regions where ultrastructural
alterations were observed (data not shown). Accordingly, these
ultrastructural abnormalities were hypothesised to result from the
toxic effects of iodine on thyroid tissue, since they were not
observed in the control animals (Fig.
8).
Discussion
The results presented in the present study partially
endorse the hypothesis that iodine has an important involvement in
the development of thyroiditis. This may be observed by the
comparison of the CG60 group, with no animals affected, with the
EG60 group, where 50% of the animals exhibited thyroiditis.
Additionally, a higher frequency of thyroiditis was observed in the
EG90 group. The etiopathogeny of autoimmune thyroiditis continues
to be broadly discussed and salt iodation and elevated urinary
iodine are frequently suggested as factors responsible for inducing
autoimmune thyroiditis (3–5). The results of the present study
support this theory since the control animals did not develop
thyroiditis except at the reported incidence of NOD mice (24).
Notably, a longer exposure to iodine did not appear
to increase the frequency of thyroiditis. This suggests that the
iodine is a stochastic rather than deterministic factor for the
development of thyroidal autoimmunity and that iodine may trigger
an earlier appearance of thyroid autoimmune process. We propose
that the ultrastructural alterations triggered by treatment with
iodine represent early signs of follicular cell lesions which
contribute to exposing the immune system to thyroidal autoantigens,
thus inducing immune cell infiltration.
The incidence of 18.7% for lymphocytic infiltration
in the CG90 group is quite similar to that reported by Damotte
et al(24) (14.3%) using
the same mouse lineage. These authors also observed that the first
lymphocytic infiltration in the wild-type NOD mice occurred within
14–15 weeks, but predominantly in the male colony.
Similar to the human thyroiditis, the mice also
exhibited lymphocyte infiltration as the most prominent
observation. Although we were able to identify some plasma
cell-like lymphocytes (Fig. 4), in
general the infiltrating cells in mouse thyroiditis were not
predominantly composed of plasma cells, as usually can occur in
human thyroiditis. The general appearance of the experimental group
EG90 appeared to exhibit increased severity of lymphocytic
infiltration compared with EG60, which may be explained, in part,
by the evolution of inflammatory process.
The frequency of necrosis observed in the EG60 group
(31.25%), is in agreement with the toxic effect of iodine in
thyroid cells, as reported by Bagchi et al(23). As anticipated, other investigators
have described necrosis as the first step to antigen exposure in
the autoimmune thyroiditis process (14,23,25).
Of note, the frequency of necrosis decreased in the EG90 mice. It
is possible that the toxic effects of iodine cannot be sustained
for a prolonged period of time or may evoke a desensitization
response following repeated exposure. A short-lived toxic effect of
iodine may explain why necrosis was considerably less frequent
following 90 days of treatment. The coexistence of necrosis and
thyroiditis possibly indicates that necrosis triggers the thyroid
autoimmunity as suggested by Many et al(14). We propose that acute iodine induces
blood flow restraint, which contributes to the necrosis of
follicular cells, resulting in the inflammatory process and release
of thyroid antigens to immune cells.
EM showed alterations compatible with cell
degeneration. Distended rough endoplasmatic reticulum and
mitochondrial lesions with a loss of cristae were described by
Nakazawa et al(13) in
humans following the use of amiodarone for 19 days. Since no
subcellular lesions were observed in the control groups (CG60 and
CG90), it is possible that the ultra-structural degeneration is an
iodine-induced event.
The amorphous spaces documented in the experimental
group have not been reported previously. There is no evidence
concerning the spaces’ nature and no reasonable explanation for
their occurrence may be proposed currently. However, the
distribution pattern suggests that these spaces are not likely to
be artefacts. The general appearance of these structures mimics the
light cells usually detected in normal and pathological tissues,
but unlike light cells no nuclei were observed. Further studies
should be considered to test the reproducibility of these findings
and their significance. Although unspecific, the cellular debris
and mitochondrial lesions, even without lymphocytic infiltration,
appear to be related to iodine and the beginning of this autoimmune
process.
Acknowledgements
The authors thank Dr Eduardo Pompeu
for the support of the Bioterism Center of FMUSP for breeding the
congenic colony, Keila da Silva for histological section
preparation and Helio Correa and the staff of the Electron
Microscopy Department of FMUSP for ultrastructure section
preparation.
References
|
1.
|
Bülow Pedersen I, Knudsen N, Jørgensen T,
Perrild H, Ovesen L and Laurberg P: Large differences in incidences
of overt hyper-and hypothyroidism associated with a small
difference in iodine intake: a prospective comparative
register-based population survey. J Clin Endocrinol Metab.
87:4462–4469. 2002.
|
|
2.
|
Teng W, Shan Z, Teng X, Guan H, Li Y, Teng
D, et al: Effect of iodine intake on thyroid diseases in China. N
Engl J Med. 354:2783–2793. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Fountoulakis S, Philippou G and Tsatsoulis
A: The role of iodine in the evolution of thyroid disease in
Greece: from endemic goiter to autoimmunity. Hormones (Athens).
6:25–35. 2007.PubMed/NCBI
|
|
4.
|
Camargo RY, Tomimori EK, Neves SC, G S
Rubio I, Galrão AL, Knobel M and Medeiros-Neto G: Thyroid and the
enviromment: exposure to excessive nutritional iodine increases the
prevalence of thyroid disorders in São Paulo, Brazil. Eur J
Endocrinol. 159:293–299. 2008.PubMed/NCBI
|
|
5.
|
Harach HR and Ceballos GA: Thyroid cancer,
thyroiditis and dietary iodine: A review based on the Salta,
Argentina model. Endocr Pathol. 19:209–220. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Li HS and Carayanniotis G: Induction of
goitrous hypothyroidism by dietary iodide in SJL mice.
Endocrinology. 148:2747–2752. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Poncin S, Gérard AC, Boucquey M, Senou M,
Calderon PB, Knoops B, Lengelé B, Many MC and Colin IM: Oxidative
stress in the thyroid gland: from harmlessness to hazard depending
on the iodine content. Endocrinology. 149:424–433. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Fassbender WJ, Vogel C, Doppl W, Stracke
H, Bretzel RG and Klör HU: Thyroid function, thyroid immunoglobulin
status and urinary iodine excretion after enteral contrast-agent
administration by endoscopic retrograde cholangiopancreatography.
Endoscopy. 33:245–252. 2001. View Article : Google Scholar
|
|
9.
|
Linder N, Sela B, German B, Davidovitch N,
Kuint J, Hegesh J, Lubin D and Sack J: Iodine and hypothyroidism in
neonates with congenital heart disease. Arch Dis Child Fetal
Neonatal Ed. 77:F239–F240. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Grubeck-Loebenstein B, Kronik G,
Mösslacher H and Waldhäusl W: The effect of iodine containing
contrast medium on thyroid function of patients undergoing coronary
angiography. Exp Clin Endocrinol. 81:59–64. 1983. View Article : Google Scholar
|
|
11.
|
del Cerro Marín M, Fernández Ruiz A,
García-Guereta L, Benito Bartolomé F, Burgueros M, Ares Segura S,
Moreno F and Gracia Bouthelier R: Thyroid function alterations in
children with congenital cardiac disease after catheterization with
iodinated contrast agents. Rev Esp Cardiol. 53:517–524. 2000.(In
Spanish).
|
|
12.
|
Gartner W and Weissel M: Do
iodine-containing contrast media induce clinically relevant changes
in thyroid function parameters of euthyroid patients within the
first week? Thyroid. 14:521–524. 2004. View Article : Google Scholar
|
|
13.
|
Nakazawa T, Murata S, Kondo T, Nakamura N,
Yamane T, Iwasa S and Katoh R: Histopathology of the thyroid in
amiodarone-induced hypothyroidism. Pathol Int. 58:55–58. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Many MC, Maniratunga S, Varis I, Dardenne
M, et al: Two-step development of Hashimoto-like thyroiditis in
genetically autoimmune prone non-obese diabetic mice: effects of
iodine-induced cell necrosis. J Endocrinol. 147:311–320. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Many MC, Mestdagh C, van den Hove MF and
Denef JF: In vitro study of acute toxic effects of high iodide
doses in human thyroid follicles. Endocrinology. 131:621–630.
1992.PubMed/NCBI
|
|
16.
|
Melo M: Autoimmune thyroiditis. Acta Med
Port. 19:387–394. 2006.(In Portuguese).
|
|
17.
|
Tanimoto C, Hirakawa S, Kaiwasaki H,
Hayakawa N and Ota Z: Apoptosis in thyroid diseases: a
histochemical study. Endocr J. 42:193–201. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Wang SH and Baker JR: The role of
apoptosis in thyroid autoimmunity. Thyroid. 17:975–979. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Arscott PL and Baker JR Jr: Apoptosis and
thyroiditis. Clin Immunol Immunopathol. 87:207–217. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Kotani T, Aratake Y, Hirai K, Fukazawa Y,
Sato H and Ohtaki S: Apoptosis in thyroid tissue from patients with
Hashimoto’s thyroiditis. Autoimmunity. 20:231–236. 1995.
|
|
21.
|
Chen X, Liu L, Yao P, Yu D, Hao L and Sun
X: Effect of excessive iodine on immune function of lymphocites and
intervention with selenium. J Huazhong Univ Sci Technolog Med Sci.
27:422–425. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Allen EM, Appel MC and Braverman LE:
Iodine-induced thyroiditis and hypothyroidism in
hemithyroidectomized BB/W rat. Endocrinology. 121:481–485. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Bagchi N, Brown TR and Sundick RS: Thyroid
cell injury is an initial event in the induction of autoimmune
thyroiditis by iodine in obese strain chickens. Endocrinology.
136:5054–5060. 1995.PubMed/NCBI
|
|
24.
|
Damotte D, Colomb E, Cailleau C, Brousse
N, Charreire J and Carnaud C: Analysis of susceptibility of NOD
mice to spontaneous and experimentally induced thyroiditis. Eur J
Immunol. 27:2854–2862. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Li HS, Verginis P and Carayanniotis G:
Maturation of dendritic cells by necrotic thyrocytes facilitates
induction of experimental autoimmune thyroiditis. Clin Exp Immunol.
144:467–474. 2006. View Article : Google Scholar : PubMed/NCBI
|