Introduction
Adjuvant intravesical Bacillus Calmette-Guerin (BCG)
therapy is a well-established and successful immunotherapy for
preventing local recurrences and tumor progression following the
transurethral resection of non-muscle invasive bladder cancer
(1,2). While the mechanism of BCG therapy
remains unclear, natural killer (NK) cells play an important role
in BCG-mediated antitumor effects (3). In vitro experiments have
demonstrated that BCG-activated killer (BAK) cells, which are
generated from peripheral blood mononuclear cells (PBMCs)
stimulated with BCG, are the main effector cells. The BAK cell
activity has been attributed to a small subpopulation of activated
lymphocytes, which belong to the
CD3−/CD8+/CD56+ NK cell phenotype
(4). The BAK cells kill cancer
cells mainly via perforin-mediated mechanisms rather than by
Fas-FasL interactions (5).
Previous clinical studies have demonstrated that
topical BCG is highly effective in the treatment of Condylomata
acuminata (6,7), including flat condyloma of the cervix
(8). While Condylomata acuminata
is associated with low-risk human papillomavirus (HPV) infection,
no study has examined the efficacy of BCG immunotherapy in
high-risk HPV-related diseases such as cervical cancer. The HPV
early proteins E6 and E7 are the major viral oncoproteins that
regulate cell proliferation in high-risk HPV-infected cancer cells
through the inactivation of the p53 and retinoblastoma (RB) tumor
suppressor proteins, respectively. The RB/E2F1 pathway is a vital
regulator of cell proliferation, differentiation, senescence and
apoptosis (9). It has been
reported that altered RB protein (pRB) expression is an independent
predictor of recurrence and progression in patients treated by
intravesical BCG (10), and pRB
underexpression is predictive of nonresponse and cancer recurrence
(11). The aim of the present
study was to determine whether BCG immunotherapy has an antitumor
effect on high-risk HPV infected cells, such as the HeLa cell line,
and whether BCG immunotherapy alters the RB/E2F1 pathway in the
HeLa cells.
Materials and methods
Cervical cancer cells
The established HeLa cell line (ATCC CCL-2) was used
as the cervical cancer cells in the present study. The HeLa cells
were grown in RPMI-1640 medium supplemented with 10% fetal bovine
serum (FBS), 100 U/ml penicillin and 100 μg/ml streptomycin
(Gibco, Grand Island, NY, USA). The cells were incubated at 37°C in
a humidified atmosphere containing 5% CO2.
Isolation and stimulation of PBMCs
PBMCs from the EDTA-mediated anticoagulated blood of
six informed healthy human donors were obtained using Lympholyte-H
(Cedarlane, Burlington, ON, Canada) density centrifuging. The
isolated PBMCs were washed twice with PBS and adjusted to a
concentration of 1×106 cells/ml in RPMI-1640 medium
containing 10% FBS. The 50 μg/ml reconstituted lyophilizate
of BCG (Connaught substrain, ImmuCyst; Sanofi Pasteur, Toronto,
Canada) was added to the PBMCs and the cells were cultured in
six-well plates at 37°C and 5% CO2 for 5 days to
generate BAK cells (12).
Unstimulated cultured PBMCs, which are equivalent to NK cells,
served as the negative controls. After 5 days, the suspended
BCG-stimulated PBMCs were collected and adjusted to a concentration
of 2×106 cells/ml as effector cells, while the
unstimulated PBMCs served as NK cells and were prepared similarly
to act as the control. The study was approved by the ethics
committee of the First Affiliated Hospital of Zhejiang
University.
Cytotoxicity assay
The cytotoxicity of BAK and NK cells against HeLa
cells was assessed by the CellTiter 96® AQueous One
Solution Cell Proliferation assay (Promega, Madison, WI, USA). The
HeLa cells were suspended at a concentration of 1x105
cells/ml. The effector (E) and target (T) cells were combined at
E/T ratios of 40:1, 20:1 and 10:1 in 96-well plates with a total
volume of 200 μl in each well. Combinations of E and T cells
are referred to as ET. The E and T cells were cultured in RPMI-1640
medium alone to determine the spontaneous release, and the wells
with 200μl RPMI-1640 medium were blank (B) wells. Each group
had three parallel replicate wells. After 20 h of incubation in a
humidified 37°C incubator with 5% CO2, 20 μl MTS
solution was added to each well and incubated for 3.5 h. The
optical density value of each well was measured at 490 nm with an
automatic ELISA reader. The average value of the three wells in
each group was used. The cytotoxicity was calculated as follows:
Cytotoxicity (%)=[1-(ODET -
ODE)/(ODT - ODB)]x100%.
Cell apoptosis assay
HeLa cells (1×105 cells/ml) were
incubated in 12-well plates with a volume of 1 ml in each well.
After 6 h, when the cells were adherent, BAK cells
(2×106 cells/ml, 1 ml) were added to the adherent HeLa
cells (2 ml total per well) and incubated for 20 h. Unstimulated
PBMCs were added to HeLa cells at the same E/T ratio to serve as
the negative control. The wells containing HeLa cells without
effector cells were supplemented with 1 ml RPMI-1640 medium to
serve as the blank control. Following incubation, the suspended
cells (BAK and NK cells) were washed three times with PBS to remove
all effector cells. The HeLa cells were trypsinized and washed with
PBS, then collected and stained using the FITC Annexin V Apoptosis
Detection kit I (BD Biosciences, San Diego, CA, USA) according to
the manufacturer’s instructions. The stained HeLa cells were
analyzed by fluorescent-activated cell sorting (FACS) using a BD
LSR II Flow Cytometer (BD Biosciences).
Cell cycle assay
The HeLa cells were incubated with the BAK or NK
cells at an E/T ratio of 20:1 in 12-well plates for 20 h. Untreated
HeLa cells served as the blank control. After removing the
suspended effector cells with PBS, the HeLa cells were trypsinized
and washed with PBS and suspended in PBS. Ethanol was added to a
final concentration of 70% and the suspension was stored at 4°C
overnight. The cells were washed with PBS to remove ethanol and
were then suspended in PBS containing 0.25 mg/ml DNase-free RNase
(Sigma-Aldrich, St. Louis, MO, USA). After nuclear staining with
propidium iodide (PI, 50 μg/ml; Sigma-Aldrich) in the dark
at room temperature for 30 min, flow cytometry was performed using
the BD LSR II Flow Cytometer system with FACSDiva software (BD
Biosciences). The data from three identical analyses were used to
confirm the results.
Real-time RT-PCR
HeLa cells were incubated with BAK or NK cells at an
E/T ratio of 20:1 in 12-well plates for 20 h. Untreated HeLa cells
served as a blank control. After removing the suspended effector
cells with PBS, total RNA was extracted from the HeLa cells
(treated or untreated) using the TRIzol (Invitrogen, Carlsbad, CA,
USA) method. The mRNAs were resuspended in RNase-free water. The
index of purity of the mRNA samples ranged between 1.8 and 2.0 by
260/280 measurement. Total RNA was used to generate cDNA with the
PrimeScript II 1st Strand cDNA Synthesis kit (Takara, Otsu, Japan)
according to the manufacturer’s instructions. This was followed by
detection of PCR products with iQ™ SYBR® Green Supermix
(Bio-Rad, Hercules, CA, USA) real-time RT-PCR with primers specific
for the HPV-E7, RB and E2F1 transcripts with an internal
amplification control of GAPDH. The nucleotide sequences of the
primers are shown in Table I. The
HPV-E7, RB and E2F1 mRNA expression levels were measured using the
Ct (cycle threshold) method, and relative fold-expression changes
were normalized to GAPDH mRNA using the equation
2−ΔΔCt.
 | Table I.Primer sequences of HPV-E7, RB, E2F1
and GAPDH. |
Table I.
Primer sequences of HPV-E7, RB, E2F1
and GAPDH.
| Gene name | | Sequence |
|---|
| HPV-E7 | Forward |
5′-ATGTCACGAGCAATTAAGC-3′ |
| Reverse |
5′-TTCTGGCTTCACACTTACAACA-3′ |
| RB | Forward |
5′-CCTCCTTAATTTGGGAAGGTTTGTG-3′ |
| Reverse |
5′-GCCTAACCCATAATGACCCTTGATT-3′ |
| E2F1 | Forward |
5′-CAATCTGCACTTTGATTTGCTTCC-3′ |
| Reverse |
5′-CCCGAAATGTTCCCAACAGA-3′ |
| GAPDH | Forward |
5′-ATGGGGAAGGTGAAGGTGG-3′ |
| Reverse |
5′-GGGGTCATTGATGGCAACAATA-3′ |
Western blotting
The HeLa cells were incubated with BAK or NK cells
at an E/T ratio of 20:1 in 12-well plates for 20 h. Untreated HeLa
cells served as a blank control. Effector cells were removed with
cold PBS and the remaining HeLa cells were lysed by placing on ice
in cell lysis buffer (Cell Signaling Technology, Inc., Beverly, MA,
USA). Cell lysates were incubated on ice for 30 min and centrifuged
at 12,000 x g for 10 min at 4°C. The proteins were applied to 8–12%
gels and separated by SDS-PAGE. The samples were then transferred
to a polyvinylidene difluoride (PVDF) transfer membrane (Bio-Rad)
for 1 h. The membranes were blocked for 2 h at room temperature
with 5% dry milk in Tris-buffered saline with Tween (TBST) and
incubated overnight at 4°C with primary antibodies against RB
(1:1,500), E2F1 (1:3,000; Epitomics, Burlingame, CA, USA) and
HPV18-E7 (1:1,000; Abcam, Cambridge, UK). Protein levels were
normalized to total GAPDH using a mouse anti-GAPDH monoclonal
anti-body (Abcam). The membranes were washed in TBST and incubated
with 1:5,000 anti-mouse or anti-rabbit IgG conjugated to
horseradish peroxidase for 1 h at room temperature before washing
again. Band signals were visualized using chemiluminescence
reagents (Millipore, Billerica, MA, USA), acquired in the linear
range of the scanner and analyzed using QUANTITY ONE software
(Bio-Rad).
Statistical analysis
All data are presented as the median ± range. The
statistical significance between treatment and control groups was
determined using the Wilcoxon signed-rank test and SPSS 18.0
software (SPSS Inc., Chicago, IL, USA). P<0.05 was considered to
indicate a statistically significant result.
Results
Cytotoxicity of BAK and NK cells
The PBMCs from six healthy human donors cultured
with or without BCG were tested for cytotoxicity against HeLa cells
by the MTS assay. The PBMCs showed increased cytotoxicity following
5 days of incubation with BCG (Fig.
1). The PBMCs stimulated with BCG (BAK cells) demonstrated
higher cytotoxicity than the unstimulated PBMCs (NK cells) at the
E/T ratios of 40:1 and 20:1. At the ratio of 10:1, no significant
difference in cytotoxicity was observed between the BAK and NK
groups (P>0.05). The cytotoxicities of the BAK and NK cells
increased as the E/T ratios increased.
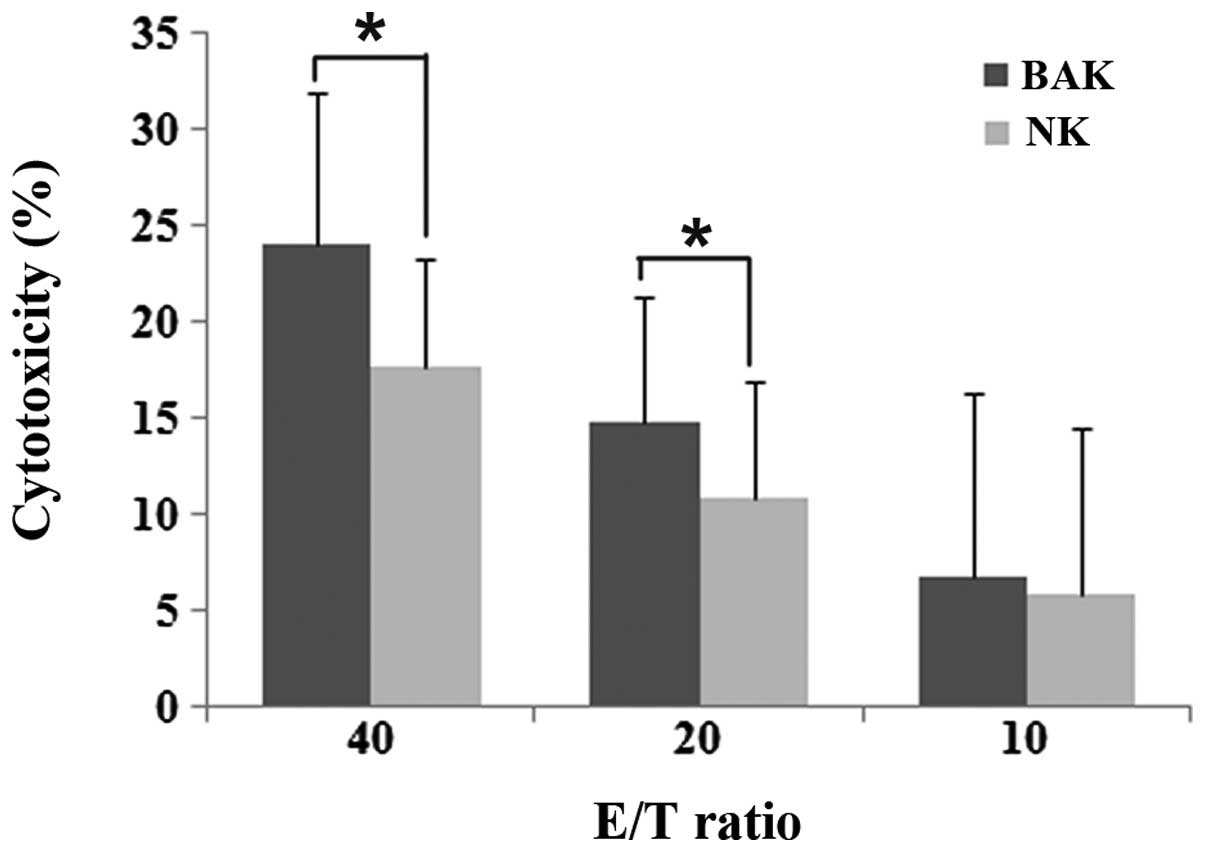 | Figure 1.Cytotoxicity of BAK and NK cells
against HeLa cells. The BAK cell cytotoxicity was 24.08±7.81,
14.74±6.61 and 6.8±9.44% and the NK cell cytotoxicity was
17.62±5.59, 10.78±6.18 and 5.8±8.7% at the E/T ratios of 40:1, 20:1
and 10:1, respectively. Between the BAK and NK groups, there was no
significant difference at the ratio of 10:1 (P= 0.249). However,
the cytotoxicity of the BAK cells was significantly increased at
the ratios of 40:1 (P=0.028) and 20:1 (P=0.046). The cytotoxicity
of the BAK and NK cells increased as the E/T ratios increased. Data
are shown as the median ± range,*P<0.05. BAK,
BCG-activated killer; NK, natural killer; E, effector; T, target
cell; BCG, Bacillus Calmette-Guerin. |
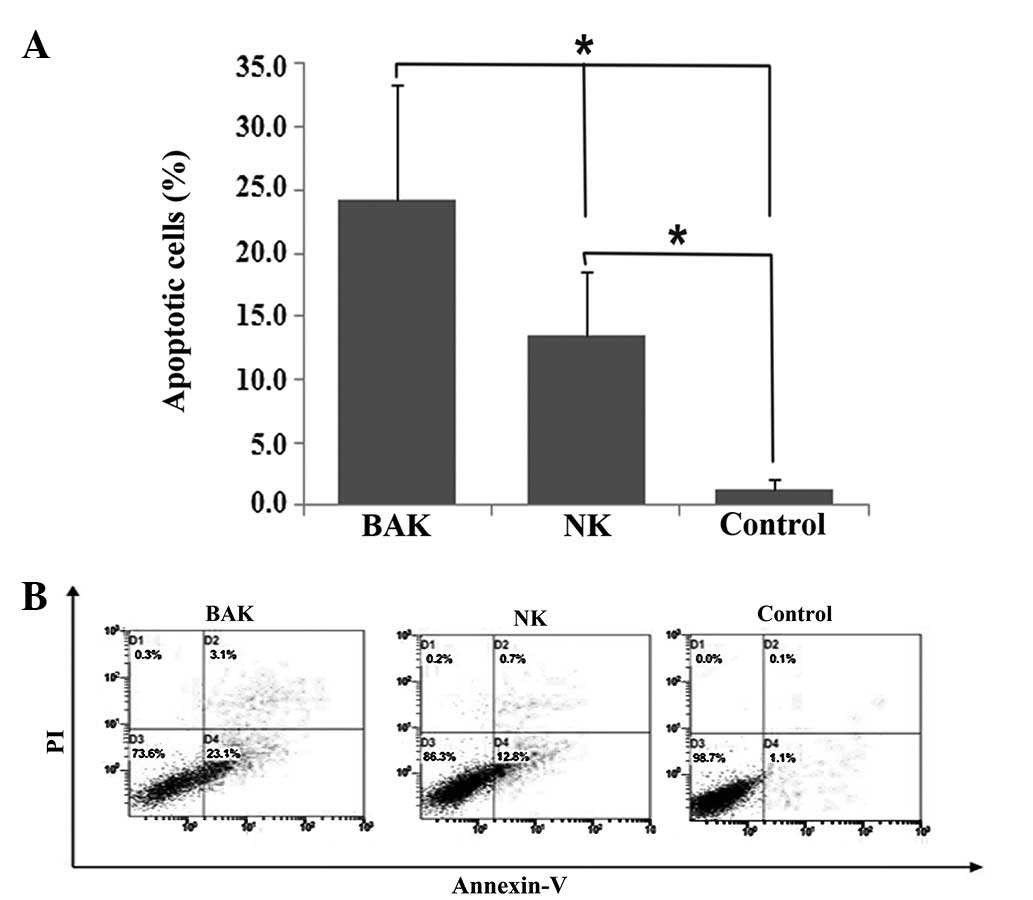
Effect of BAK and NK cells on the
apoptosis of HeLa cells
Post-incubation HeLa cells were analyzed for
effector cell effects on apoptosis by flow cytometry. Although the
blank controls exhibited 1.25% apoptosis and the NK cells exhibited
13.45% apoptosis, BAK cells had a significant impact on the level
of apoptosis (24.2%). NK cells also showed a significant effect on
the apoptosis of HeLa cells compared with the blank control
(Fig. 2, P<0.05). This result
suggested that PBMCs promote apoptosis of HeLa cells following
stimulation with BCG.
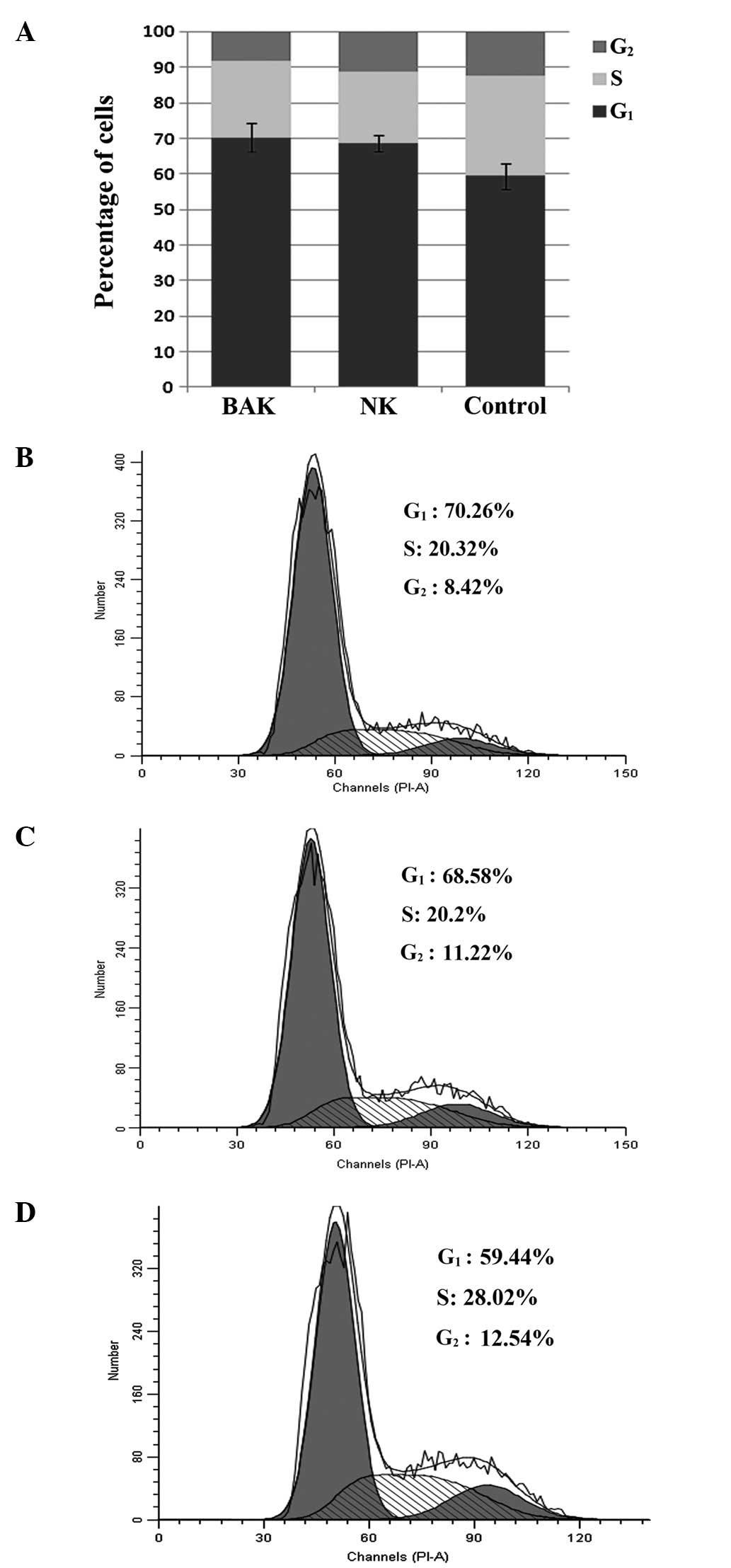
Effect of BAK and NK cells on the cell
cycle of HeLa cells
Post-incubation HeLa cells were analyzed for
effector cell effects on the cell cycle by flow cytometry.
Incubation of effector and HeLa cells induced a shift in cell cycle
arrest with enhanced G1 phase arrest (Fig. 3). Compared with the normal HeLa
cell cycle distribution where 59.4% of cells are in G1,
BAK cells increased the level of G1/S arrest to 70.3%
(P<0.05) and NK cells increased it to 68.6% (P<0.05). While
BAK and NK cells each had a statistically significant effect on
HeLa cell cycle distribution, no significant difference was
observed between the BAK and NK groups (P>0.05). This result
showed that PBMCs may inhibit the proliferation of HeLa cells
independently of BCG stimulation; BAK cells did not exert a greater
effect than NK cells.
mRNA expression of RB, E2F1 and HPV-E7 in
HeLa cells following treatment with BAK and NK cells
The HPV-E7 mRNA expression levels were very similar
in the BAK, NK and control groups (Fig. 4A). RB mRNA expression in the HeLa
cells increased following treatment with either BAK or NK cells
(Fig. 4B, P<0.05), but no
significant difference existed between the two groups (P>0.05).
The E2F1 mRNA expression showed the opposite result compared with
RB; E2F1 mRNA expression was reduced almost 3-fold following
treatment with BAK or NK cells (Fig.
4C, P<0.05). However, there was also no significant
difference between the effects of BAK and NK cells on the
expression levels of either RB or E2F1. RB and E2F1 are associated
with the cell cycle, therefore, this result was consistent with the
results of the cell cycle assay.
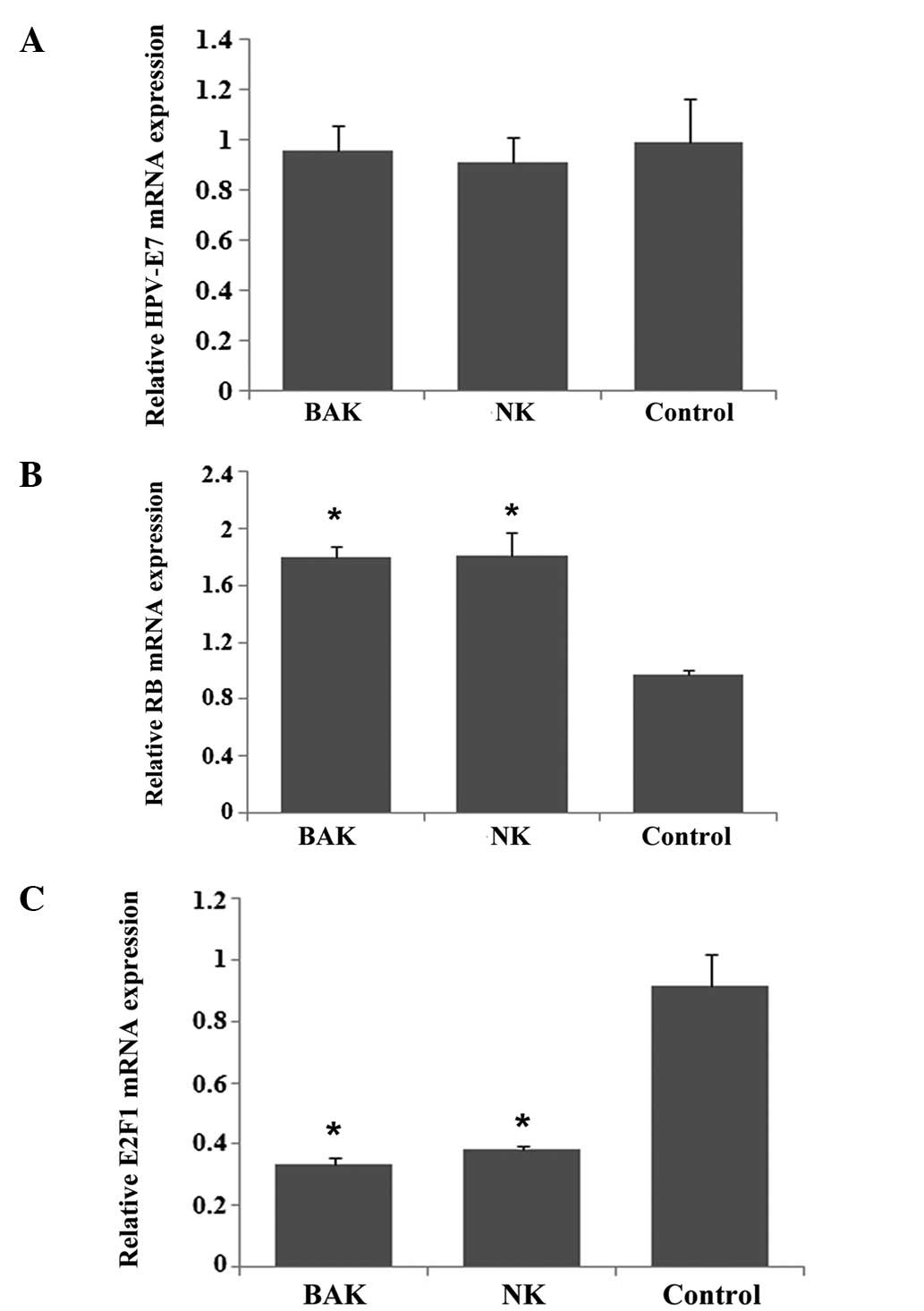 | Figure 4.mRNA expression levels of RB, E2F1 and
HPV-E7 in HeLa cells following treatment with BAK and NK cells. (A)
HPV-E7 mRNA expression was very similar among BAK, NK and control
groups. (B) RB mRNA expression by HeLa cells increased following
treatment with BAK or NK cells, but no significant difference was
observed between the two groups (P>0.05). (C) E2F1 mRNA
expression decreased almost 3-fold after treatment with BAK or NK
cells, but there was also no significant difference between the BAK
and NK groups (P>0.05).*P<0.05, compared with the
blank control. RB, retinoblastoma; HPV, human papillomavirus; BAK,
BCG-activated killer; NK, natural killer; BCG, Bacillus
Calmette-Guerin. |
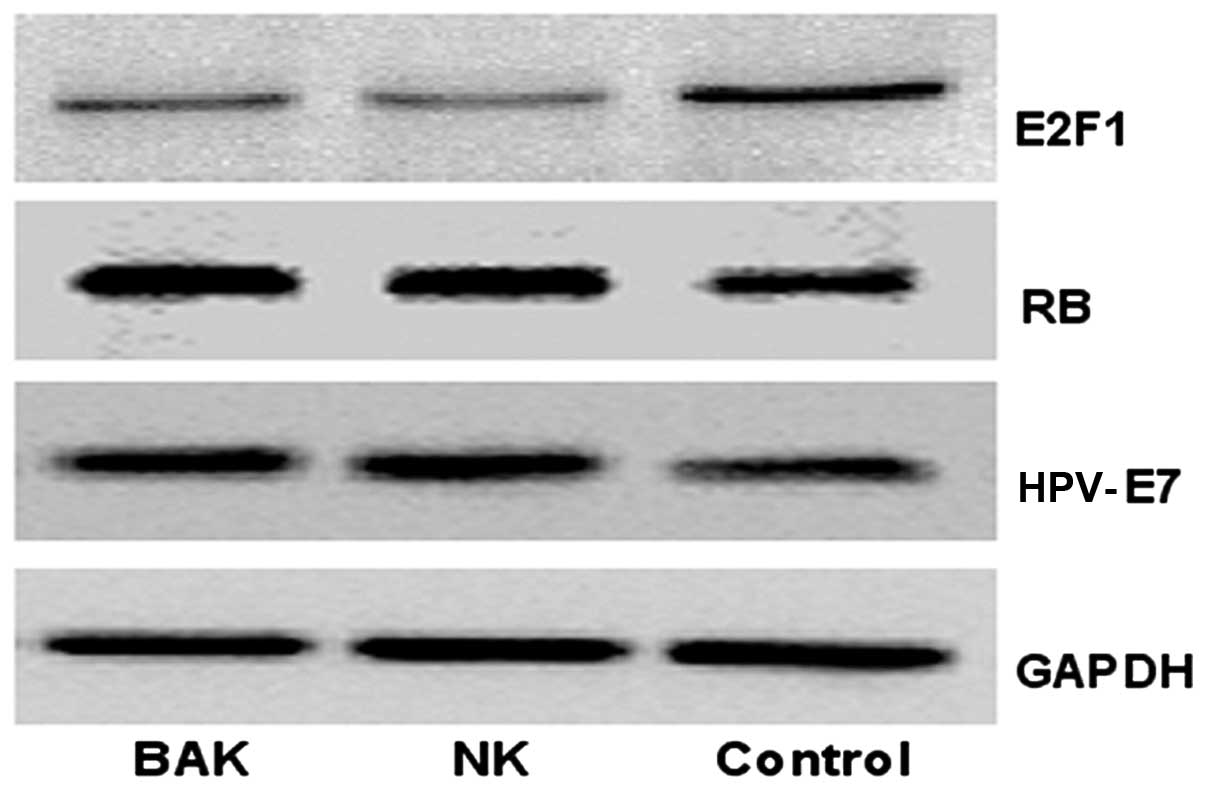
Expression of RB, E2F1 and HPV-E7
proteins in HeLa cells following treatment with BAK and NK
cells
The RB and HPV-E7 protein expression levels
increased in HeLa cells following treatment with BAK and NK cells
compared with their levels in the blank control. By contrast, the
E2F1 protein expression level decreased. However, the differences
in RB, E2F1 and HPV-E7 protein expression levels between the BAK
and NK cell groups were not significant (P>0.05) (Fig. 5). The results concerning the RB and
E2F1 proteins were also consistent with the cycle assay, and the
result showed that the transcription and translation of RB and E2F1
were consistent.
Discussion
Intravesical BCG immunotherapy is a well-established
treatment for human bladder cancer and is commonly used as the
first-line adjuvant treatment. The mechanism of BCG immunotherapy
is complex and remains unclear, however, it is mainly dependent on
the activation of a number of immunocytes (including macrophages,
NK cells, CD4+ and CD8+ T cells) and cytokines
(including IFN-γ, IL-2, IL-12, TNF-α and TNF-β) (13). NK cells are essential for effective
BCG immunotherapy (3). BAK cells
have the
CD3−/CD8+/CD56+/CD16+
phenotype of a subpopulation of NK cells and possibly NK T
lymphocytes (4). It has been
reported that topical BCG for the treatment of genital warts
attained a high success rate (6,7),
even in flat condyloma of the cervix (8). Condylomata acuminata (genital warts)
are caused by HPV. Infection with oncogenic HPV is the leading
cause of cervical carcinoma. In this study, we investigated the
feasibility of BCG immunotherapy for the treatment of high-risk
HPV-infected cervical cancer by examining its cytotoxicity on HeLa
cells. The HeLa cell line is an immortal cervical cancer cell line
which is infected with HPV18. PBMCs stimulated with BCG have been
shown to generate BAK cells in a previous study (12).
In the current study, PBMCs stimulated with BCG
showed more cytotoxicity against HeLa cells compared with PBMCs
that were not stimulated with BCG. We observed that the apoptotic
cell ratio was significantly higher in the BAK group than in the NK
and control groups. However, it was also shown that BCG-stimulated
and untreated PBMCs were able to increase the apoptotic index of
HeLa cells, although the effect was more pronounced for the
BCG-stimulated PBMCs. Perforin and Fas ligand (FasL) are the major
cytolytic molecules of cytotoxic lymphocytes (14). The cellular mediators of BCG
effector mechanisms kill targets via perforin and independently of
the FasL pathway. BCG-activated lymphocytes express higher levels
of perforin (5), and this may be
the mechanism of the increased apoptosis observed in HeLa
cells.
We investigated whether any associated influence on
HPV-E7 protein expression and the RB/E2F1 pathway results from the
BCG treatment. The high risk HPVs (such as HPV-16 and HPV-18) that
are associated with specific anogenital cancers encode two
oncoproteins E6 and E7, which are expressed in HPV-positive
cancers. High-risk HPV-E7 is a major oncoprotein that plays a
crucial role in the development of cervical cancer. The HPV-E7
protein functions in cellular transformation via interactions with
pRB (15). The important roles of
RB have been demonstrated in the suppression of cellular
proliferation (16), stimulation
of differentiation and senescence (17,18),
cellular survival (19) and the
maintenance of stem cell quiescence (20). RB plays a key role in the
regulation of cell cycle progression and it is essential for the
proper modulation of G1/S transition. pRB exerts its
cell cycle regulatory functions mainly by targeting the E2F family
of transcription factors and has been shown to physically interact
with E2F1, 2 and 3, repressing their transcriptional activity.
Multiple genes involved in DNA synthesis and cell cycle progression
are regulated by E2Fs, and RB prevents their expression by
inhibiting E2F activity and thereby inducing growth arrest
(9).
In the present study, flow cytometric cell cycle
analysis showed that HeLa cells treated with BAK or NK cells
demonstrated a shift in the cell population from G1 to S
arrest. However, BAK cells did not show a more pronounced effect
than NK cells on the G1/S arrest. It is known that RB
plays a major role in the regulation of cell cycle progression, it
is essential for the proper modulation of G1/S
transition. We measured the changes of RB and E2F1 at the
transcriptional and translational levels. The results of
quantitative real-time PCR (qRT-PCR) and western blotting showed
consistent changes in RB and E2F1. BAK cells may suppress E2F1
expression in HeLa cells by increasing the expression of RB. E2F1
is a member of the E2F family of transcription factors and plays a
crucial role in the cell cycle during the G1/S
transition. This may be the reason why HeLa cells arrest at
G1/S following incubation with BAK and NK cells.
However, the effect on RB and E2F1 was not significantly different
between the BAK and the NK groups.
It has been established that inactivation of RB by
interaction with HPV-E7 leads to a release of the repression of E2F
activity by RB, and thereby facilitates cell cycle progression
(21). We also hypothesized that
RB/E2F1 pathway alteration was associated with HPV-E7. We predicted
that HPV-E7 protein expression would decrease partly to activate
more RB in order to suppress E2F1 in the BAK cell-treated HeLa
cells. However, the HPV-E7 mRNA expression presented at a
consistent level among the BAK, NK and blank control groups. The
expression level of HPV-E7 protein in the BAK and NK groups was
slightly higher than in the blank control in which the HeLa cells
were not treated with effector cells. In addition, HPV-E7 protein
levels were not significantly different between the BAK and NK
cell-treated groups. The HPV-E7 protein was previously supposed to
be decreased in the HeLa cells following treatment with BAK or NK
cells. By contrast, it was increased in our study. The reason may
be that HPV-E7 viral DNA was randomly integrated into the host
genome and increased following the overexpression of certain
proteinases during the progress of apoptosis. We suggest that
HPV-E7 proteins were inactive, and they could not be combined with
RB proteins or that the expression level of HPV-E7 was less than
that of pRB. We conclude that the BAK or NK cells may affect the
RB/E2F1 pathway during the process of killing the HeLa cells by
increasing the expression of RB and reducing the expression of
E2F1, but the alterations of pRB and E2F1 were not correlated with
the expression of HPV-E7 protein.
In addition, it has been reported that altered pRB
expression is an independent predictor of the recurrence and
progression of non-muscle invasive bladder cancer following BCG
treatment (10). The nuclear pRB
underexpression may be predictive of nonresponse and cancer
recurrence following intravesical BCG+IFN-α therapy (11). Genetic alterations of the RB gene
and aberrant post-translational modifications of the RB protein
have also been implicated in invasive bladder cancer. Alterations
in the RB gene or protein are becoming candidate targets for novel
therapeutics (22). In the current
study, changes of RB transcription and translation were detected in
HeLa cells following treatment with BAK or NK cells. We consider
that the immunotherapy may be an effective therapy for cervical
carcinoma based on this study, and BCG immunotherapy, which
promoted apoptosis of target cancer cells, is an alternative
method.
In summary, our study demonstrates that PBMCs
inhibit the proliferation of the human cervical carcinoma cell
line, HeLa, by G1/S arrest and the promotion of
apoptosis of HeLa cells following stimulation with BCG. The
mechanism of G1/S arrest may be correlated with the
RB/E2F1 pathway, but the RB and E2F1 alterations are not caused by
HPV-E7. This study showed that BCG immunotherapy is a potential
treatment for cervical cancer. Our study is limited and
preliminary, and further experiments and clinical trials are
required to verify this effect.
Acknowledgements
This study was supported by grants
from the health department of Zhejiang province, China (No.
2008A069).
References
|
1.
|
Babjuk M: New insights in intravesical
treatment for intermediate- and high-risk non-muscle-invasive
urothelial bladder carcinoma. Eur Urol. 57:774–776. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Shelley MD, Mason MD and Kynaston H:
Intravesical therapy for superficial bladder cancer: a systematic
review of randomised trials and meta-analyses. Cancer Treat Rev.
36:195–205. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Brandau S, Riemensberger J, Jacobsen M, et
al: NK cells are essential for effective BCG immunotherapy. Int J
Cancer. 92:697–702. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Brandau S and Böhle A: Activation of
natural killer cells by Bacillus Calmette-Guérin. Eur Urol.
39:518–524. 2001.
|
|
5.
|
Brandau S, Suttmann H, Riemensberger J, et
al: Perforin-mediated lysis of tumor cells by Mycobacterium
bovis Bacillus Calmette-Guérin-activated killer cells. Clin
Cancer Res. 6:3729–3738. 2000.PubMed/NCBI
|
|
6.
|
Metawea B, El-Nashar AR, Kamel I, Kassem W
and Shamloul R: Application of viable bacille Calmette-Guérin
topically as a potential therapeutic modality in condylomata
acuminata: a placebo-controlled study. Urology. 65:247–250.
2005.PubMed/NCBI
|
|
7.
|
Böhle A, Büttner H and Jocham D: Primary
treatment of condylomata acuminata with viable bacillus
Calmette-Guerin. J Urol. 165:834–836. 2001.PubMed/NCBI
|
|
8.
|
Fayed ST, Amer M, Ammar E and Salam MA:
Local BCG injection administered to patients with flat condyloma of
the cervix. Int J Gynaecol Obstet. 107:253–254. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Singh S, Johnson J and Chellappan S: Small
molecule regulators of RB-E2F pathway as modulators of
transcription. Biochim Biophys Acta. 1799:788–794. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Cormio L, Tolve I, Annese P, et al:
Altered p53 and pRB expression is predictive of response to BCG
treatment in T1G3 bladder cancer. Anticancer Res. 29:4201–4204.
2009.PubMed/NCBI
|
|
11.
|
Esuvaranathan K, Chiong E, Thamboo TP, et
al: Predictive value of p53 and pRB expression in superficial
bladder cancer patients treated with BCG and interferon-alpha.
Cancer. 109:1097–1105. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Thänhauser A, Böhle A, Flad HD, Ernst M,
Mattern T and Ulmer AJ: Induction of
bacillus-Calmette-Guérin-activated killer cells from human
peripheral blood mononuclear cells against human bladder carcinoma
cell lines in vitro. Cancer Immunol Immunother. 37:105–111.
1993.
|
|
13.
|
Suttmann H, Jacobsen M, Reiss K, Jocham D,
Böhle A and Brandau S: Mechanisms of bacillus Calmette-Guerin
mediated natural killer cell activation. J Urol. 172:1490–1495.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Moretta A: Molecular mechanisms in
cell-mediated cytotoxicity. Cell. 90:13–18. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Münger K and Howley PM: Human
papillomavirus immortalization and transformation functions. Virus
Res. 89:213–228. 2002.PubMed/NCBI
|
|
16.
|
Cobrinik D: Pocket proteins and cell cycle
control. Oncogene. 24:2796–2809. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Bremner R and Zacksenhaus E: Cyclins,
Cdks, E2f, Skp2, and more at the first international RB Tumor
Suppressor Meeting. Cancer Res. 70:6114–6118. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Deshpande A, Sicinski P and Hinds PW:
Cyclins and cdks in development and cancer: a perspective.
Oncogene. 24:2909–2915. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Chau BN and Wang JY: Coordinated
regulation of life and death by RB. Nat Rev Cancer. 3:130–138.
2003. View
Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Ruiz S, Santos M, Segrelles C, et al:
Unique and overlapping functions of pRB and p107 in the control of
proliferation and differentiation in epidermis. Development.
131:2737–2748. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Wise-Draper TM and Wells SI:
Papillomavirus E6 and E7 proteins and their cellular targets. Front
Biosci. 13:1003–1017. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Mitra AP, Birkhahn M and Cote RJ: p53 and
retinoblastoma pathways in bladder cancer. World J Urol.
25:563–571. 2007. View Article : Google Scholar : PubMed/NCBI
|