Introduction
The high recrudescence rate of drug addiction has
received attention worldwide and its mechanisms remains to be
determined and elucidated.
Drug craving in addiction maturation involves
multiple memory circuits, including working, fragmentary and
emotional memories (1). In
addition, addiction modifies a number of morphological changes in
the hippocampus, including organelle reduction, mitochondrial
swelling, chromatin margination, karyopyknosis and necrosis, which
have been observed in rats with morphine addiction (2,3).
Craving extinction is a condition in which an individual with a
drug addiction does not present drug craving and seeking behaviour
for a certain period after drug withdrawal. However, craving
extinction is only an occult state of drug dependency, which is
transformed into a recrudescent state through stimulation (4). The recrudescence of drug addiction
refers to a condition in which an occult state of drug craving and
seeking behaviour turns into an apparent state by ignition, thereby
leading to addiction recrudescence (5). The recrudescence of drug addiction is
the reinstatement of drug craving following extinction, which is
largely associated with learning and memory (6). The hippocampus is the memory centre,
an important part of which is the Papez circuit, a key addiction
maturation circuit (7).
Effective animal models, including locomotor
sensitization, conditioned place preference (CPP), drug
discrimination and self-administration models play a crucial role
in the study of drug addiction. Locomotor sensitization is easy to
perform and has good sensitivity. However, this model does not
reflect the subjective desires of the animals involved. CPP is also
easy to implement and has a short experimental period; however, it
requires a large number of animals and also does not reflect the
subjective desires of the animals involved. Drug discrimination is
classified as a behavioural test that exhibits the advantages of
reflecting the desires of the animals studied, as well as easy
animal model maintenance. However, this model has poor sensitivity
(5). By contrast,
self-administration efficiently reflects the subjective demands of
the animal and simulates the process of human addictive behaviour;
however, it has a complicated procedure and requires laborious
animal maintenance (8,9).
In light of such procedures, intravenous
self-administration animal models were established in the present
study to investigate the changes in hippocampal protein expression
during the recrudescence of morphine addiction.
Recrudescence-related specific proteins were then identified. Using
these procedures, this study intends to lay a theoretical
foundation for research into the molecular biological mechanism of
recrudescence and its treatment targets.
Materials and methods
Animals
Sixteen adult male, clean-grade Sprague-Dawley rats
were supplied by the laboratory animal centre of Zhejiang, China.
These animals weighed 240±10 g before the experiment and 220±10 g
by the end of the experiment. Specific experimental cages were
designed and software devices were programmed according to the
SuperState Borland Delphi software. This study was conducted in
strict accordance with the recommendations in the Guide for the
Care and Use of Laboratory Animals of the National Institutes of
Health and the animal use protocol was reviewed and approved by the
Institutional Animal Care and Use Committee (IACUC) of the First
Affiliated Hospital of Chongqing Medical University.
Model establishment
Models were established according to the methods
used by Weeks (9); however, a
number of adaptations were conducted according to the requirements
of the current experiment. The experimental procedure was divided
into five stages as follows: i) the animals were fed naturally for
3–5 days for acclimation prior to the experiment. Spontaneous nose
poke screening tests were carried out 1 day before training. The
animals were kept away from food 24 h before the screening. Those
rats with a spontaneous nose poke count of >10/4 h during the
observation period were excluded. ii) The animals were subjected to
a venous cannula treatment of the neck and then allowed to recover
for 7 days. During the recovery period, the rats were fed ad
libitum and received an anti-infection treatment with
penicillin for 3 days. iii) The animals were placed into specific
cages and were allowed to run for 15 min. They were given water
ad libitum but were prohibited food during the training. A
green light was turned on at the beginning of each training cycle.
Whenever an effective nose poke occurred, the light would be turned
off. Immediately, the automatic equipment injected morphine (purity
98%, purchased from Zhejiang Provincial Public Security Department,
Zhejiang, China) intravenously at 1.0 mg/kg with a pump injection
sound. A red light was turned on for 5 sec to indicate an effective
nose poke. A 20 sec refractory period ensued after each injection,
during which nose pokes were counted but without drug injections
administered. The control group was treated under the same method
using physiological saline injections at 1.0 mg/kg instead of drug
injections. iv) A natural withdrawal method was adopted. The
animals were trained for 2 h each day. During the training, the
rats were given water ad libitum but no food and were placed
in an environment without light signals and injections. The
training lasted for 12 days. v) The animals were returned to the
training cages, in which they were again stimulated by lights and
pump injection sounds. Conditions were the same as during the
addiction training but without morphine injections. Effective nose
poke counts were recorded.
Hippocampal tissue handling
The animals were decapitated rapidly after
anesthetisation. Brain tissue blood was flushed through using
pre-chilled physiological saline (4°C). The skull was isolated and
the brain tissues were then extracted completely. The brain tissues
and fascia on the surface of the hippocampus were removed and
placed in an ice bath. The hippocampus was collected, weighed and
frozen in liquid nitrogen for 2 min prior to storing at −80°C.
Tissue protein extraction
The samples in the cryopreservation tube were
swirled with pre-chilled deionised water (4°C) and then centrifuged
at 3,000 × g for 5 min. The supernatant was removed and 0.1 ml 40
mmol/l Tris was added (Sigma-Aldrich, St. Louis, MO, USA). Each
sample was frozen and thawed three times for 1 min. An enzyme was
added to obtain the homogenate on ice. The homogenate was allowed
to react with the enzyme for 25 min at 4°C. Lysate was added and
the homogenate was obtained on ice. After another 25-min reaction
period at 4°C, the obtained sample was centrifuged at 13,000 × g
for 30 min and the supernatant was collected. The protein
concentration was determined using the Bradford method.
Immobilised pH gradient-based
two-dimensional gel electrophoresis (2-DE)
Isoelectric focusing (first dimension) was performed
by diluting the sample in heavy rehydration buffer. Rehydration and
focusing were conducted automatically at 18°C. The total voltage ×
working time was ∼75,000 V/h. Then, the following procedure was
performed: i) gel strip equilibration. The focused gel strips were
equilibrated twice in equilibration buffer and washed in
electrophoresis buffer for 1 min.ii) Sodium dodecylsulphate
polyacrylamide gel electrophoresis (SDS-PAGE; second dimension) was
carried out. iii) Silver nitrate staining. The gels were fixed in
stationary liquid overnight, rinsed, sensibilised, washed and kept
away from light for 25 min. The gels were washed three times and
then stained. Protein expression in the morphine and physiological
saline groups were determined repeatedly to obtain stable graphic
spectra for the comparison of the protein spots. Repeatability was
assessed. iv) Gel image analysis. Gel images were obtained using a
GS-800 scanner and Quality One scanning software ChemiDoc XRS
(Bio-Rad, Hercules, CA, USA). The intensity correction, spot
detection, background subduction, matching and ID correction of the
images were performed using PDQuest 2D analysis software (Bio-Rad).
Disparate protein spots were obtained by comparing the images of
the morphine and physiological saline groups using SPSS software
(SPSS Inc., Chicago, IL, USA).
Mass spectrometry identification
The disparate protein points were cut and digested
with trypsin in gel. Peptide fingerprint data were obtained using
matrix-assisted laser desorption/ionisation time-of-flight mass
spectrometry. Following removal of the interference peaks, the
Swiss-Prot database was accessed. The type of rat was limited and
an error within 1 Da was allowed. At least four peptides were
matched and the formylation and partial oxidation of methionine
were allowed. Fifteen disparate proteins were identified.
Statistical analysis
Data were analysed using SPSS 15.0 software.
Effective nose poke counts were presented as the means ± standard
error. Paired t-tests were performed for comparisons between groups
and one-factor analysis of variance (ANOVA) was used for
comparisons within a group during recrudescence. P<0.05
indicated a statistically significant difference.
Results
Addiction phase
Compared with the saline group, the morphine group
showed a significant difference from 2 days of addiction training
(P<0.01). The morphine group arrived at a relatively stable
plateau phase of self-administration after 3 days, with a nose poke
count of >20/4 h, which was also significantly higher than the
saline group (P<0.001). These results are summarised in Table I.
 | Table I.Effective nose poke counts in the
morphine and physio logical saline groups at different time points
in the addiction maturation phase. |
Table I.
Effective nose poke counts in the
morphine and physio logical saline groups at different time points
in the addiction maturation phase.
| Day | Morphine group | Physiological saline
group |
|---|
| 1 | 7.62±3.41 | 5.17±2.11 |
| 2 | 13.13±3.31a | 8.21±2.36 |
| 3 | 21.73±4.05b | 7.26±3.93 |
| 4 | 27.26±4.63b | 6.04±1.56 |
| 5 | 22.04±4.55b | 5.64±1.24 |
| 6 | 25.54±3.66b | 5.86±1.90 |
| 7 | 25.05±3.14b | 7.54±3.21 |
| 8 | 27.18±5.17b | 5.51±1.66 |
| 9 | 28.25±3.83b | 5.60±1.43 |
| 10 | 31.65±3.37b | 5.80±1.96 |
| 11 | 30.09±3.55b | 7.31±2.62 |
| 12 | 34.22±5.61b | 7.09±2.75 |
| 13 | 31.08±5.14b | 6.82±1.40 |
Extinction phase
A significant difference in the nose poke count
between the two groups was observed 2 days before drug withdrawal
(P<0.001). After 3 days extinction, the nose poke count in the
morphine group dropped below the spontaneous nose poke count (10/4
h). No significant difference in this count was observed compared
with the saline group (P>0.05). This state continued until the
end of the 12 days of the extinction training. These results are
summarised in Table II.
 | Table II.Effective nose poke counts in the
morphine and physio logical saline groups at different time points
in the addiction extinction phase. |
Table II.
Effective nose poke counts in the
morphine and physio logical saline groups at different time points
in the addiction extinction phase.
| Day | Morphine group | Physiological saline
group |
|---|
| 1 | 13.60±1.3b | 6.16±1.62 |
| 2 | 10.71±1.6b | 4.99±1.26 |
| 3 | 3.72±1.52a | 2.88±1.12 |
| 4 | 2.23±0.65 | 2.01±1.03 |
| 5 | 2.41±0.47 | 2.43±0.84 |
| 6 | 3.04±0.84 | 2.46±0.56 |
| 7 | 1.08±1.12 | 1.24±0.63 |
| 8 | 2.09±0.72 | 0.89±0.39 |
| 9 | 2.12±0.91 | 1.17±0.36 |
| 10 | 1.32±0.33 | 0.96±0.45 |
| 11 | 2.02±1.23 | 2.16±1.17 |
| 12 | 2.11±1.14 | 1.3±0.9 |
Recrudescence phase
The effective post-recrudescence nose poke count in
the morphine group significantly increased compared with the
pre-recrudescence count (P<0.001). Furthermore, the effective
pre- and post-recrudescence counts in the morphine group varied
significantly from the effective pre- and post-recrudescence counts
in the physiological saline group (P<0.001). Although the
effective post-recrudescence nose poke count in the physiological
saline group also increased compared with the pre-recrudescence
count, the difference was not significant. These results are
summarised in Table III.
 | Table III.Effective nose poke counts in the
morphine and physiological saline groups in the extinction and
recrudescence phases. |
Table III.
Effective nose poke counts in the
morphine and physiological saline groups in the extinction and
recrudescence phases.
| Phases | Morphine group | Physiological saline
group |
|---|
| Extinction | 2.6±1.3 | 2.2±1.1 |
| Recrudescence | 26.2±6.2a,b | 3.2±1.4 |
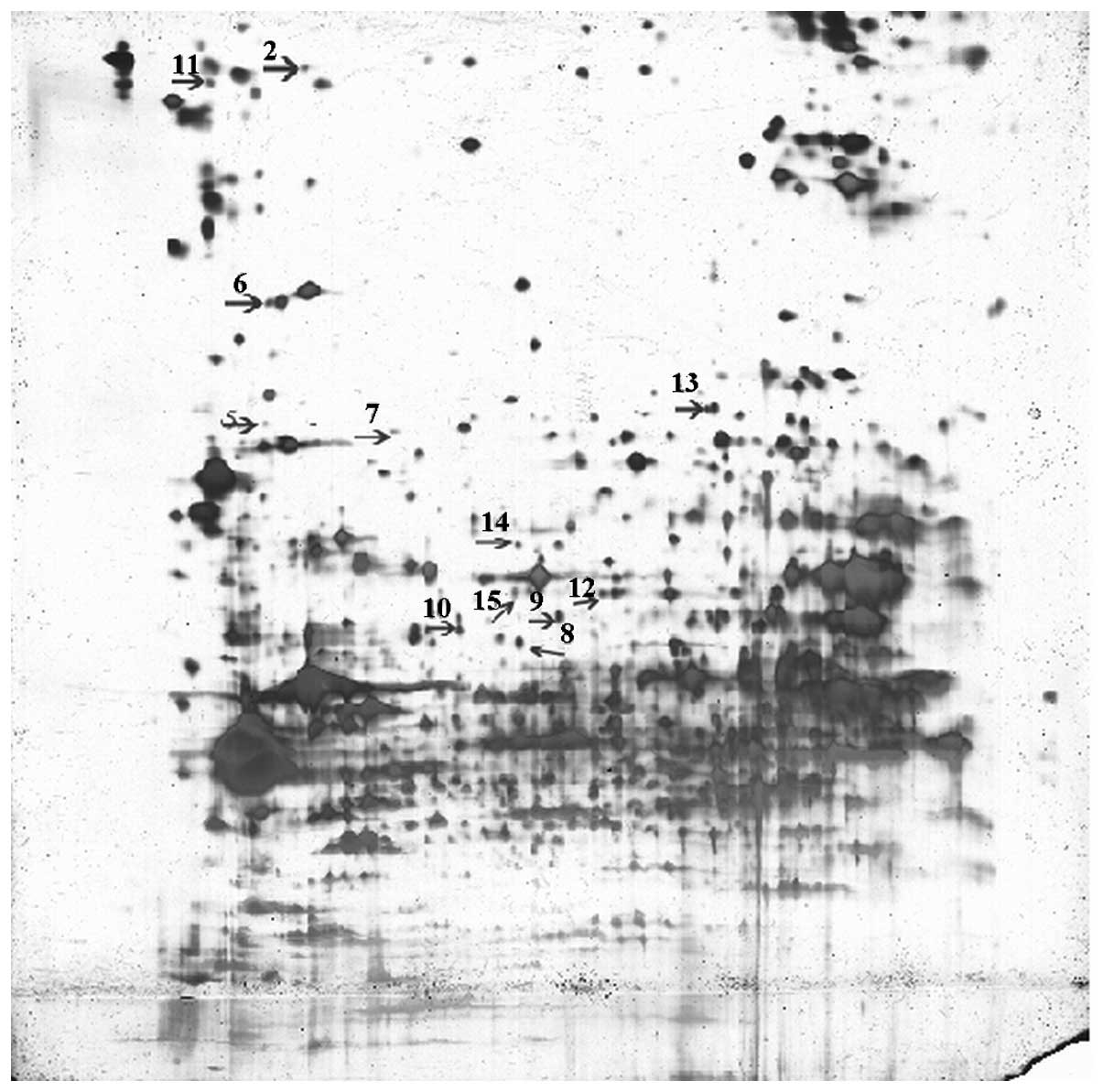
Distributions of amphitropic protein
spots
The matching rate between the gels of the groups was
76%. The gel images of the two groups were compared using PDQuest
software, taking one sheet from each group as a reference.
Significant differences in 23 spots were observed (P<0.05;
Figs. 1 and 2).
Disparate protein spots
Fifteen disparate proteins were identified,
including three enzymes related to energy metabolism, nucleoside
diphosphate (NDP) kinase A, fatty acid-binding protein and
nicotinamide adenine dinucleotide (NADH) dehydrogenase (ubiquinone)
1 α subcomplex subunit 10; two ionic channel regulatory proteins,
K+ channel-interacting protein 2 and mitogen-activated
protein kinase 11, as well as carboxylesterase 3,
cytoskeleton-associated protein 4 and 14-3-3 protein β/α. These
results are summarised in Table
IV.
 | Table IV.Disparate protein spots in the
morphine and physiological saline recrudescence groups. |
Table IV.
Disparate protein spots in the
morphine and physiological saline recrudescence groups.
| Protein point
group | Serial number | Name | Molecular
weight/isoelectric point (Da) | Peptides
matching | Expression changes
with morphine |
|---|
| 1 | O70139 | cAMP-dependent
protein kinase inhibitor γ | 7944/4.1 | 5/11 | Increase |
| 2 | P55051 | Fatty acid-binding
protein | 14864/5.5 | 11/32 | Decrease |
| 3 | Q61908 | Protein p8
MTCP-1 | 7743/8.7 | 4/27 | Decrease |
| 4 | Q05982 | Nucleoside
diphosphate kinase A | 17193/6.0 | 4/15 | Increase |
| 5 | Q9D3N2 | EF-hand
calcium-binding domain-containing protein 1 | 24606/4.9 | 7/23 | Decrease |
| 6 | Q9D217 | Prune homolog
2 | 20677/4.8 | 9/69 | Decrease |
| 7 | Q9JM59 | K+
channel-interacting protein 2 | 30993/4.9 | 6/18 | Increase |
| 8 | Q561S0 | NADH dehydrogenase
(ubiquinone) 1 α subcomplex subunit 10 | 40493/7.6 | 15/45 | Decrease |
| 9 | Q8VCT4 | Carboxylesterase
3 | 61788/6.2 | 7/23 | Decrease |
| 10 | Q8BMK4 |
Cytoskeleton-associated protein 4 | 63693/5.5 | 10/30 | Decrease |
| 11 | P51879 | Oncomodulin | 12260/4.0 | 4/12 | None |
| 12 | Q920V68 | 14-3-3 protein
β/α | 39371/8.1 | 17/50 | None |
| 13 | Q64270 | Translation
initiation factor eIF-2B subunit α | 33679/8.4 | 5/14 | None |
| 14 | P14659 | Heat shock-related
70 kDa protein 2 | 69642/5.5 | 6/27 | None |
| 15 | Q9WUI1 | Mitogen-activated
protein kinase 11 | 41358/5.4 | 5/22 | None |
Discussion
The present study has demonstrated that
self-administration models exhibit a relatively stable drug
addiction plateau, extinction and recrudescence phases, which
satisfies the current primary criteria for recrudescence models
(10,11). Thus, this study succeeded in
establishing self-administration rat models and simulating the
three phases of addiction, maturation, extinction and
recrudescence.
Fifteen disparate proteins were identified. Among
these proteins, three are associated with energy metabolism, NDP
kinase A, fatty acid-binding protein and NADH dehydrogenase
(ubiquinone) 1 α subcomplex subunit 10. These proteins participate
in the energy supply process through oxidation reduction. The
results of this study revealed that their expression significantly
decreased in the morphine group during the recrudescence phase.
This finding suggests a decrease in hippocampal energy metabolism
during the morphine addiction maturation or addiction recrudescence
process. NDP kinase A is highly expressed in the sera of stroke
patients and therefore serves as a marker for apoplectic seizure.
This indicates that its overexpression is a manifestation of an
increase in brain function compensation and a stress-induced change
due to brain injury (12). NDP
kinase A, together with a series of other individual proteins, is
linked to performance in the Morris water maze (13). Serotonin 1A receptor knockout mice
are commonly used in anxiety and cognitive function tests. NDP
kinase A expression decreases in these rat models (14).
Of the disparate proteins identified in this study,
two were ionic channel regulatory proteins: K+
channel-interacting protein 2 and mitogen-activated protein kinase
11. The former regulates the density of potassium ion receptors in
cell membranes and promotes complex formation in the ionic channel
(15) and the latter transmits the
pressure on the cell membranes and induces gliocytes to activate
the p38 mitogen-activated protein kinase channel (16). Thus, the two proteins function in
intra and extracellular substance transportation. This study has
demonstrated that the expression of K+
channel-interacting protein 2 and mitogen-activated protein kinase
11 decreased in the morphine recrudescence group. This finding
indicates a decrease in substance transport ability and cell
metabolism during the morphine recrudescence phase. Heat
shock-related 70 kDa protein 2 is expressed in the hippocampus of
normal rats (17). The expression
of this protein also significantly decreased in the morphine
recrudescence group. Heat shock-related 70 kDa protein 2 functions
in the accurate assembly, folding and transport of proteins. It
also participates in the maintenance of normal projection
transmission and inhibits apoptosis (18). A decrease in its expression may
therefore accelerate apoptosis. This finding is in line with the
hippocampal atrophy observed following long-term addiction
(19).
A decrease in carboxylesterase 3 expression was also
observed in the morphine recrudescence group. Carboxylesterase 3
performs a significant role in exogenous material metabolism and
specifically binds with morphine in the liver (20). It is also involved in cocaine and
morphine metabolism. Therefore, the significant decrease in its
expression in the morphine recrudescence group may be closely
correlated with the mechanism of recrudescence. In addition, the
expression of cytoskeleton-associated protein 4 and 14-3-3 protein
β/α decreased in the experimental group, which is consistent with a
previous study reporting the decreased expression of these proteins
in rats with morphine addiction (20). Presumably, these changes are
associated with neural cell reduction and protein fibre
demyelination. However, whether the low expression of these two
proteins occurs in whole brain tissues or is confined to the
hippocampus remains to be explored.
The present study has a number of limitations.
Firstly, the changes in brain proteins following addiction are
complicated, involving other brain regions, including the
hippocampus, nucleus accumbens septum and mesencephalic ventral
tegmental area. This study only focused on the hippocampus, thus
investigation into these other brain sections should be conducted.
Secondly, protein databases are not complete. Consequently, certain
proteins may still not be listed. Additionally, the roles of some
proteins remain uncertain. Further role verifications for key
proteins are therefore necessary.
Acknowledgements
This study was supported by the
National Natural Science Foundation of China (grant no.
30570658).
References
|
1.
|
Hadjiconstantinou M and Neff NH: Nicotine
and endogenous opioids: neurochemical and pharmacological evidence.
Neuropharmacology. 60:1209–1220. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Sussman S and Sussman AN: Considering the
definition of addiction. Int J Environ Res Public Health.
8:4025–4038. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Morales M and Pickel VM: Insights to drug
addiction derived from ultrastructural views of the
mesocorticolimbic system. Ann N Y Acad Sci. 1248:71–88. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Gudehithlu KP, Duchemin AM, Tejwani GA,
Neff NH and Hadjiconstantinou M: Nicotine-induced changes of brain
beta-endorphin. Neuropeptides. 46:125–131. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Zhang F, Zhou W, Tang S, Lai M, Liu H and
Yang G: Motivation of heroin-seeking elicited by drug-associated
cues is related to total amount of heroin exposure during
self-administration in rats. Pharmacol Biochem Behav. 79:291–298.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Hu Z, Deng Y, Hu C, et al: 1H
NMR-based metabonomic analysis of brain in rats of morphine
dependence and withdrawal intervention. Behav Brain Res. 231:11–19.
2012. View Article : Google Scholar
|
|
7.
|
Goldstein RZ and Volkow ND: Drug addiction
and its underlying neurobiological basis: neuroimaging evidence for
the involvement of the frontal cortex. Am J Psychiatry.
159:1642–1652. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Zhou W, Zhang F, Tang S, Liu H, Gu J and
Yang G: The dissociation of heroin-seeking patterns induced by
contextual, discriminative, or discrete conditioned cues in a model
of relapse to heroin in rats. Psychopharmacology (Berl).
181:197–206. 2005. View Article : Google Scholar
|
|
9.
|
Weeks JR: Experimental morphine addiction:
method for automatic intravenous injections in unrestrained rats.
Science. 138:143–144. 1962. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Robinson TE: Neuroscience. Addicted rats.
Science. 305:951–953. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Vanderschuren LJ and Everitt BJ: Drug
seeking becomes compulsive after prolonged cocaine
self-administration. Science. 305:1017–1019. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Marginean IC, Stanca DM, Vacaras V,
Soritau O, Margiean M and Muresanu DF: Plasmatic markers in
hemorrhagic stroke. J Med Life. 4:148–150. 2011.PubMed/NCBI
|
|
13.
|
Li K, Muller I, Patil S, et al:
Strain-independent global effect of hippocampal proteins in mice
trained in the Morris water maze. Amino Acids. 43:1739–1749. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Li L, Whittle N, Klug S, et al: Serotonin
(1A)-receptor-dependent signaling proteins in mouse hippocampus.
Neuropharmacology. 57:556–566. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Foeger NC, Marionneau C and Nerbonne JM:
Co-assembly of Kv4 alpha subunits with K+
channel-interacting protein 2 stabilizes protein expression and
promotes surface retention of channel complexes. J Biol Chem.
285:33413–33422. 2010.PubMed/NCBI
|
|
16.
|
Hur J, Kim SY, Kim H, Cha S, Lee MS and
Suk K: Induction of caspase-11 by inflammatory stimuli in rat
astrocytes: lipopolysaccharide induction through p38
mitogen-activated protein kinase pathway. Febs Lett. 507:157–162.
2001. View Article : Google Scholar
|
|
17.
|
McClung CA: The molecular mechanisms of
morphine addiction. Rev Neurosci. 17:393–402. 2006. View Article : Google Scholar
|
|
18.
|
Bierczynska-Krzysik A, Bonar E, Drabik A,
et al: Rat brain proteome in morphine dependence. Neurochem Int.
49:401–406. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Tsuchida H, Nishimura I and Fukui K:
Alcohol and substance dependence. Brain Nerve. 64:163–173. 2012.(In
Japanese).
|
|
20.
|
Brzezinski MR, Spink BJ, Dean RA, Berkman
CE, Cashman JR and Bosron WF: Human liver carboxylesterase hCE-1:
binding specificity for cocaine, heroin, and their metabolites and
analogs. Drug Metab Dispos. 25:1089–1096. 1997.PubMed/NCBI
|