Introduction
Diabetic retinopathy (DR) is a common
blindness-causing retinal vascular disease. The pathogenesis of DR
is complex and has yet not been clarified. Advanced glycation end
products (AGEs) are a class of complex products. AGEs are the
results of a reaction between carbohydrates and free amino groups
on proteins. AGEs are toxic and may induce bacteria to undergo
mutagenesis. AGEs are formed in excess during aging, diabetes
mellitus and renal failure (1). A
key characteristic of certain reactive or precursor AGEs is their
ability to form covalent crosslinks between proteins which alters
their structure and function, as observed in the cellular matrix,
basement membranes and vessel-wall components (2). In addition, AGEs are associated with
the development of DR. However, the pathogenic mechanisms are
poorly defined. Vascular endothelial growth factor (VEGF) is widely
recognized as the most influential factor that induces mitosis and
regulates the permeability of endothelial cells, and increases
vascular permeability. VEGF levels are increased in ischemic and
nonischemic diabetic retina, and VEGF is required for the
development of retinal and iris neovascularization. In addition,
VEGF alone may induce the majority of the concomitant pathology of
DR. Intraocular VEGF levels are increased in diabetic patients
(3). Furthermore, the specific
intravitreous injected inhibition of VEGF may reduce the symptoms
of DR (4). Hypoxia-inducible
factor-1 (HIF-1) is a key transcription factor, involved in the
regulation of intracellular metabolism, which induces the
expression of the downstream gene VEGF (5). In addition, HIF-1α plays a
pathogenetic role in DR (6).
However, the mechanism by which AGEs modulate the expression VEGF
and HIF-1α, and the correlation between VEGF and HIF-1α is not
clear. With reference to these data, we examined the role of AGEs
in the induction of VEGF and HIF-1α expression in vitro.
Materials and methods
Materials
Fetal bovine serum (FBS) was purchased from PAA
Laboratories (Colbe, Germany). DMSO was obtained from Sigma (St.
Louis, MO, USA), anti-mouse polyclonal antibody HIF-1α was
purchased from Novus Biologicals (NB100-105; Littleton, CO, USA)
and 4% paraformaldehyde was purchased from Wuhan Boster Biological
Technology, Ltd. (Wuhan, China). The bicinchoninic acid (BCA) and
VEGF ELISA kit were obtained from Beijing Dingguo Changsheng
Biotechnology Co. Ltd. (Beijing, China). The anti-mouse
immunohistochemistry kit was purchased from Jingmei Inc. (Shanghai,
China), anti-β-actin secondary antibody was purchased from Beijing
Zhongshan Jinqiao Biotechnology Co. Ltd. (Beijing, China) and
HRP-goat anti-mouse IgG was obtained from Beyotime Institute of
Biotechnology (Shanghai, China).
Cell culture
Rhesus monkey retinal fovea vascular endothelial
cells (RF/6A) were obtained from our laboratory (Department of
Ophthalmology, The Second Xiangya Hospital, Xiangya School of
Medicine, Central South University, Changsha). The RF/6A cells were
maintained in DME containing 10% FBS. The cells were incubated with
5% CO2 at 37°C. The study was approved by the
Institutional Review Board of The Second Xiangya Hospital, Central
South University (Changsha, China).
Preparation of AGEs
Bovine serum albumin (BSA) was glycated by
incubation with glucose-6-phosphate in phosphate-buffered saline
(PBS) for 6 weeks at 37°C, as described previously (7). Dialyzed glycated protein was
characterized based on fluorescence at 450 nm upon excitation at
390 nm using a fluorescence spectrometer (model LS-3B; Perkin-Elmer
Corp., Norwalk, CT, USA). The endotoxin content in each sample was
measured by the Limulus amebocyte lysate assay (E-Toxate; Sigma)
and observed to be below detectable levels (0.2 ng/ml). For the
control, non-glycated albumin consisted of the same initial
preparations of albumin incubated at 37°C in the absence of sugar.
The glycation process was performed twice, and the two AGE
preparations yielded similar results.
Experimental groups
The cells were divided into two groups: the AGE
experimental group and the non-glycated control group. The RF/6A
cells were incubated with different concentrations of AGEs (0, 50,
100, 200, 400 and 800 mg/l) for 24 h.
Conditioned media VEGF
measurements
The conditioned media VEGF levels were determined
using a sandwich ELISA assay according to the manufacturer’s
instructions. Briefly, cells were cultured for 24 h, the
supernatant was removed and transferred to wells which were coated
with a monoclonal antibody to VEGF. VEGF proteins levels were
normalized to cell counts.
Detection of HIF-1α protein
expression
Briefly, after the cells were cultured for 24 h, the
culture solution was removed and cells were washed in PBS three
times. The cells were fixed using 4% paraformaldehyde for 10 min.
The cells were then incubated with polyclonal antibody to HIF-1α
(1:100) and stained with DAB.
Western blot assay of HIF-1α
protein
After the cells were cultured with various
concentrations of AGEs for 24 h, the culture solution was discarded
and cells were washed with PBS three times. The cells were scraped
and diverted into a 1.5 ml microtube which contained RIPA buffer
with protease inhibitor. After being put on ice for 3 min, the
mixture was agitated, dissolved and put on ice for a further 30
min. The mixture was centrifuged at 4°C for 25 min [5,220 × g;
Microcentrifuge 5415R (5415D); Eppendorf, Stevenage, UK], the top
clear liquid layer was removed and western blotting was performed
in triplicate.
Statistical analysis
Values were expressed as the mean ± standard
deviation (SD). Statistical analyses were performed using SPSS
software V11.5 (SPSS, Inc., Chicago, IL, USA). Continuous data were
analyzed by the Levene method. The significance of a difference
between groups was evaluated using one-way ANOVA with a post hoc
Student-Newman-Keuls multiple comparisons test. The experimental
and control groups were compared using a matched Student’s t-test.
The correlation between VEGF and HIF-1α expression was analyzed
with double variable regression and correlation analysis. P<0.05
was considered to indicate a statistically significant result.
Results
Morphological observation of RF/6A
cells
RF/6A cells are a type of monolayer-adherent cell.
Under a microscope, the cells represented two different shapes. One
type of cell was short and ‘shuttle-like’ with a diminished cell
volume. In addition, the cytoplasm was particularly reflective,
presented a typical ‘cobblestone’ appearance and was small in
number. The other type of cell had a larger volume and was
polygonal. This type of cell grew well, was greater in number, had
extensive cytoplasm and one or more nucleoli (Fig. 1).
Changes in VEGF protein
expression
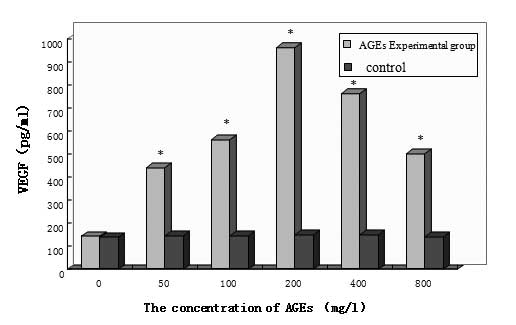
As shown in Fig. 2,
the non-glycated control groups secreted low levels of VEGF and
there was no statistical significance between the cells treated
with different concentrations of non-glycated albumin (P>0.05).
By contrast, VEGF secretion was significantly higher in all AGE
experimental groups compared with the control groups (P<0.05).
In addition, VEGF secretion increased as the AGE concentration
increased and the VEGF level reached its highest point at 200 mg/l
AGE. At higher concentrations of AGEs, the VEGF secretion gradually
decreased, but there remained a significant difference compared
with the control group (P<0.05). In summary, VEGF expression was
AGE concentration-dependent.
HIF-1α protein expression in RF/6A
cells
Immunocytochemical analysis of HIF-1α
protein expression
As shown in Fig. 3,
HIF-1α protein expression was observed in the AGE experimental
groups, where the majority of HIF-1α was located in the nucleus and
a small quantity was located in the cytoplasm, which was
significantly different from the control group (P<0.05).
However, no significant differences (P>0.05) were observed among
the experimental groups. In addition, the difference in HIF-1α
expression between the AGE group and blank control group was not
statistically significant (P>0.05). The non-glycated control
groups had low HIF-1α protein expression levels, and there were no
significant differences (P>0.05) among the non-glycated control
groups. However, the HIF-1α expression levels of the AGE
experimental groups were significantly higher compared with those
of the non-glycated control groups.
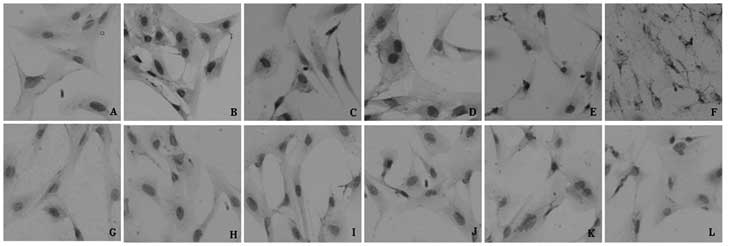 | Figure 3Expression of HIF-1α in RF/6A cells by
immunocytochemical staining analysis. (A) Blank control group;
(B–F) 50, 100, 200, 400 and 800 mg/l AGEs, respectively. (G)
Negative control group; (H–L) contain 50, 100, 200, 400 and 800
mg/l non-glycated control group, respectively (magnification x200).
AGEs, advanced glycation end products; HIF-1α, hypoxia-inducible
factor-1α; blank control, AGEs were replaced by PBS; negative
control, first antibody was replaced by PBS. |
Western blot analysis of HIF-1α
protein expression
In the present study, we also detected the HIF-1α
protein expression by western blot analysis. As shown in Fig. 4, the level of HIF-1α expression was
lower in all non-glycated control groups than in the AGE
experimental groups. However, different levels of HIF-1α protein
expression were detected, and the HIF-1α expression level increased
along with the AGE concentration. The maximum level of HIF-1α
expression occurred when the cells were treated with 200 mg/l AGEs.
The HIF-1α expression level then decreased with increasing AGE
concentration. These results were consistent with VEGF expression
in the AGE experimental groups.
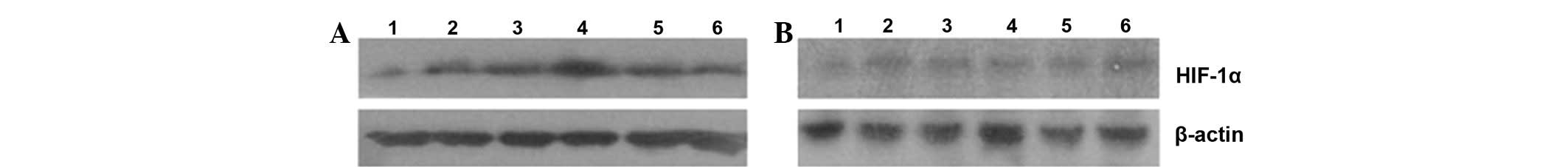 | Figure 4Western blot analysis of HIF-1α
protein expression in RF/6A cells. (A) AGE experimental groups; (B)
non-glycated control groups. Lanes 1, 2, 3, 4, 5 and 6 represent 0
(blank control group), 50, 100, 200, 400 and 800 mg/l AGEs or
non-glycated albumin, respectively. AGEs, advanced glycation end
products; HIF-1α, hypoxia-inducible factor-1α. |
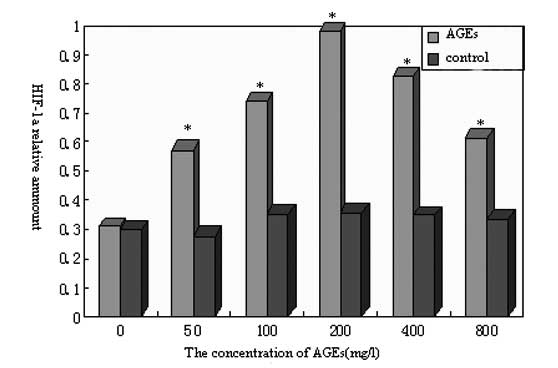
As shown in Fig. 5,
the levels of HIF-1α protein expression in the non-glycated control
groups were low, and no significant difference was observed among
the control groups (P>0.05). However, the level of HIF-1α
expression was significantly higher in all AGE experimental groups
than in the control groups (P<0.05). Accordingly, as the AGE
concentration increased, HIF-1α protein expression was promoted;
the maximum HIF-1α expression occurred when the AGE concentration
was 200 mg/l. The expression level then decreased, but with no
significant difference (P>0.05). In summary, these results
suggest that the HIF-1α expression level was dependent on AGE
concentration.
Association between VEGF and HIF-1α
expression of RF/6A cells with different concentrations of
AGEs
The association between VEGF and HIF-1α was analyzed
by a bivariate vector autoregressive model and correlation
analysis. The results showed there was a positive correlation
between VEGF and HIF-1α, and the regression equation was y =
1198.2× − 241.78, R=0.9881, P<0.01.
Discussion
ssThe HIF-1 transcription factor mediates adaptive
responses to alterations in tissue oxygenation. HIF-1 is a
heterodimer that consists of two subunits; HIF-1β which is
constitutively expressed and HIF-1α which is highly regulated. The
level of HIF-1α expression is determined by the rates of protein
synthesis and degradation. The synthesis of HIF-1α is regulated by
O2-independent mechanisms. HIF-1α degradation is
regulated mainly via O2-dependent mechanisms (11). A previous study indicated that
HIF-1α expression is increased in DR mice compared with wild type
mice, which shows that HIF-1α plays an important role in DR
pathogenesis (12). Another study
(13) has shown that HIF-1α mRNA
and protein expression levels are increased in human breast cancer
tissue. In the present study using AGEs, the majority of HIF-1α
expression was located in the cell nucleus and less in the
cytoplasm. The HIF-1α protein expression level was increased by AGE
in a concentration-dependent manner, and the levels in the AGE
groups were significantly different (P<0.05) from those in the
control groups.
HIF-1 activates the transcription of genes that are
involved in angiogenesis, which enables the body to rectify the
oxygen deficit. VEGF is the most significant stimulating factor for
vascularization and is the important target gene of HIF-1 (14). Previous studies have demonstrated
that the HIF-1α and VEGF levels in the vitreous humor of DR
patients and retinas of DR animals were increased, and that there
is a correlation between the two (15,16).
In the current study, HIF-1α and VEGF levels were observed to be
increased markedly in RF/6A cells treated with AGEs, and the
increase occurred in an AGE concentration-dependent manner. HIF-1α
and VEGF expression levels increased gradually with increasing AGE
concentration. The maximum level of protein expression appeared
when the AGE concentration reached 200 mg/l. However, the
expression of the two proteins decreased with further increase of
the AGE concentration. A positive correlation was identified in our
study. We suggest that the cell surface receptors reach a saturated
state following combination with AGEs, which results in surplus
AGEs that have no effect. In addition, excess AGEs are toxic to
endothelial cells, so this result is associated with necrocytosis
or apoptosis.
In conclusion, AGEs cause ischemia and oxygen
deficit in retinal tissue, and this results in vascular leakage,
vascular endothelial cell hyperplasia and neovascularization
through increasing HIF-1α and following activation of the
transcription of genes that are involved in angiogenesis such as
VEGF. These results indicate that inhibition of the HIF-1 pathway
may be a novel approach for the prevention of neovascularization in
DR.
References
|
1.
|
Schleicher ED, Wagner E and Nerlich AG:
Increased accumulation of the glycoxidation product
N(epsilon)-carboxymethyl) lysine in human tissues in diabetes and
aging. J Clin Invest. 99:457–468. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Peppa M, Uribarri J and Vlassara H:
Glucose, advanced glycation end products and diabetes
complications: what is new and what works. Clin Diabetes.
21:186–187. 2003. View Article : Google Scholar
|
|
3.
|
Lu M, Kuroki M, Amano S, et al: Advanced
glycation end products increase retinal vascular endothelial growth
factor expression. J Clin Invest. 101:1219–1224. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Simó R and Hernández C: Intravitreous
anti-VEGF for diabetic retinopathy: hopes and fears for a new
therapeutic strategy. Diabetologia. 51:1574–1580. 2008.PubMed/NCBI
|
|
5.
|
Bracken CP, Whitelaw ML and Peet DJ: The
hypoxia-inducible factors: key transcriptional regulators of
hypoxic responses. Cell Mol Life Sci. 60:1376–1393. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Poulaki V, Joussen AM, Mitsiades N, et al:
Insulin-like growth factor-I plays a pathogenetic role in diabetic
retinopathy. Am J Pathol. 165:457–469. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Makita Z, Vlassara H, Cerami A and Bucala
R: Immunochemical detection of advanced glycosylation end products
in vivo. J Biol Chem. 267:5133–5138. 1992.PubMed/NCBI
|
|
8.
|
Hammes HP, Alt A, Niwa T, et al:
Differential accumulation of advanced glycation end products in the
course of diabetic retinopathy. Diabetologia. 42:728–736. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Aiello LP, Avery RL, Arrigg PG, et al:
Vascular endothelial growth factor in ocular fluid of patients with
diabetic retinopathy and other retinal disorders. N Engl J Med.
331:1480–1487. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Song E, Li TY, Xu Q, et al: Expression of
vascular endothelial growth factor in retinopathy of diabetic rats.
Int J Ophthalmol. 3:9–12. 2003.(In Chinese).
|
|
11.
|
Semenza GL: Targeting HIF-1 for cancer
therapy. Nat Rev Cancer. 3:721–732. 2003. View Article : Google Scholar
|
|
12.
|
Lin M, Chen Y, Jin J, et al:
Ischaemia-induced retinal neovascularisation and diabetic
retinopathy in mice with conditional knockout of hypoxia-inducible
factor-1 in retinal Müller cells. Diabetologia. 54:1554–1566.
2011.PubMed/NCBI
|
|
13.
|
Wu HX and You ZP: Study progress of HIF-1
regulation mechanisms in diabetic retinopathy and neuropathy. Int J
Ophthalmol. 9:102–105. 2009.(In Chinese).
|
|
14.
|
Okada K, Osaki M, Araki K, et al:
Expression of hypoxia-inducible factor (HIF-1α), VEGF-C and VEGF-D
in non-invasive and invasive breast ductal carcinomas. Anticancer
Res. 25:3003–3009. 2005.
|
|
15.
|
Treins C, Giorgetti-Peraldi S, Murdaca J
and Van Obberghen E: Regulation of vascular endothelial growth
factor expression by advanced glycation end products. J Biol Chem.
276:43836–43841. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Wang X, Wang G and Wang Y: Intravitreous
vascular endothelial growth factor and hypoxia-inducible factor-1α
in patients with proliferative diabetic retinopathy. Am J
Ophthalmol. 148:883–889. 2009.
|