Introduction
Ankle fractures, are the fifth most common type of
fracture and account for ∼9.0% of all fractures in the human body,
are common worldwide (1). In the
United States, it is estimated that ∼260,000 individuals suffer
from ankle fractures each year (2). Ankle fractures are most often caused
by simple falls, athletic injuries and underlying pathology
(2). Every year ∼25% patients with
ankle fractures are treated with surgery (3).
Implants, including screws and rods, play an
important role in the internal fixation of ankle fractures. Almost
all the displaced fractures of the posterior malleolus are fixed
with screws and fractures of the medial malleolus are partially
fixed (4). Conventional implants
made of metal are widely used. However, reoperation is essential to
remove the internal fixation, which may cause additional damage to
the patients and increase the risk of infection, as well as other
complications.
Absorbable implants (AIs) made of polyglycolide
(PGA) or polylactide (PLA) have been developed to avoid reoperation
(5), which may result in a
reduction in costs and psychological benefits. Furthermore, AIs
lose their strength gradually and the stress is transferred to the
healing bone. Thus, the stress-shielding effect of metal implants
(MIs) is reduced. AIs are used for a variety of situations,
particularly for joints that are not suitable for repeated surgery,
including the reconstruction of the anterior cruciate ligament
(ACL) (6) and the fixation of
calcaneal fractures (7). The most
common use for absorbable materials is displaced ankle fractures
(8).
The aim of the current study was to evaluate the
efficiency and complications of AIs used for ankle fractures. We
consider that this meta-analysis provides strong evidence for the
selection of different implants in ankle fractures.
Materials and methods
Study design and search strategy
A systematic search of PubMed, Embase, Cochrane
Library, SinoMed and Wanfang Data was performed by two authors
independently for randomized controlled trials in which metal and
AIs are compared for ankle fractures. The search terms used were:
‘absorbable’, ‘bioabsorbable’, ‘biodegradable’, ‘biodegradation’,
‘degradable’, ‘degradation’, ‘polylactide’, ‘polylactic’,
‘polylevolactide’, ‘polylevolactic’, ‘polyglycolide’,
‘polyglycolic’, ‘ankle’, ‘malleolar’ and ‘malleolus’, singly or in
combination. When searched in SinoMed and Wanfang Data, related
terms were translated into Chinese. There were no limitations on
time and publication language.
Inclusion and exclusion criteria
Studies were included according to the following
criteria: i) study design was a randomized controlled trial (RCT),
including randomized and quasi-randomized trials; ii) included
patients with ankle fractures of all ages; iii) provided
comparative information between MIs and AIs for the fixation of the
ankle; and iv) no language and time limits set. Studies were
excluded when meeting the following criteria: i) studies on ankle
fractures with syndesmosis rupture; ii) study designs were case
reports, case series, retrospective studies, cohort studies or
controlled clinical studies; iii) studies that were redundant or
duplicate publications; and iv) studies with <20 patients.
Data extraction
Data were extracted onto a pre-designed table
independently by two reviewers. Then, the tables were exchanged to
verify consistency. Discrepancies in outcome extraction were
resolved by discussions or a senior reviewer’s opinion. Measurement
data and count data in all trials were extracted for meta-analyses,
as well as the general characteristics (first author, age, gender,
number of patients, study design and intervention) and the
descriptive data (average surgery time, length of hospital stay and
Olerud and Molander scores) (9).
Methodological assessment
The methodological quality of the included studies
was assessed by the modified Jadad scale (10). During this procedure, eight items,
including randomization, blind method, withdrawals, dropouts,
inclusion/exclusion criteria, adverse effects and statistical
analysis were assessed. The score of the study ranged from 0
(lowest quality) to 8 (highest quality). Studies with scores of 4–8
were considered to be of high quality, while scores of 0–3 were
considered poor quality. The strict assessment was performed by one
reviewer and verified by the other.
Outcomes for meta-analysis
The primary outcome measures were excellent and good
recovery rate, reoperation, foreign body reaction, infection rate,
osteoarthritis and pain. Other complications, including refracture,
skin necrosis, deep vein thrombosis (DVT), nerve injury and
palpable implants were also assessed. The secondary outcome was a
sensitive analysis performed by excluding the studies of low
quality (score 0–3).
Statistical analysis
When the data provided was not appropriate for
meta-analysis, the outcome was performed descriptively. Otherwise,
the relative risk (RR) and mean difference (MD), with 95%
confidence interval (CI), were used as statistical measures to
analyze dichotomous variables and continuous data, respectively.
Between-study heterogeneity was evaluated using I2
statistics. When I2>50%, substantial heterogeneity
could not be ignored and a random-effects model was adopted.
Otherwise a fixed-effects model was used. Statistical analysis was
conducted using RevMan 5.1 software for outcome measures. P<0.05
was considered to indicate a statistically significant
difference.
Results
Identification of relevant
literature
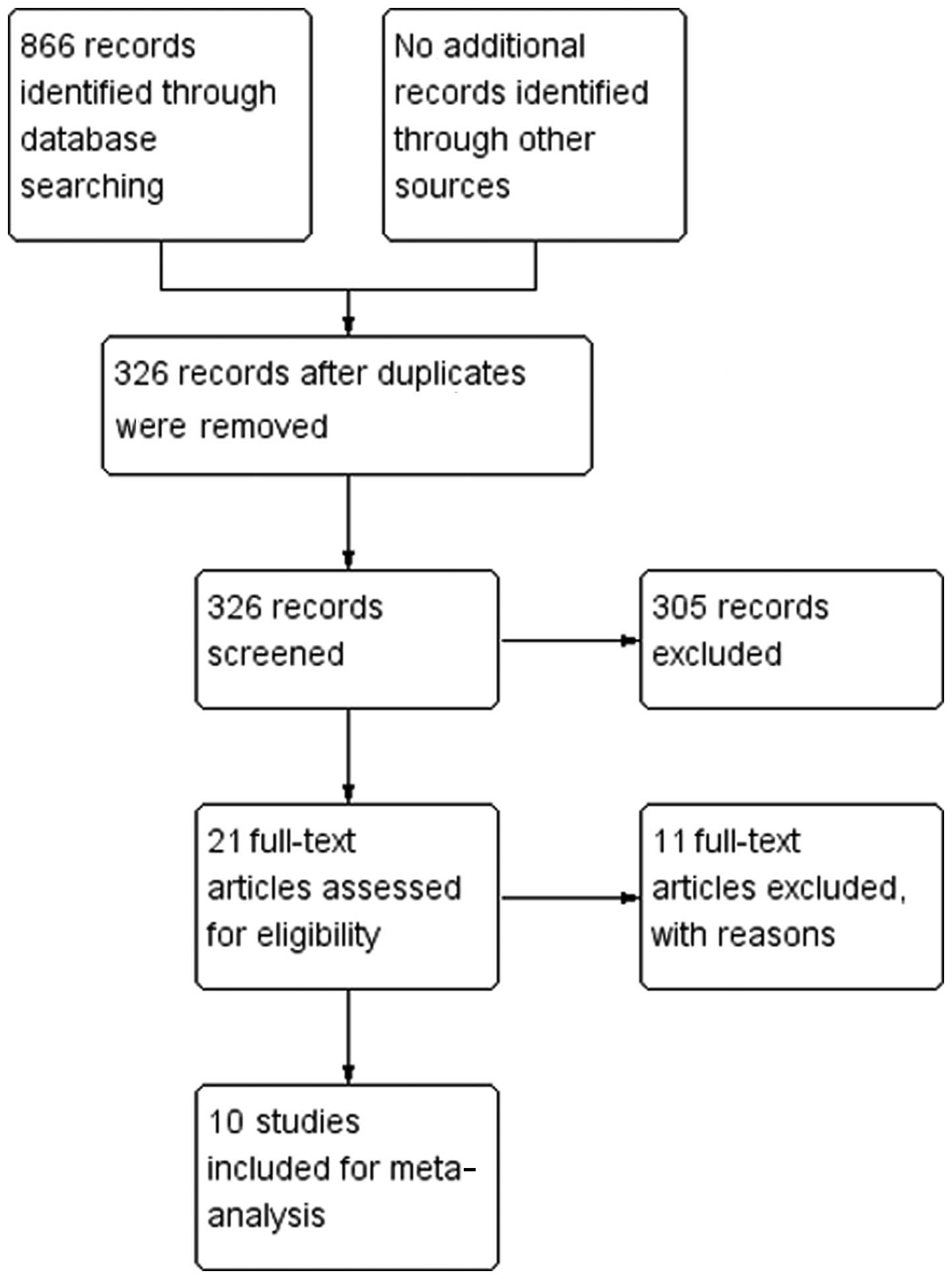
A flow diagram and results of the literature
screening are presented in Fig. 1.
The final review included 10 RCTs (11–20)
with a total of 762 patients. The general characteristics of the 10
included studies are summarized in Table I.
 | Table IGeneral characteristics of included
studies. |
Table I
General characteristics of included
studies.
| Study | Location | Cases | Average age
(years) | Study design | Randomized
method | Intervention | Comparison | Jadad score |
|---|
| Jung 2012 | Korea | 53/56 | >16 | RCT | Sealed
envelope | FreedomPlate,
screw | Metallic plate,
screw | 6 |
| Song 2011 | China | 89/89 | 18–68 | Quasi RCT | Registration
order | Absorbable
screw | Titanium alloy
screw | 4 |
| Shi 2011 | China | 30/30 | 36 (17–55) | RCT | | Absorbable
screw | Metal screw | 2 |
| Yun 2008 | China | 38/25 | 16–76 | RCT | | Absorbable
screw | Metal screw | 2 |
| Springer 1998 | Netherlands | 22/19 | 16–75 | RCT | | Biofix implant | Standard AO
fixation | 4 |
| Kankare 1996 | Finland | 16/19 | 72 (65–86)/73
(65–90) | RCT | | PGA implant | Metallic
implant | 4 |
| Kankare 1995 | Finland | 16/13 | 46 (29–68)/46
(31–62) | RCT | Sealed
envelope | PLA screw | Stainless steel
screw | 5 |
| Bucholz 1994 | America | 83/72 | 40/39 | Quasi RCT | Date (odd or
even) | PGA screw; PLLA
screw | AO implant | 4 |
| Dijkema 1993 | Netherlands | 21/22 | 16–70 | RCT | | Biofix implant | Metal implant | 3 |
| Bostman 1987 | Finland | 28/28 | 38.3/41.6 | RCT | | Biodegradable
implant | Metal implant | 4 |
Methodological quality assessment
The scores of the study are presented in Table I. The majority of the studies had a
score of 4–6 (16), indicating
that they are of high quality, while three studies were of low
quality with a score of 3 (19) or
2 (13,14). Randomization was described in all
studies. However, two (11,18)
were stated to have used a sealed envelope system. In one of the
two quasi-RCTs, the patients were randomized by the date of the
injury (17), while in the other,
they were randomized by registration order (12). None of the studies used the blind
method. For the measurements that were descriptive, statistical
analysis was described only in three studies (11,12,18).
Radiological assessment
Radiological outcomes, including the redisplacement
of fractures, were mentioned in the majority of studies. In one of
the studies, judgments made from radiographs indicated that the
redisplacements in the two groups were similar in number as well as
extent (18). By contrast,
patients in the study by Kankare et al(16) suffered smaller redisplacements with
a similar number in the two groups. Contrastingly, another study by
Kankare et al(17)
demonstrated that 8/16 patients in the PGA group and 1/13 patients
in the Arbeitsgemeinschaft für Osteosynthesefragen (Association for
the Study of Internal Fixation, ASIF) (AO) group had
redisplacements. However, these were not due to iatrogenic reasons,
but due to poor bad compliance following treatment. The results
from the study by Dijkema et al(19) revealed that all fractures healed
without any displacement, which further confirmed the effect of
compliance.
Functional assessment
Functional outcomes, including Olerud and Molander
functional score, excellent and good recovery rate, range of motion
and return to preoperative level, are mentioned in these studies.
Five studies (15–18,20)
compared the Olerud and Molander scores with different implants.
The scores in the AI group tend to be higher than those of the MI
group; however, there was no statistically significant difference.
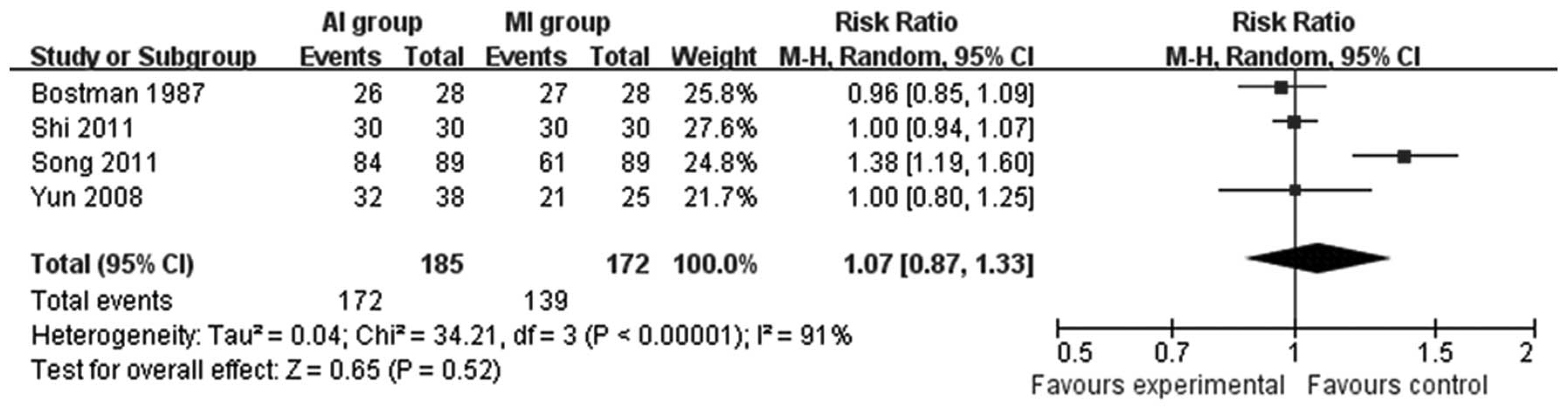
Functional outcomes were evaluated by an excellent and good
recovery rate in four studies (12–14,20).
As shown in Fig. 2, no significant
difference was detected.
Following surgery, the range of ankle motion is
significantly reduced; however, as indicated in one study (15), the reduction has no correlation
with the type of the screw used. This is in disagreement with the
study by Kankare et al(16), which observed that 2/16 patients in
the self-reinforced polyglycolide (SR-PGA) group and 8/19 patients
in the metal implant group had limited motion. Another study
(17) also stated that 3 patients
with AO implants had limited motion. Thus, no consensus was
established based on the current evidence.
The study by Bucholz et al(18) demonstrated that the majority of
patients in the two groups returned to pre-injury work status. No
significant difference was detected in the two groups in the
ability to walk, run, jump and climb stairs. Outcomes from Kankare
et al(17) revealed that
one patient in the PGA group lost dorsiflexion. Differences were
not statistically significant among all these studies.
Meta-analysis
The excellent and good functional recovery rate in
the AI and MI groups were compared in four studies (12–14,20)
and were observed to be similar (RR=1.07; 95% CI= 0.87–1.33; P=
0.52; Fig. 2). The number of
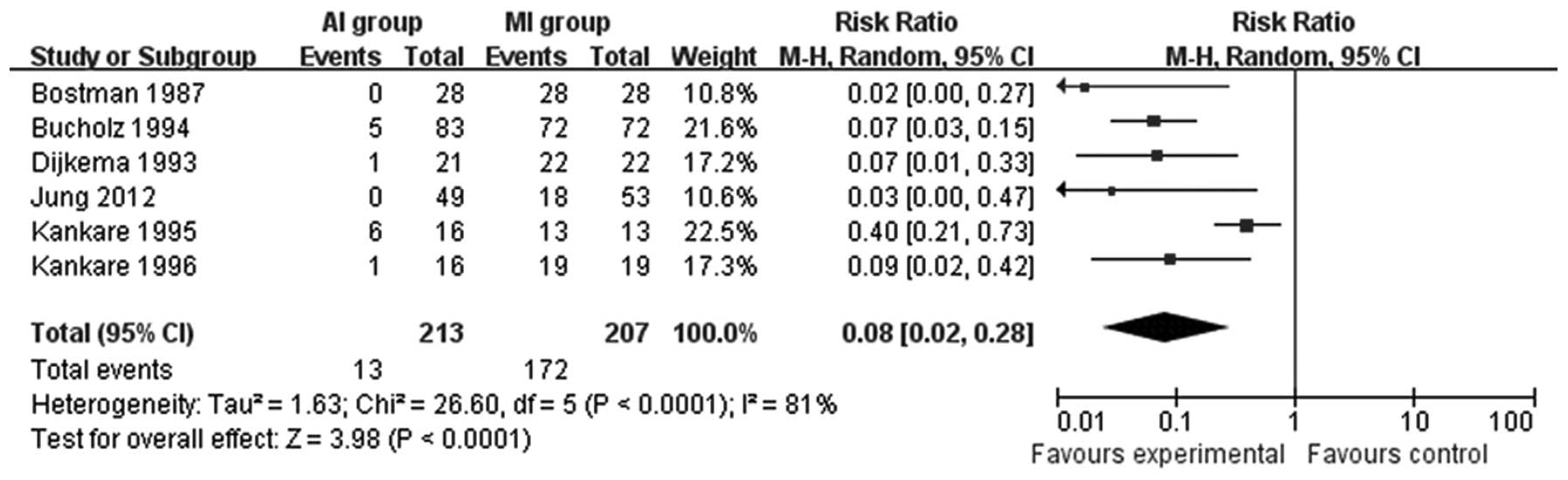
patients that required reoperation was counted in six studies
(11,16–20).
The AI group required significantly fewer reoperations compared
with the MI group (RR=0.08; 95% CI=0.02–0.28; P<0.0001; Fig. 3).
Complications following surgery were mentioned in
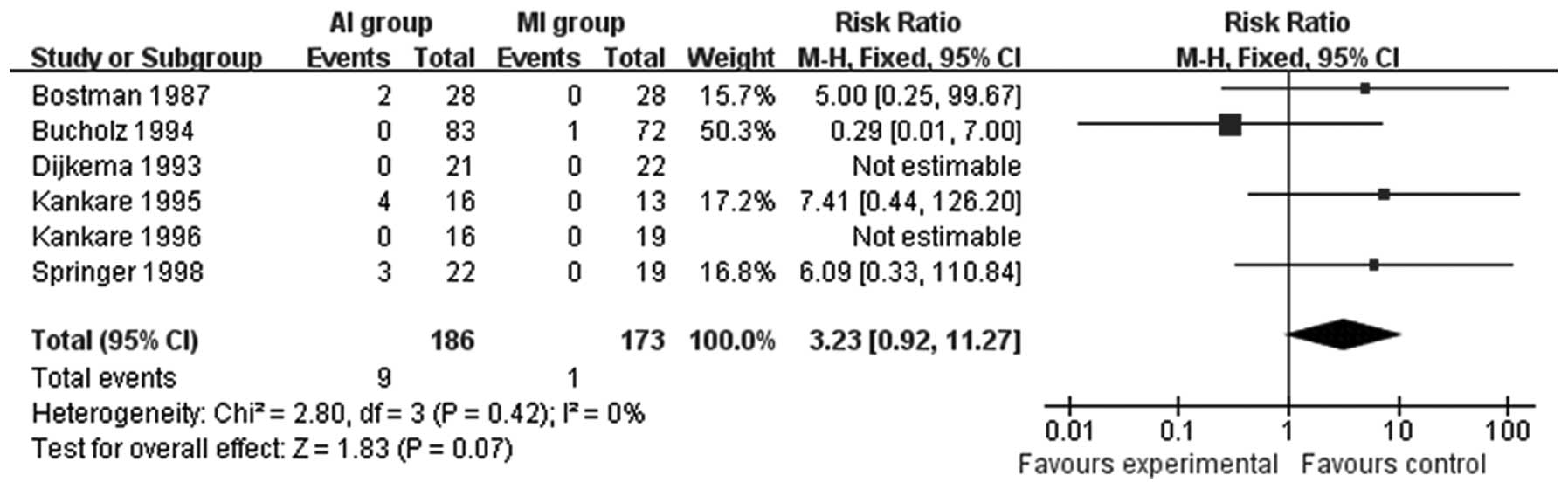
all included studies. Foreign body reactions were compared in six
studies (14–19). Patients treated with absorbable
materials are more likely to suffer sterile effusion, sinus
formation and osteolysis (RR=3.23; 95% CI=0.92–11.27; P=0.07;
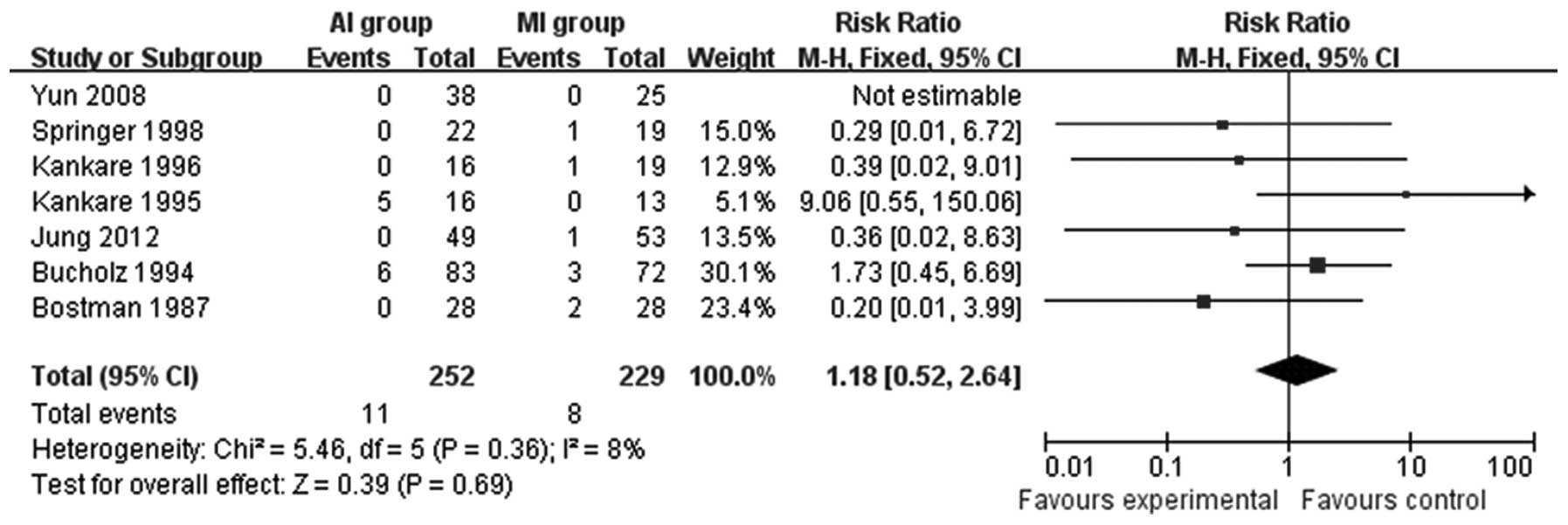
Fig. 4). Seven studies (11,14–18,20)
compared the infection rate between the MI group and the AI group.
The results revealed that there was no statistically significant
difference between the two groups (RR=1.18; 95% CI=0.52–2.64;
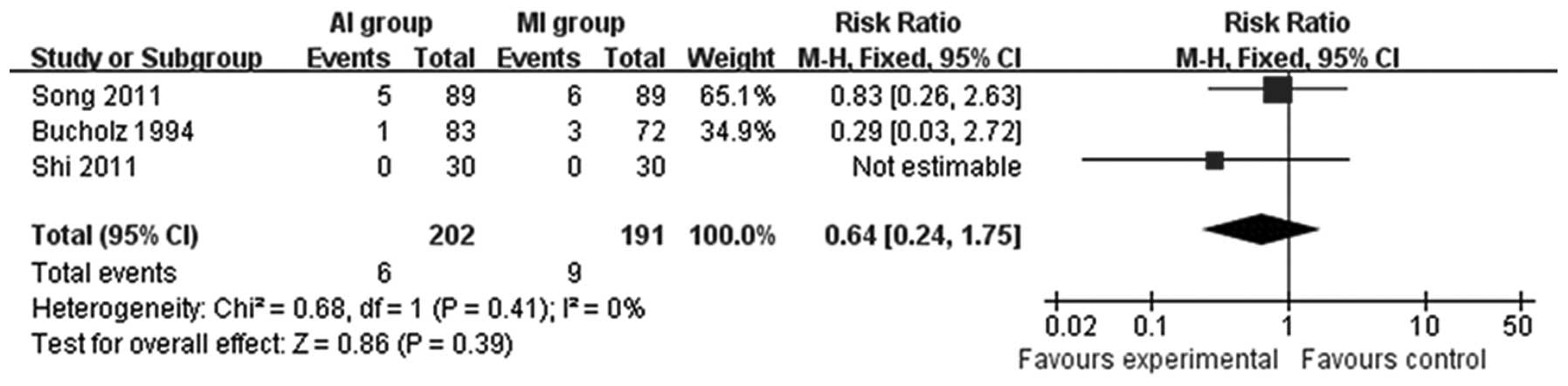
P=0.69; Fig. 5). Osteoarthritis,
recorded in three studies (12,13,18),
was not significantly different (RR= 0.64; 95% CI= 0.24–1.75; P=
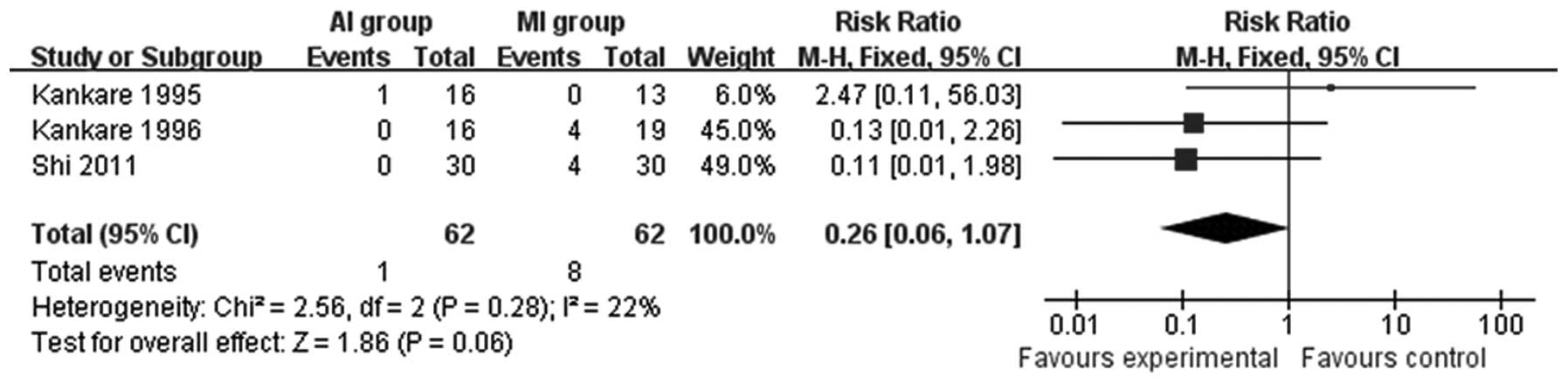
0.39; Fig. 6). Patients treated
with AIs had improved results with regard to the incidence of pain
(RR=0.26; 95% CI=0.06–1.07; P=0.06; Fig. 7). Furthermore, as shown in Table II, no significant differences were
detected with regard to refracture (RR=0.68; 95% CI=0.12–3.92;
P=0.67), skin necrosis or sloughs (RR=1.01; 95% CI=0.15–6.92;
P=0.99), DVT (RR= 0.31; 95% CI= 0.05–1.91; P=0.21) and nerve injury
(RR=0.94; 95% CI=0.19–4.74; P=0.94); however, a significant
difference was observed in the incidence of palpable implants
(RR=0.68; 95% CI=0.50–0.93; P= 0.02).
 | Table IIMeta-analysis of selected
outcomes. |
Table II
Meta-analysis of selected
outcomes.
| All included
studies
|
|---|
| Outcomes | No. | Cases | I2
(%) | RR (95% CI) | P-values |
|---|
| Refracture | 2 | 99 | 35 | 0.68
(0.12–3.92) | 0.67 |
| Skin necrosis | 2 | 190 | 0 | 1.01
(0.15–6.92) | 0.99 |
| DVT | 3 | 105 | 0 | 0.31
(0.05–1.91) | 0.21 |
| Nerve injury | 3 | 219 | 0 | 0.94
(0.19–4.74) | 0.94 |
| Palpable
implant | 4 | 352 | 0 | 0.68
(0.50–0.93) | 0.02 |
Sensitivity analysis
Sensitivity analysis was conducted by excluding the
studies (13,14,19)
of low quality (scores 0–3). The study data did not change with
respect to the outcomes of infection, skin necrosis, DVT and nerve
injury following exclusion, respectively, and there was only one
study with respect to refracture. Thus, sensitivity analyses of
these outcomes were not performed. I2, RR and 95% CI of
all outcomes are shown in Table
III. The results suggest that all of the excluded studies had
no bias on the outcomes and the results reported in this study are
acceptable.
 | Table IIISensitivity analysis. |
Table III
Sensitivity analysis.
| All included
studies | Studies of high
quality |
|---|
|
|
|---|
| Outcomes | No. | Cases | I2
(%) | RR (95% CI) | P-value | No. | Cases | I2
(%) | RR (95%CI) | P-value |
|---|
| Excellent and good
rate | 4 | 357 | 91 | 1.07
(0.87–1.33) | 0.52 | 2 | 234 | 95 | 1.15
(0.76–1.74) | 0.51 |
| Reoperation | 6 | 420 | 81 | 0.08
(0.02–0.28) | <0.0001 | 5 | 377 | 85 | 0.08
(0.02–0.36) | 0.0008 |
| Foreign body
reaction | 6 | 359 | 0 | 3.23
(0.92–11.27) | 0.07 | 5 | 316 | 0 | 3.23
(0.92–11.27) | 0.07 |
| Osteoarthritis | 3 | 393 | 0 | 0.64
(0.24–1.75) | 0.39 | 2 | 333 | 0 | 0.64
(0.24–1.75) | 0.39 |
| Pain | 3 | 124 | 22 | 0.26
(0.06–1.07) | 0.06 | 2 | 64 | 47 | 0.40
(0.07–2.20) | 0.29 |
| Palpable
implant | 4 | 352 | 0 | 0.68
(0.50–0.93) | 0.02 | 3 | 292 | 0 | 0.73
(0.53–1.00) | 0.05 |
Discussion
The results of our study revealed that the
functional outcomes were not significantly different between the AI
and MI groups. Reoperation was seldom necessary for ankle fractures
fixed with AIs, unless refractures occurred, implants broke or the
local response was serious. This benefits the patients financially
and physiologically. However, aside from the incidence of palpable
implants, the rate of foreign body reaction, infection,
osteoarthritis, pain, refracture, skin necrosis, DVT and nerve
injury were similar in the two groups.
The incidence of ankle fractures is gender-related
(21). For males the peak age
range is 15–24 years, whereas it is 65–75 years for females. This
is in accordance with the results in our study (data not shown). In
the study by Shi et al(13), the gender ratio was 49/11
(male/female) with an average age of 36 years, while another study
(16) with 9 males and 26 females
had an average age of 72/73 years (absorbable/metal).
When treated with different implants, the excellent
and good recovery rates were not significantly different in the
ankle functional evaluation (Fig.
2). Rangdal et al conducted a prospective study
(22) to assess the functional
recovery of ankle fractures treated with AIs. With plaster
immobilization and no bearing of weight, the results were
satisfactory. However, before concluding that AIs are similar or
even slightly better than metal ones in function, the
heterogeneity, study design and the number of patients included
should not be ignored.
The lower reoperation rate of the AI group compared
with the MI group may be due to the biodegradable and absorbable
charactistics of the implant used in vivo without removal.
Yet, there were several patients that required reoperations for the
following two reasons: i) the non-specific tissue response elicited
by the degradation and absorption of the materials in the tissue
and ii) AIs made of polymer materials are not as strong as metal
ones. Refracture is a great threat following surgery (23). As Kankare et al(17) declared, certain patients did not
follow the post-operative instructions and their screws broke,
which required immediate surgical removal.
Absorbable materials made of PGA or PLA are broken
down via hydrolysis in the body (24). With the accumulation of the
breakdown products, foreign body reactions, including sterile
effusion, sinus formation and osteolysis around the implants, are
triggered (25). The results
indicated that foreign body reactions are more likely to occur with
absorbable materials, although no significant difference was
detected. The incidence of foreign body reactions in our study was
4.8% (9/186), which is slightly lower than that of 6.1% reported in
the study by Böstman (26).
Although foreign body reactions occurred and fluid accumulated
topically, the fracture healing was uneventful (27). AIs had no specific inhibitory and
stimulatory effects on bone compared with metal materials (28).
The difference in the incidence of infection is not
statistically significant. However, patients in the AI group had a
tendency of reduced infection compared with those in the MI group.
This may be due to the small sample size. A study (29) with a large sample size compared the
infection rate between AIs and metal fixation and detected no
significant difference. Theoretically, the degradation of the
polymer results in the accumulation of sterile effusion, which
leads to topical susceptibility to infection. However, the risk of
infection may increase along with incidence of reoperation in the
MI group. Therefore, it is doubtful whether a significant
difference would be detected if the sample size was enlarged.
There were no statistical differences in refracture,
skin necrosis, DVT, nerve injury, palpable implants and pain,
presented in Table II and Fig. VII, between the two groups. We
consider that this is due to the following reasons: i) no
difference exists substantially; ii) the weakness of the included
studies, including the small sample size, large number of patients
lost to follow-up and the incompleteness of measurements; and iii)
these complications are nonspecific and are affected by a number of
factors, including the types of fractures and the body mass index
(BMI) (30). However, the
incidence of palpable implants was higher in the MI group. To a
certain degree, the biodegradable property of AIs accounts for
this. Another cause may be that the determination of palpable
implants is subjective.
As shown in Table
III, the results of the sensitivity analyses indicate that the
meta-analysis results are stable and accurate. Following exclusion
of an article, although the results for palpable implants were
determined to not be significantly different, this may be explained
by the sample size allowing for the existence of a clear tendency.
Furthermore, following exclusion of the three studies, sensitivity
analyses caused slight changes in the results; however, they were
not conclusive.
Heterogeneity must be noted as a weakness of our
study. Clinical heterogeneity may exist objectively owing to the
complexity of ankle fractures, distribution of age, subjective
evaluation and heterogeneity of treatments. Once the patients were
admitted, treatments may have differed due to variations in the
specific degree of injury, age and the willingness of the patients,
despite the use of the same surgeon. There was a high statistical
heterogeneity (I2=81%) for the results of reoperation
(Fig. 3). By excluding a study of
low quality, the heterogeneity was even higher (I2=85%).
Following exclusion of the other article (16), the heterogeneity decreased
dramatically (I2= 0%) with an extremely slight change in
the result (RR=0.06; 95% CI=0.03–0.12; P<0.00001). This may be
explained by the poor compliance of the patients included. AIs are
not as strong as MIs and are prone to breakage. Thus, compliance
and post-operative nursing are important for the uneventful
recovery of patients.
Although the results of our study are based on the
best evidence currently available, there remain limitations that
need to be addressed. Firstly, the total number of cases is small,
which may be a possible reason for the lack of a significant
difference. Secondly, the follow-up times, the majority of which
were 12 months or less, were relatively short. The time until the
occurrence of adverse tissue reactions to PGA and PLA was 11 weeks
and 4.3 years, respectively, following surgery (31). Certain chronic complications,
including post-traumatic osteoarthritis with a latency time of 20.9
years (32), would not be
detected. Although osteoarthritis was reported in a study (18), it was likely to be incomplete. The
relatively large number of patients lost to follow-up may also
affect the validity of the study. Furthermore, all studies were
conducted in different places without a blinding method. This
determined the differences in the incompleteness of the reported
results and the inconsistency of the scoring criteria, particularly
the subjective ones. In addition, several studies described the
statistical methods; however, only means without standard
deviations were provided in a number of cases with quantitative
data, particularly the information on functional measurements.
Thus, these data were analyzed descriptively without meta-analysis.
Finally, the majority of the articles included in our review are
relatively old. We expanded the research; however, this produced
only reviews and case series that did not meet the inclusion
criteria.
There has been a wide range of applications for AIs
in orthopedic use, including reconstruction of the ACL (7), fixation of type II odontoid fracture
(33) and even maxillofacial
surgery (34), which are not
weight bearing. However, the ankle joint is weight bearing. In the
view of the majority of doctors, the fixation of implants is not
strong enough to secure and stabilize the ankle. Thus, the risk of
refracture may increase. In consideration of this, patients were
immobilized with plaster for six weeks routinely and weight bearing
was allowed gradually following surgery. Therefore, the activities
of the ankle and the weight bearing of implants and the ankle were
reduced to a certain extent, which reduced the rate of refracture.
However, immobilization also leads to poor circulation and topical
hemodynamic changes. The potential risk of DVT increases. Among all
relevant studies, the incidence rate of DVT was low and similar in
the two groups. This may be a false finding, caused by the small
sample size and the weakness of the design. Therefore, large
strictly designed and high-quality multi-center, randomized,
double-blinded studies are required to confirm the results of the
current study.
AIs for the treatment of ankle fractures do not
typically require reoperation and result in similar functional
outcomes, as well as complications, compared with MIs. These
implants are safe and efficient enough for the management of ankle
fractures. More high-quality, larger scale, randomized controlled
trials are required to confirm this conclusion.
References
|
1.
|
Court-Brown CM and Caesar B: Epidemiology
of adult fractures: A review. Injury. 37:691–697. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Scott AM: Diagnosis and treatment of ankle
fractures. Radiol Technol. 81:457–475. 2010.
|
|
3.
|
Ganesh SP, Pietrobon R, Cecilio WA, Pan D,
Lightdale N and Nunley JA: The impact of diabetes on patient
outcomes after ankle fracture. J Bone Joint Surg Am. 87:1712–1718.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Hopton BP and Harris NJ: Fractures of the
foot and ankle. Surgery (Oxford). 28:502–507. 2010. View Article : Google Scholar
|
|
5.
|
Böstman O, Hirvensalo E, Vainionpää S,
Mäkelä A, Vihtonen K, Törmälä P and Rokkanen P: Ankle fractures
treated using biodegradable internal fixation. Clin Orthop Relat
Res. 195–203. 1989.PubMed/NCBI
|
|
6.
|
Gaweda K, Walawski J, Weglowski R and
Krzyzanowski W: Comparison of bioabsorbable interference screws and
posts for distal fixation in anterior cruciate ligament
reconstruction. Int Orthop. 33:123–127. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Zhang J, Xiao B and Wu Z: Surgical
treatment of calcaneal fractures with bioabsorbable screws. Int
Orthop. 35:529–533. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Rokkanen PU, Böstman O, Hirvensalo E, et
al: Bioabsorbable fixation in orthopaedic surgery and traumatology.
Biomaterials. 21:2607–2613. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Olerud C and Molander H: A scoring scale
for symptom evaluation after ankle fracture. Arch Orthop Trauma
Surg. 103:190–194. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Jiang N, Wang B, Chen A, Dong F and Yu B:
Operative versus nonoperative treatment for acute Achilles tendon
rupture: a meta-analysis based on current evidence. Int Orthop.
36:765–773. 2012. View Article : Google Scholar
|
|
11.
|
Jung HN, Roh YH, Yang BG, Kim SW, Lee JS
and Oh MK: Outcomes of operative treatment of unstable ankle
fractures: a comparison of metallic and biodegradable implants. J
Bone Joint Surg Am. 94:e1662012.PubMed/NCBI
|
|
12.
|
Song ZL: The choice of fixators for
surgery of trimalleolar fractures. Chinese Journal of Primary
Medicine and Pharmacy. 18:2935–2936. 2011.(In Chinese).
|
|
13.
|
Shi WY, Huang JJ and Huang L: Clinical
efficiency of absorbable screws for the treatment of internal
malleolus fractures. Journal of Minimally Invasive Medicine.
6:139–140. 2011.(In Chinese).
|
|
14.
|
Yun C, Zhu JH, He JS and Yang T: Treating
ankle joint fracture by simplified surgical techniques. Journal of
Clinical Orthopaedics. 11:269–270. 2008.(In Chinese).
|
|
15.
|
Springer MA, van Binsbergen EA, Patka P,
Bakker FC and Haarman HJ: Resorbable rods and screws for fixation
of ankle fractures. A randomized clinical prospective study.
Unfallchirurg. 101:377–381. 1998.(In German).
|
|
16.
|
Kankare J, Partio EK, Hirvensalo E,
Böstman O and Rokkanen P: Biodegradable self-reinforced
polyglycolide screws and rods in the fixation of displaced
malleolar fractures in the elderly: A comparison with metallic
implants. Ann Chir Gynaecol. 85:263–270. 1996.PubMed/NCBI
|
|
17.
|
Kankare J, Hirvensalo E and Rokkanen P:
Malleolar fractures in alcoholics treated with biodegradable
internal fixation: 6/16 reoperations in a randomized study. Acta
Orthop Scand. 66:524–528. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Bucholz RW, Henry S and Henley MB:
Fixation with bioabsorbable screws for the treatment of fractures
of the ankle. J Bone Joint Surg Am. 76:319–324. 1994.PubMed/NCBI
|
|
19.
|
Dijkema AR, van der Elst M, Breederveld
RS, Verspui G, Patka P and Haarman HJ: Surgical treatment of
fracture-dislocations of the ankle joint with biodegradable
implants: a prospective randomized study. J Trauma. 34:82–84. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Böstman O, Vainionpää S, Hirvensalo E,
Mäkelä A, Vihtonen K, Törmälä P and Rokkanen P: Biodegradable
internal fixation for malleolar fractures: A prospective randomised
trial. J Bone Joint Surg Br. 69:615–619. 1987.PubMed/NCBI
|
|
21.
|
Westerman RW and Porter K: Ankle fractures
in adults: an overview. Trauma. 9:267–272. 2007. View Article : Google Scholar
|
|
22.
|
Rangdal S, Singh D, Joshi N, Soni A and
Sament R: Functional outcome of ankle fracture patients treated
with biodegradable implants. Foot Ankle Surg. 18:153–156. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Pina S and Ferreira JMF: Bioresorbable
plates and screws for clinical applications: a review. J Healthc
Eng. 3:243–260. 2012. View Article : Google Scholar
|
|
24.
|
Spitalny AD: Bioabsorbable implants. Clin
Podiatr Med Surg. 23:673–694. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Stroud CC: Absorbable implants in fracture
management. Foot Ankle Clin. 7:495–499. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Böstman OM: Osteoarthritis of the ankle
after foreign-body reaction to absorbable pins and screws: a three-
to nine-year follow-up study. J Bone Joint Surg Br. 80:333–338.
1998.PubMed/NCBI
|
|
27.
|
Givissis PK, Stavridis SI, Papagelopoulos
PJ, Antonarakos PD and Christodoulou AG: Delayed foreign-body
reaction to absorbable implants in metacarpal fracture treatment.
Clin Orthop Relat Res. 468:3377–3383. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Pihlajamäki HK, Salminen ST, Tynninen O,
Böstman OM and Laitinen O: Tissue restoration after implantation of
polyglycolide, polydioxanone, polylevolactide, and metallic pins in
cortical bone: an experimental study in rabbits. Calcif Tissue Int.
87:90–98. 2010.PubMed/NCBI
|
|
29.
|
Sinisaari I, Pätiälä H, Böstman O, et al:
Metallic or absorbable implants for ankle fractures: a comparative
study of infections in 3,111 cases. Acta Orthop Scand. 67:16–18.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Lübbeke A, Salvo D, Stern R, Hoffmeyer P,
Holzer N and Assal M: Risk factors for post-traumatic
osteoarthritis of the ankle: an eighteen year follow-up study. Int
Orthop. 36:1403–1410. 2012.PubMed/NCBI
|
|
31.
|
Böstman OM and Pihlajamäki HK: Adverse
tissue reactions to bioabsorbable fixation devices. Clin Orthop
Relat Res. 216–227. 2000.PubMed/NCBI
|
|
32.
|
Horisberger M, Valderrabano V and
Hintermann B: Posttraumatic ankle osteoarthritis after
ankle-related fractures. J Orthop Trauma. 23:60–67. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Nourbakhsh A, Patil S, Vannemreddy P,
Ogden A, Mukherjee D and Nanda A: The use of bioabsorbable screws
to fix type II odontoid fractures: a biomechanical study. J
Neurosurg Spine. 15:361–366. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Buijs GJ, van Bakelen NB, Jansma J, et al:
A randomized clinical trial of biodegradable and titanium fixation
systems in maxillofacial surgery. J Dent Res. 91:299–304. 2012.
View Article : Google Scholar : PubMed/NCBI
|