Introduction
As percutaneous nephrolithotomy continues to
advance, the majority of upper urinary calculi do not require open
surgical treatments. Percutaneous nephrolithotomy has become the
main method of upper urinary calculi treatment, particularly for
Staghorn calculi (1,2). However, percutaneous nephrolithotomy
has its disadvantages. It requires persistent washing to maintain a
clear field of vision during surgery. This procedure causes the
washing liquid to be absorbed and the renal pelvic pressure to
increase, thereby resulting in a backflow problem (3,4).
When the kidney is injured or in a purulent infection status, the
renal pelvic pressure increases, which causes backflow of bacteria
into the blood, causing septicaemia of urinary origin and a higher
mortality rate (5–8). Therefore, when the kidney is
infected, the majority of medical doctors do not conduct one-stage
percutaneous nephrolithotomy. Instead, they initially conduct
percutaneous nephrostomy, followed by two-stage percutaneous
nephrolithotomy once the disease condition has become stable
(9–11). However, under certain conditions,
conducting one-stage percutaneous nephrolithotomy is also feasible,
even if the kidney has purulent infection (12). Thus, several questions arise,
particularly concerning the procedures involved in conducting
surgery when renal infection is present, as well as on the
correlation between renal pelvic pressure and renal backflow.
Currently, studies on these issues are rare. Therefore,
investigating the correlation between renal pelvic pressure and
renal backflow during renal infection is important. This study
presents a pathological biopsy by preparing the porcine
pyonephrosis model and implementing various perfusion pressures to
observe the pathological changes in the kidney under different
pressures and to investigate the effects of pressure on the
infected kidney. This study also aims to provide information
concerning the clinical surgical treatment of calculus
pyonephrosis.
Materials and methods
Animals
Five miniature common male test pigs aged 2 months
with body weights ranging from 25 to 30 kg were selected. They were
fed conventionally. This study was performed in strict accordance
with the recommendations in the Guide for the Care and Use of
Laboratory Animals of the National Institutes of Health. The animal
use protocol was reviewed and approved by the Institutional Animal
Care and Use Committee (IACUC) of the 303rd Hospital of the Chinese
People’s Liberation Army.
Methods
Five miniature test pigs were used to prepare the
pyonephrosis model. After the pigs had been adaptively fed for 5
days, the experiment was conducted. Prior to surgery, 10 mg/kg
ketamine hydrochloride was administered by intra-muscular injection
for anaesthesia. Next, 5 mg/kg ketamine hydrochloride was
administered by intravenous injection for maintenance. Once the
pigs were anaesthetised, they were laid on the left side while
surgery was performed on the right ureter. Each pig was
coeliotomised using a right waist incision to expose the right
kidney and right ureter. At 5 cm from the right kidney, 2 ml
supernatant of pig manure and normal saline was injected into the
right ureter. When the injection had been administered, the
abdominal cavity was closed. Following surgery, the pigs were fed
normally and no antibiotic was administered.
After 3 days, the miniature test pigs were
anaesthetised using the aforementioned method. The pigs were
coeliotomised to expose the bilateral kidneys and ureters.
Bilateral ureters were ligated 5 cm from the renal pelvis and a
piezometer and a water fill tube were inserted from the
ligation-proximal ureter towards the renal pelvis. At this time,
the water fill tube was opened. The model was qualified after
purulent urine was drained from the right injured kidney and clear
urine was drained from the left healthy kidney.
Prior to perfusion, punctures were performed on the
healthy and purulent sides of the kidneys to obtain tissues (as
controls). Normal saline was perfused into the renal pelvis to
allow the renal pelvic pressure to increase rapidly to 10 mmHg;
this pressure was maintained for 10 min. Subsequently, the healthy
and injured kidneys were punctured using one needle to conduct
biopsy. Next, the perfusion liquid was drained and normal saline
was perfused again to increase the pressure to 20 mmHg. When this
pressure had been maintained for 10 min, the kidneys were punctured
again. Similarly, puncture biopsy was performed at renal pelvic
pressures of 10, 20, 30, 40 and 50 mmHg. The obtained tissues were
restored in 10% formalin and electron microscopy fixation
solutions.
Optical microscopy
Haematoxylin and eosin (H&E) staining of a
specimen was conducted. Afterwards, the morphological structures of
the renal capsule, renal glomerulus and renal tubule were observed
under an optical microscope. At the same time, images were
captured.
Electron microscopy
A specimen was cut into ultra-thin sections of 60–68
nm. A JEM-1010 model transmission electron microscope was used to
observe the ultra-microstructures of the renal capsule, renal
glomerulus and renal tubule. At the same time, images were
captured.
Results
Pathological examination
When the renal pelvic pressure increased, healthy
and injured kidneys presented pathological changes, including renal
tubule and renal capsule dilations and renal glomerulus
compression. At the same time, the purulent infected kidney
exhibited interstitial oedema and inflammatory cell infiltration.
When the perfusion pressure exceeded 20 mmHg, the purulent infected
kidney exhibited degenerative necrosis of the renal tubule. When
the perfusion pressure was increased to 40 mmHg, damage in the
renal glomerulus and renal tubule became more evident and local
structures of the renal glomerulus and renal tubule were damaged
and destroyed. Under the same pressure, the healthy kidney
exhibited dilations of the renal tubule and capsule and compression
of the renal glomerulus. Less damage and fewer changes were
observed in the healthy kidney (Fig.
1).
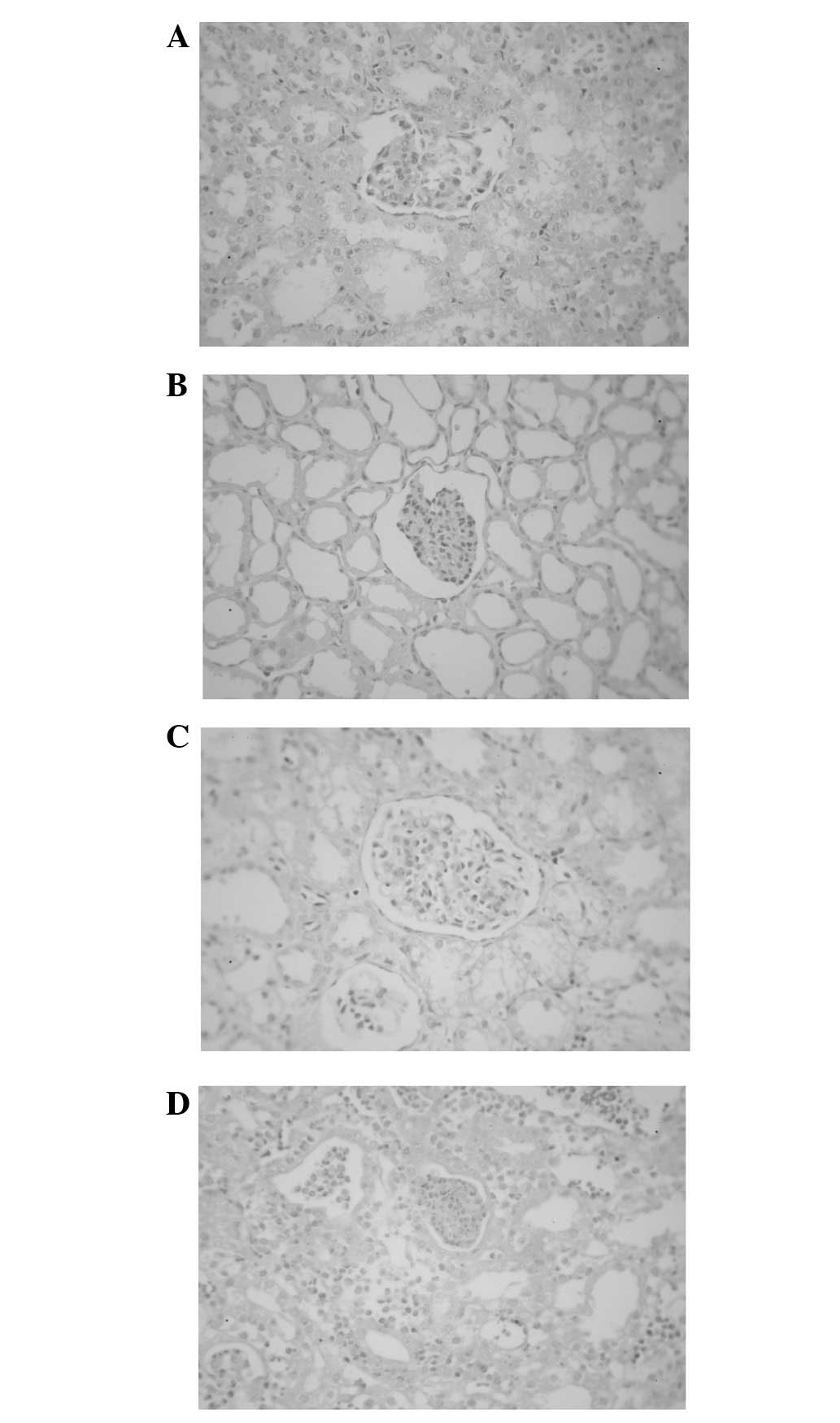 | Figure 1Pathological examination results. (A)
When the pressure in the healthy side increased to 20 mmHg, the
renal tubule dilated (H&E staining; optical microscope
magnification, ×200). (B) When the pressure in the healthy side
increased to 40 mmHg, the renal tubule and renal capsule were
clearly dilated and the nephron structure was complete (H&E
staining, magnification, ×200). (C) When the pressure in the
infected side increased to 20 mmHg, the renal tubule and renal
capsule were dilated and the continuity of the renal tubule
basement membrane was partially interrupted (H&E staining,
magnification, ×200). (D) When the pressure in the infected side
increased to 40 mmHg, the renal tubule and renal capsule were
clearly dilated and the continuities of the renal capsule and renal
tubule were interrupted (H&E staining, magnification, ×200).
H&E, haematoxylin and eosin. |
Electron microscopy
Different results were observed as the pressure was
increased. The podocyte gap widened and the epithelial cells in the
renal capsule separated from the basement membrane. The basement
membrane thickness became uneven and its continuity was interrupted
at multiple positions. The renal tubule microvillus arrangement
became disorganised. The manifestations in the pyonephrosis model
were more evident compared with those in the healthy kidney. In the
renal capsule, inflammatory cells and transudatory erythrocytes
were visible. In addition, microvillus disorder and shedding,
mitochondrial vacuolisation, reduction in mitochondrial cristae and
lysosomal expansion were visible at multiple sites (Fig. 2).
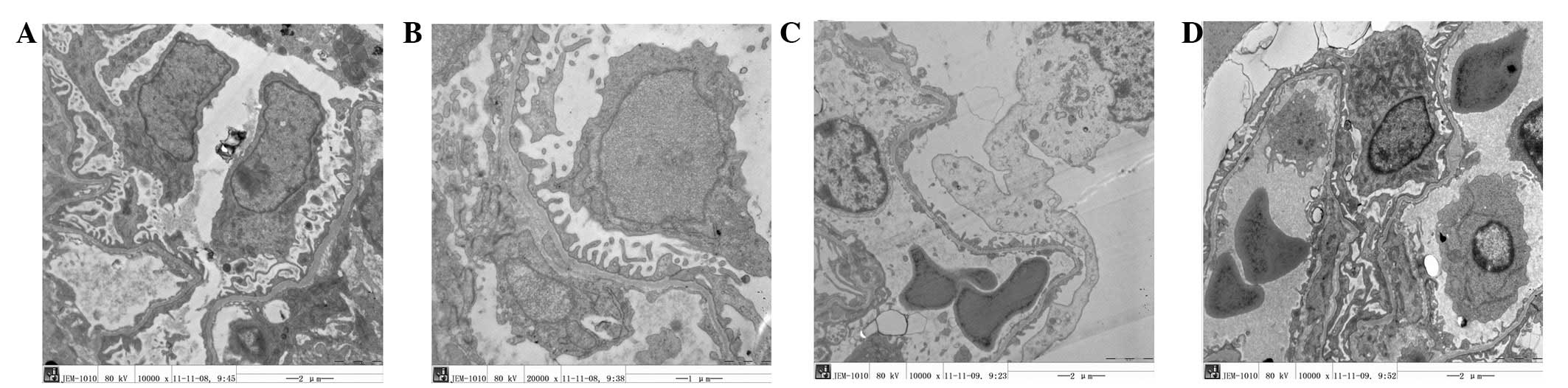 | Figure 2Electron microscopy results. (A) When
the pressure in the healthy side increased to 20 mmHg, the
separation of podocytes from the basement membrane was increased
(transmission electron microscope; magnification, ×10,000). (B)
When the pressure in the healthy side increased to 40 mmHg, the
separation of podocytes from the basement membrane continued to
increase and morphological disorders appeared (transmission
electron microscope; magnification, ×10,000). (C) When the pressure
in the injured side increased to 20 mmHg, the podocytes protruded
and were clearly separated from the basement membrane,
protuberances were flattened and the structure became disordered.
Additionally, podocyte protuberance fragments were visible
(transmission electron microscope; magnification, ×10,000). (D)
When the pressure in the infected side increased to 40 mmHg,
morphological changes of podocyte protuberances were acutely
aggravated. Additionally, erythrocyte leakage occurred and
leukocytes appeared (transmission electron microscope;
magnification, ×10,000). |
Discussion
Upper urinary calculus is a common disease that
causes kidney obstruction and hydrops. In physiological status, the
renal pelvic pressure is ∼0.98 kPa (7.35 mmHg). In the early stage
of kidney obstruction, renal blood flow perfusion increases and the
renal pelvic pressure gradually increases until it reaches a
maximum (8.82 kPa) (13).
Following this, the renal blood flow begins to decrease, the renal
glomerulus filtration rate consequently drops and the renal pelvic
pressure gradually decreases, at times becoming reduced to the
baseline or to a level slightly above the normal baseline.
Furthermore, ureter dilatation and renal pathological changes occur
(13). Therefore, a basic method
of relieving renal obstructive lesions is the prompt removal of the
obstruction. Percutaneous nephrolithotomy is a common treatment
method for the removal of calculus obstruction. However, it
requires persistent perfusion and washing during surgery. Thus,
renal pelvic pressure is likely to increase rapidly within a short
time, which easily affects the renal structure and function. The
effect is most evident in cases of complicated purulent
infection.
According to the results of an in vitro study
(14), a renal pelvic pressure
>35 mmHg causes persistent reverse flow in the renal pelvic
veins and lymphatic vessels. In cases of infection, a renal pelvic
pressure of 15–18 mmHg may cause reverse flow. According to Kukreja
et al(15), perfusion
liquid absorption occurs during percutaneous nephrolithotomy. Even
when renal pelvic pressure is <30 mmHg, perfusion liquid
absorption continues to occur. During high-pressure perfusion, the
perfusion liquid is absorbed through the impaired collection system
mucosae and the open vessel of the puncture channel and the maximum
amount of reabsorbed liquid may reach 474 ml. Furthermore,
post-operative fever, pain and reductions in haemoglobin levels are
all related to perfusion liquid absorption. For certain patients
with complications involving heart and lung diseases and renal
inadequacy, or even children, large amounts of absorbed liquids
cause body fluid levels to increase excessively (16). If the calculus is complicated with
bacterial infection, the absorbed bacteria cause bacteraemia.
Therefore, administering furosemide following surgery is helpful in
post-operative recovery. Using the radioactive labelling method,
Stenberg et al(17)
identified that liquid backflow sites are located in the renal
calyceal fornix region. The liquid also undergoes backflow through
the renal veins, instead of the lymphatic system. In vitro
renal model studies (18) have
shown that high pressure in the renal pelvis is related to the size
and length of the puncture sheath and its position in the kidney.
Therefore, decreasing the renal pelvic pressure during surgery is
necessary to expand the puncture channel or decrease the height of
the perfusion liquid (19). Ng
et al(20) conducted
micro-channel percutaneous nephrolithotomy on patients in a supine
position. When the working sheath was placed at a position lower
than the renal collecting system, high-pressure perfusion liquid
flowed from the gap between the working sheath and the percutaneous
nephroscope due to gravity and the renal pelvic pressure was
significantly less than that when the patient was in a prone
position. Furthermore, effective intra-operative gravel washing was
conducted and the post-operative complications were slightly
reduced. These observations indicate that perfusion liquid
absorption or backflow caused by an increase in the renal pelvic
pressure is an important factor that causes various post-operative
complications. Minimising the corresponding complications by
decreasing the intra-operative renal pelvic pressure also becomes
possible.
The current study investigated the effects of renal
pelvic pressure on the kidney. The study was specifically designed
to investigate the correlation between nephron damage and renal
pelvic pressure with renal purulent infection. Since a porcine
kidney is extremely similar to a human kidney, the porcine renal
model efficiently represents the progressive situation of the same
lesions in humans. This study shows that an increase in the renal
pelvic pressure within a certain range (<50 mmHg) for a healthy
kidney causes renal tubule and renal capsule dilation and renal
glomerulus compression. Less damage and fewer changes were also
observed. For a kidney with purulent infection, renal tubule
degenerative necrosis was observed at a perfusion pressure >20
mmHg. When the pressure increased to 40 mmHg, damage in the renal
glomerulus and renal tubule became more evident and local
structures in the renal glomerulus and renal tubule were damaged
and destroyed. The same changes were visible under an electron
microscope. When the renal pelvic perfusion pressure was increased
to 20 mmHg, the thickness of the renal glomerulus and renal tubule
basement membrane became uneven and the continuity of the basement
membrane was interrupted at multiple positions. The experimental
results demonstrate that the pressure resistance of a kidney with
purulent infection is significantly lower than that of the normal
kidney. If treatment is conducted at this time, pathogenic bacteria
invade the vessels at the damaged basement membrane and cause
bacteraemia or septicaemia through the blood circulation.
Therefore, when percutaneous nephrolithotomy is performed for renal
calculus complicated with infection, strictly control of the
intra-operative renal pelvic pressure is necessary. Maintaining the
pressure below 20 mmHg is also relatively safe. This study
investigated only the effects of renal pelvic pressure on a kidney
with acute infection. However, chronic renal infection is more
common in clinical practice. Therefore, further studies on the
effect of chronic renal infection are required to determine whether
our results would be consistent with those of acute infection.
References
|
1.
|
Ganpule AP and Desai M: Management of
staghorn calculus: multiple-tract versus single-tract percutaneous
nephrolithotomy. Curr Opin Urol. 18:220–223. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Soucy F, Ko R, Duvdevani M, Nott L,
Denstedt JD and Razvi H: Percutaneous nephrolithotomy for staghorn
calculi: a single center’s experience over 15 years. J Endourol.
23:1669–1673. 2009.
|
|
3.
|
Shao Y, Shen ZJ, Zhu YY, Sun XW, Lu J and
Xia SJ: Fluid-electrolyte and renal pelvic pressure changes during
ureteroscopic lithotripsy. Minim Invasive Ther Allied Technol.
21:302–306. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Mohta M, Bhagchandani T, Tyagi A, Pendse M
and Sethi AK: Haemodynamic, electrolyte and metabolic changes
during percutaneous nephrolithotomy. Int Urol Nephrol. 40:477–482.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Troxel SA and Low RK: Renal intrapelvic
pressure during percutaneous nephrolithotomy and its correlation
with the development of postoperative fever. J Urol. 168:1348–1351.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Bag S, Kumar S, Taneja N, Sharma V, Mandal
AK and Singh SK: One week of nitrofurantoin before percutaneous
nephrolithotomy significantly reduces upper tract infection and
urosepsis: a prospective controlled study. Urology. 77:45–49. 2011.
View Article : Google Scholar
|
|
7.
|
Korets R, Graversen JA, Kates M, Mues AC
and Gupta M: Post-percutaneous nephrolithotomy systemic
inflammatory response: a prospective analysis of preoperative
urine, renal pelvic urine and stone cultures. J Urol.
186:1899–1903. 2011. View Article : Google Scholar
|
|
8.
|
Labate G, Modi P, Timoney A, et al: The
percutaneous nephrolithotomy global study: classification of
complications. J Endourol. 25:1275–1280. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Hemal AK and Mishra S:
Retroperitoneoscopic nephrectomy for pyonephrotic nonfunctioning
kidney. Urology. 75:585–588. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Ng CK, Yip SK, Sim LS, et al: Outcome of
percutaneous nephrostomy for the management of pyonephrosis. Asian
J Surg. 25:215–219. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Lojanapiwat B and Kitirattrakarn P: Role
of preoperative and intra-operative factors in mediating infection
complication following percutaneous nephrolithotomy. Urol Int.
86:448–452. 2011. View Article : Google Scholar
|
|
12.
|
Tu MQ, Shi GW and He JY: Treatment of
pyonephrosis with upper urinary tract calculi. Zhonghua Yi Xue Za
Zhi. 91:1115–1117. 2011.(In Chinese).
|
|
13.
|
Shokeir AA, Shoma AM, Abubieh EA, Nasser
MA, Eassa W and El-Asmy A: Recoverability of renal function after
relief of acute complete ureteral obstruction: clinical prospective
study of the role of renal resistive index. J Urology. 59:506–510.
2002. View Article : Google Scholar
|
|
14.
|
Hinman F and Redewell FH: Pyelovenous
backflow. JAMA. 87:1287–1293. 1926. View Article : Google Scholar
|
|
15.
|
Kukreja RA, Desai MR, Sabnis RB and Patel
SH: Fluid absorption during percutaneous nephrolithotomy: does it
matter? J Endourol. 16:221–224. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Dogan HS, Kilicarslan H, Kordan Y, Celen S
and Oktay B: Percutaneous nephrolithotomy in children: does age
matter? World J Urol. 29:725–729. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Stenberg A, Bohmant S, Morsing P,
Müller-Suur C, Olsen L and Persson AE: Back-leak of pelvic urine to
the bloodstream. Acta Physiol Scand. 134:223–234. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Low RK: Nephroscopy sheath characteristics
and intrarenal pelvic pressure: human kidney model. J Endourol.
13:205–208. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Zhong W, Zeng G, Wu K, Li X, Chen W and
Yang H: Does a smaller tract in percutaneous nephrolithotomy
contribute to high renal pelvic pressure and postoperative fever? J
Endourol. 22:2147–2151. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Ng MT, Sun WH, Chen CW and Chan ES: Supine
position is safe and effective for percutaneous nephrolithotomy. J
Endourol. 18:469–474. 2004. View Article : Google Scholar : PubMed/NCBI
|
















