Introduction
Peirce (1) and
Sparks et al(2)
independently attempted to apply the wrapping phenomenon to induce
the generation of a tubular wrapping tissue as a vascular
substitute using an allotransplant implanted in the subcutaneous
tissue. However, these attempts failed. All vessels were occluded
during early implantation as the lumenal face of this type of graft
has directly exposed collagen fibers and eventually promotes
thrombosis (3–5).
Tissue engineering technology provides a method for
developing novel vascular graft materials. However, existing
synthetic and natural materials are not able to meet the demands of
this process. Traditional vascular tissue engineering technology
uses a polymer material as a stent. However, this polymer material
has a mechanical property which makes it difficult to mould.
Moreover, its waste products have varying degrees of local toxicity
(6). Increasing attention has
therefore been awarded to biologically sourced materials.
Xenogeneic or allogeneic acellular materials have unique advantages
in mechanical strength and compliance. However, the risks of
infectious diseases, antigen residues and other problems exist
(7,8). Tissue calcification is another
significant reason for limiting the application of these materials
(9). The collagen-base frame,
examined on behalf of recombination extracellular matrix materials,
has a traditional material-incomparable advantage in biological
compatibility. However, existing problems with mechanical
properties and compliance greatly restrict the applications of this
type of material in tissue engineering. Several scholars have
conducted cross-linking of the collagen-base frame using physical
or chemical methods and also by blending the frame with other
materials to modify its properties. However, the collagen-base
frame remains unsuitable for constructing tissue engineering
vessels (7,8,10,11).
An ideal tissue graft should only include a
patient’s own cells and extracellular matrix components, as well as
having the appropriate mechanical properties and shapes. The tissue
materials of the recipients themselves have attracted attention in
recent years due to their unique advantages. The wrapping tissue
generated by the allotransplant-induced package is a type of
cell-free autogenous living tissue material with incomparable
biological superiorities beyond those of synthetic materials. In
theory, this wrapping tissue completely avoids the rejection
reaction and the destructive effect of the non-autograft immune
system. This tissue may also be prepared according to the user’s
requirements. Therefore, the tissue materials of the recipients
themselves are expected to solve the problem of constructing tissue
engineering vessels. However, no studies exist on the histological
and mechanical properties of such material. The present study
investigated the histological components, structure, tensile
strength and elongation rate at the breaking point, bursting
strength, suture serviceability and other mechanical
characteristics of this novel living tissue material.
Materials and methods
Animals
In total, 4 adult female mongrel dogs weighing 20–30
kg were used in the present study. The animals were provided by the
Beijing Keyu Experimental Animal Breeding Centre (License No. SCXK
2000-0015; Animal breeding level, grade 1). All procedures were
approved by ethics committee of Xuanwu Hospital (Beijing,
China).
Living tissue preparation
Subsequent to administering mixed anesthesia to the
experimental animals via intramuscular injection, the dorsal and
abdominal skins were prepared. Each animal was placed in a prone
position and the dorsal incision area was disinfected three times
with 2% iodophor solution. A surgical knife was used to make two
longitudinal small incisions measuring 1 cm each and then two small
cavities were formed by conducting blunt separation towards two
opposite sides of the subcutaneous tissue. An aseptic medical
silicone tube (Jinan silicone factory, Shandong, China) with a
length of 4 cm and a diameter of 4 mm was then implanted into each
cavity. The incisions were closed with silk sutures. Afterwards,
each animal was turned over into the supine position. Following the
disinfection of the table covering, an incision measuring 1–2 cm
long was made in the abdominal area. A total of 8 aseptic medical
silicone tubes were implanted into the abdominal cavities of the
animals. The incisions were closed layer by layer. Following one
month of post-operative procedures, surgery was performed to
completely remove the silicone tube pod that was now wrapped by the
peritoneum or subcutaneous tissue. The two ends of the closed pod
were cut off following appropriate trimming under sterile
conditions. The silicone tubes were taken out to form the final
tubular structure.
Histological testing of living tissue
biological tubes
Specimen processing
The specimens were removed at one week or one month
subsequent to tube implantation and fixed with 10% neutral formalin
buffer for 48 h. The specimens were then gradually dehydrated with
alcohol, embedded in paraffin wax and cut into slices 4-μm
thick.
Common hematoxylin-eosin (HE) staining.
Subsequent to the specimens being dewaxed with dimethylbenzene,
dehydrated with alcohol and stained with HE, the general cell
compositions and arrangement distributions of the specimens were
observed. The thickness of the living tissue biological tube was
measured under an optical microscope.
Masson staining. Subsequent to the specimen
slices being dewaxed and placed in water, the slices were stained
with Masson’s compound staining solution (Fuzhou Wallace New
Biological Technology Development Co., Ltd., Fujian, China) for 5
min and washed under running water for 10 min. The specimen slices
were then washed with distilled water and soaked in 1%
phosphomolybdic acid for 3 min prior to being dried. The slices
were soaked in 2% aniline blue solution for 5 min, washed with
distilled water, soaked in 1% glacial acetic acid solution for 5
min and conventionally dehydrated and made transparent. Finally,
the specimen slices were mounted with neutral gum. Canine natural
femoral arteries were collected for the control group.
Picrosirius red staining. First, 0.1 g
picrosirius red (Sirius Red F3B; Sigma, St. Louis, MO, USA) was
dissolved in 100 ml picric acid saturated solution to prepare a
picrosirius red solution. The specimens were cut into slices
5-μm thick. The specimen slices were then stained with 0.1%
picrosirius red solution for 1 h at 37°C subsequent to being
dewaxed and placed in water. The slices were washed under running
water for 5 min, made transparent and mounted. Canine natural
femoral arteries were also collected for the control group.
Observations were conducted using a micropolariscope under
darkfield conditions. A closely arranged type I collagen fiber
showed strong birefringence and presented yellow, orange and red
crude fibers under polarized light. The fiber diameter was larger
and the red color was more marked. A type III collagen fiber,
arranged in a sparse network shape, presented weak birefringence
and green, slim fibers. A type II collagen fiber showed weak
birefringence and presented sparse networks with varying
colors.
Observations of the surface structure of the
living tissue biological tubes. The obtained living tissue
biological tube was longitudinally split. The inner surface was
faced upward, stuck onto dry filter paper, double fixed with 2.5%
glutaraldehyde and 1% osmic acid, gradually dehydrated with
alcohol, dried at the CO2 critical point and sprayed
with metal in a vacuum. The ultramicrostructure of the endocoele
surface of the biological tube was observed under a scanning
electron microscope (SEM; Hitachi S-520, Tokyo, Japan). Canine
natural femoral arteries were also collected for the control group.
The experimental specimens in the present study were sent to the
ultrastructural pathology laboratory of the Affiliated Tiantan
Hospital of the Capital Medical University (Beijing, China), for
complete ultrastructural observations.
Immunohistochemical staining of the muscular
components of the living tissue biological tubes. The
immunohistochemical method was used to conduct anti-α-smooth muscle
actin (anti-α-SMA) and desmin staining to understand the
distribution situations of the muscular components in the
biological tubes. Immunohistochemical specimens were prepared. The
specimens were fixed with neutral formalin, embedded in paraffin
wax, cut into slices 4-μm thick, dewaxed with
dimethylbenzene, dehydrated with a series of alcohols, repaired
with antigen, incubated with 3% H2O2 at room
temperature for 10 min and washed three times with phosphate
buffered saline (PBS). Anti-α-SMA antibody (1:100) and anti-desmin
antibody (1:100) (Lab Vision-NeoMarkers, Fremont, CA, USA) working
liquids were added to the specimens. The mixture was incubated
overnight at 4°C and washed three times with PBS. The mixture was
then incubated for 20 min and washed with PBS following the
addition of agent 1 (polymer helper; Invitrogen, Carlsbad, CA,
USA). Agent 2 (polyperoxidase-anti-mouse/rabbit IgG; Zymed) was
then added and the mixture was incubated at room temperature for 30
min. The mixture was then washed with PBS, developed with a
developer, washed under running water, restained with HE,
dehydrated, made transparent and mounted. Canine serum was used to
replace the first antibody for the negative control.
Mechanical testing of the living tissue
biological tubes
Detection of tensile strength and
elongation rate at the breaking point
The specimens were cut into 5-mm wide test strips.
The initial length of the tensile machine was set as 2 cm and the
specimens were pulled by a Shimadzu AG-5000A Universal Material
Testing Machine (Shimadzu, Kyoto, Japan) until they broke. The
specimens were frozen and sectioned prior to measurement. The
thickness was measured under an optical microscope. Canine natural
femoral arteries were also collected for the control group. The
measured maximum force was divided by the cross-sectional area of
the experimental material to obtain the material tensile strength
at the breaking point. The change in the length of the specimen
from the beginning of elongation to the breaking point was recorded
and divided by the original specimen length to obtain the
percentage length change, i.e., the elongation rate of the specimen
material at the breaking point.
Detection of bursting strength. A living
tissue tube specimen was connected to a bursting pressure gauge
(Nanjing Wanda instrument and meter plant, Jiangsu, China). The
free end was ligatured. The device was filled with PBS solution to
soak the specimen. The compression bolt was adjusted to a rate of
50 mmHg/sec at room temperature to increase the pressure in the
tube system, which was being recorded by the pressure gauge. Canine
natural femoral arteries were used as a positive control in this
group. Bursting pressure was defined as the highest pressure in the
tube material prior to rupture, representing the tolerance of the
material to pressure changes.
Detection of specimen suture strength. One
end of a specimen was clamped on the tensile machine (Shimadzu
AB-I; Shimadzu), while the other end was attached to another
fixture of the tensile machine with a 6-0 nylon suture. The
distance between the two fixtures was 2 cm. The specimen was pulled
at a constant speed of 1 mm/min. The pulling force at the breaking
point was recorded. Canine natural femoral arteries were used as
the positive control group. The suture strength represented the
material suture serviceability.
Results
Biological tube preparation
Sampling was conducted one week subsequent to the
silicone tubes being implanted into the subcutaneous tissue and
abdominal cavity of the experimental animals. The wrapping tissue
was thin and the wrapping of the partial silicone tubes in the
abdominal cavity was incomplete and fragile. The silicone tubes
were therefore not yet suitable for use as in vitro
materials. After one month, all silicone tubes implanted into the
subcutaneous tissue and abdominal cavity of the experimental
animals were completely wrapped. The subcutaneous wrapping tissue
markedly adhered to the surrounding fascia and was easily damaged
during sampling. The majority of the silicone tubes implanted into
the abdominal cavity, however, were wrapped in omental tissue
(25/32 specimens) and only a few silicone tubes were free in the
abdominal cavity (5/32 specimens), thus demonstrating pedicle
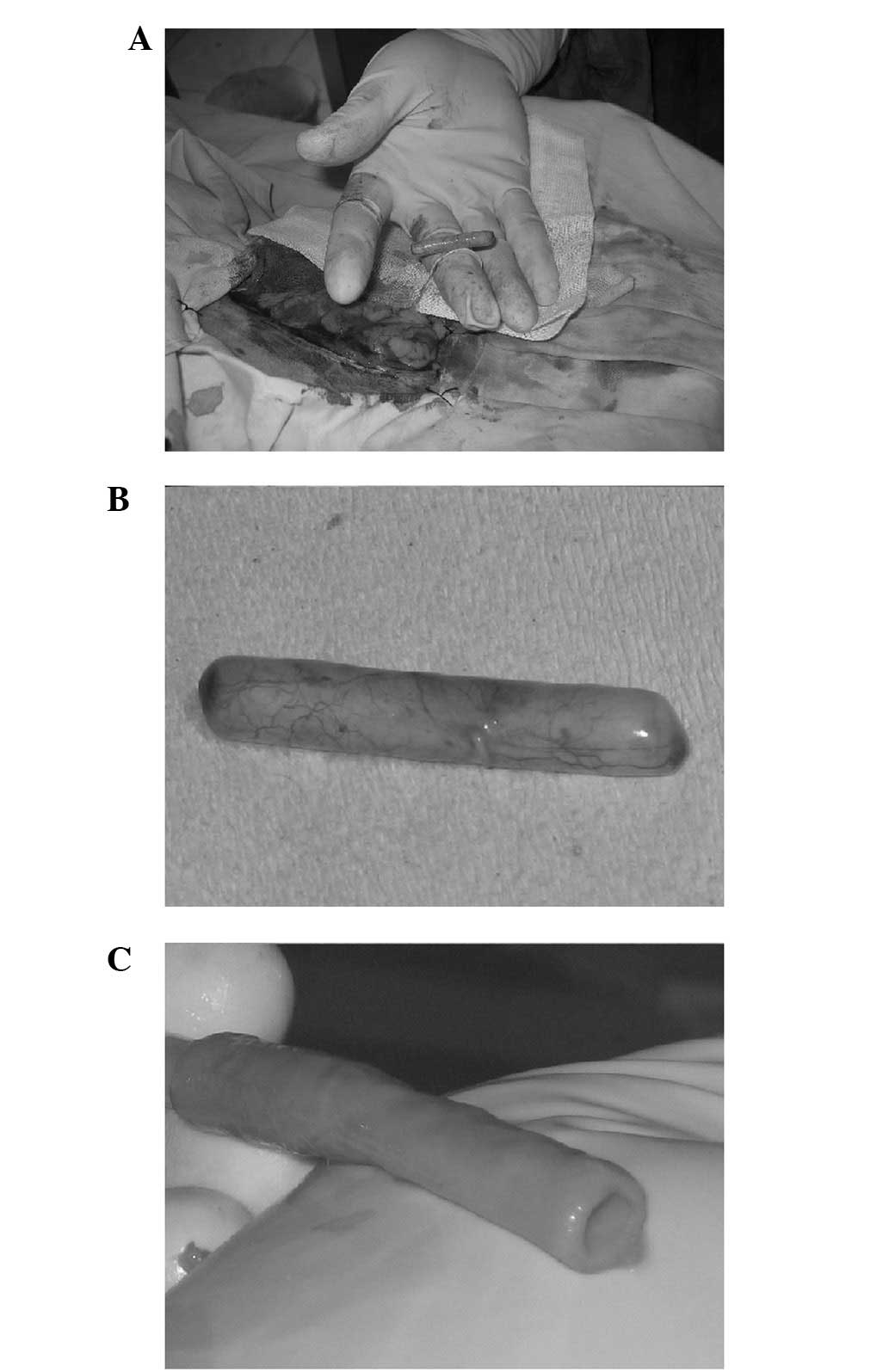
adhesion with the peritoneal tissue (Fig. 1A and B). In addition, adhesion with
the parietal peritoneum was rare (2/32 specimens). When one end of
the pod was cut open, no adhesion between the internal silicone
tubes and wrapping tissue was observed and the silicone tubes were
easily removed. The living tissue biological tubes that were
obtained were relatively thick, solid and strong. However, the
tubes were pliable in texture, their external surface was covered
with a few soft fatty tissues that were easy to remove and the
endocoele surface was smooth (Fig.
1C). In addition, all the biological tubes that were obtained
were able to better maintain their open status in solution and the
endocoele diameter was close to the external diameter of the
implanted silicone tubes (∼4 mm). Therefore, the biological tubes
implanted into the abdominal cavity for one month were selected as
the objects of investigation in the present study.
Histological testing of the living
tissue biological tubes
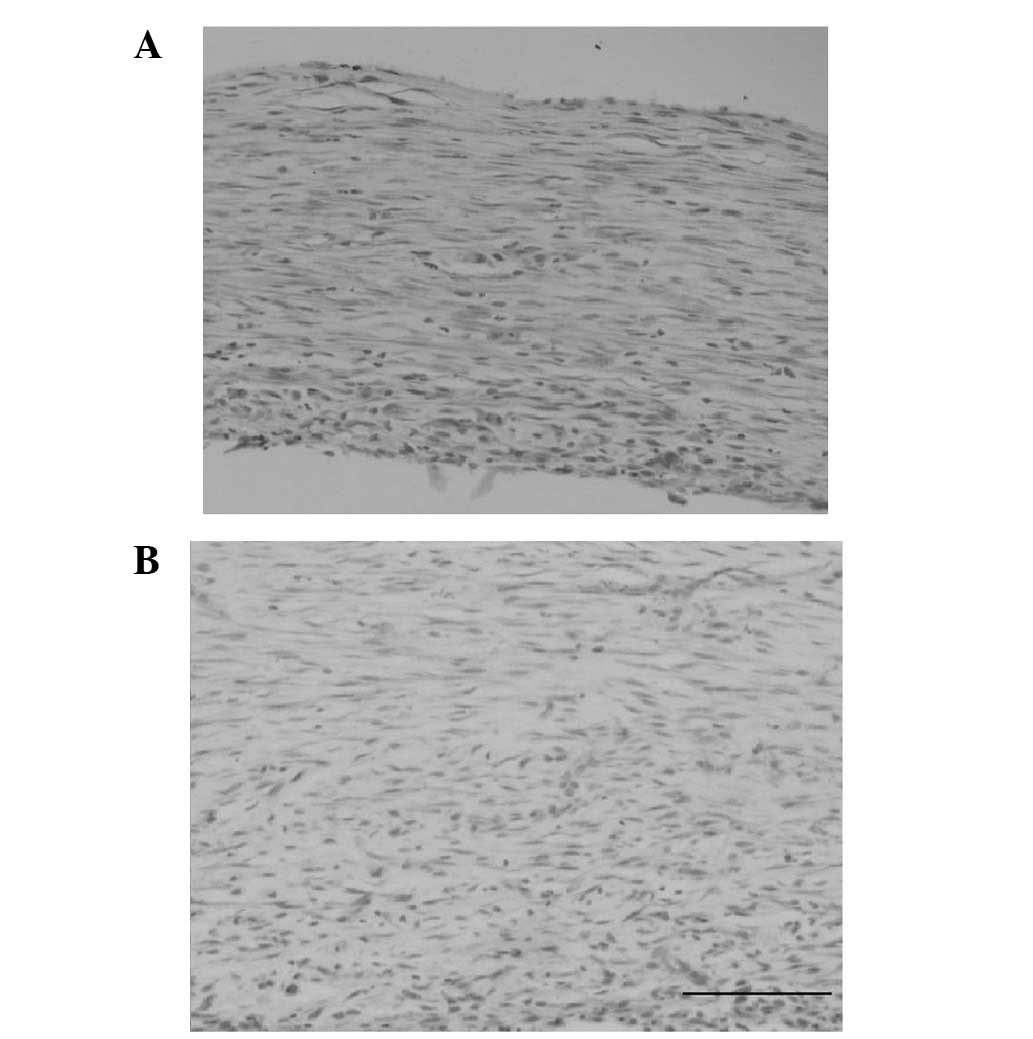
Common HE staining was conducted for the slices of
the living tissue biological tubes. The wall thickness was nearly
uniform under an optical microscope. The tube wall thickness was
observed to differ (70–250 μm) between animals or between
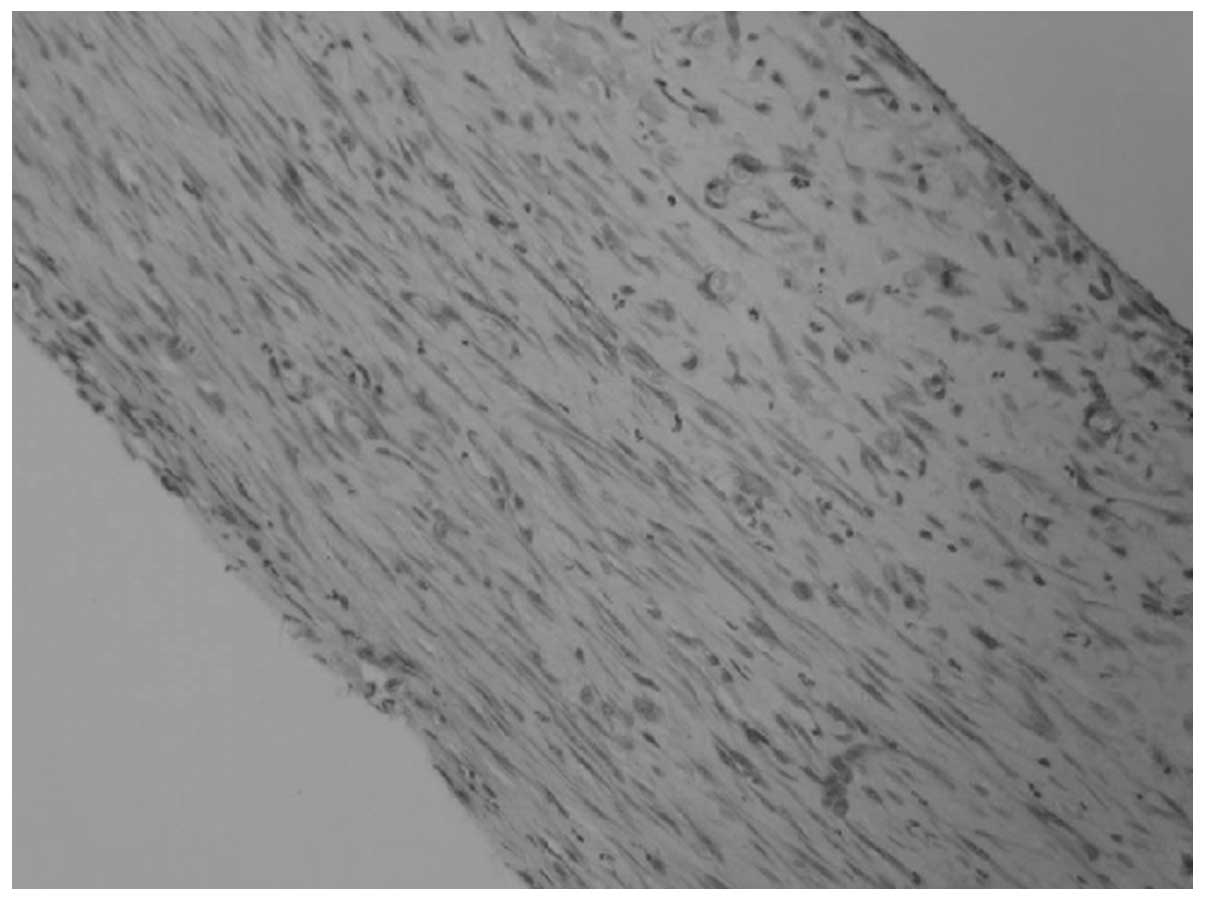
different sites of the same animal. Histological examination showed
that a great number of collagen fibers were arranged in a compact
lamellar structure. Spindle cells arranged in concentric circles
were visible among them. A small amount of inflammatory cell
infiltration was also visible in the tube wall (Fig. 2).
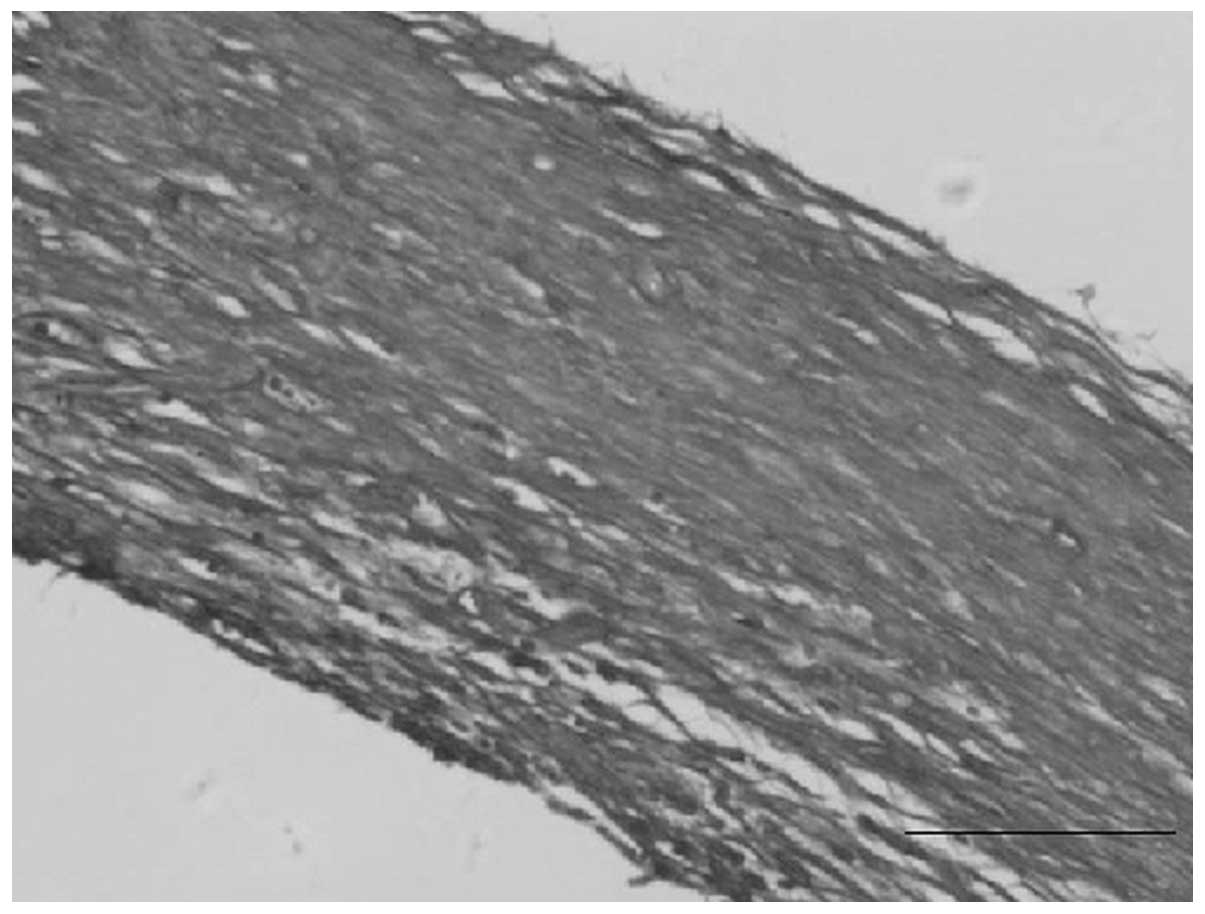
In the fresh autogenous living tissue biological
tubes, the collagen fibers turned blue following Masson’s staining
and were arranged in a wave shape, thus presenting concentric
ring-like structures. Red-stained, smooth muscle-like cells were
visible among them (Fig. 3).
Micropolariscope observation was conducted under
dark-field conditions. The fresh autogenous living tissue
biological tube walls showed a wide distribution of type III
collagen fibers with green, weak refraction. The scattered
distribution of the type I collagen fibers with red-yellow strong
refraction was similar to that of the natural blood vessels
(Fig. 4).
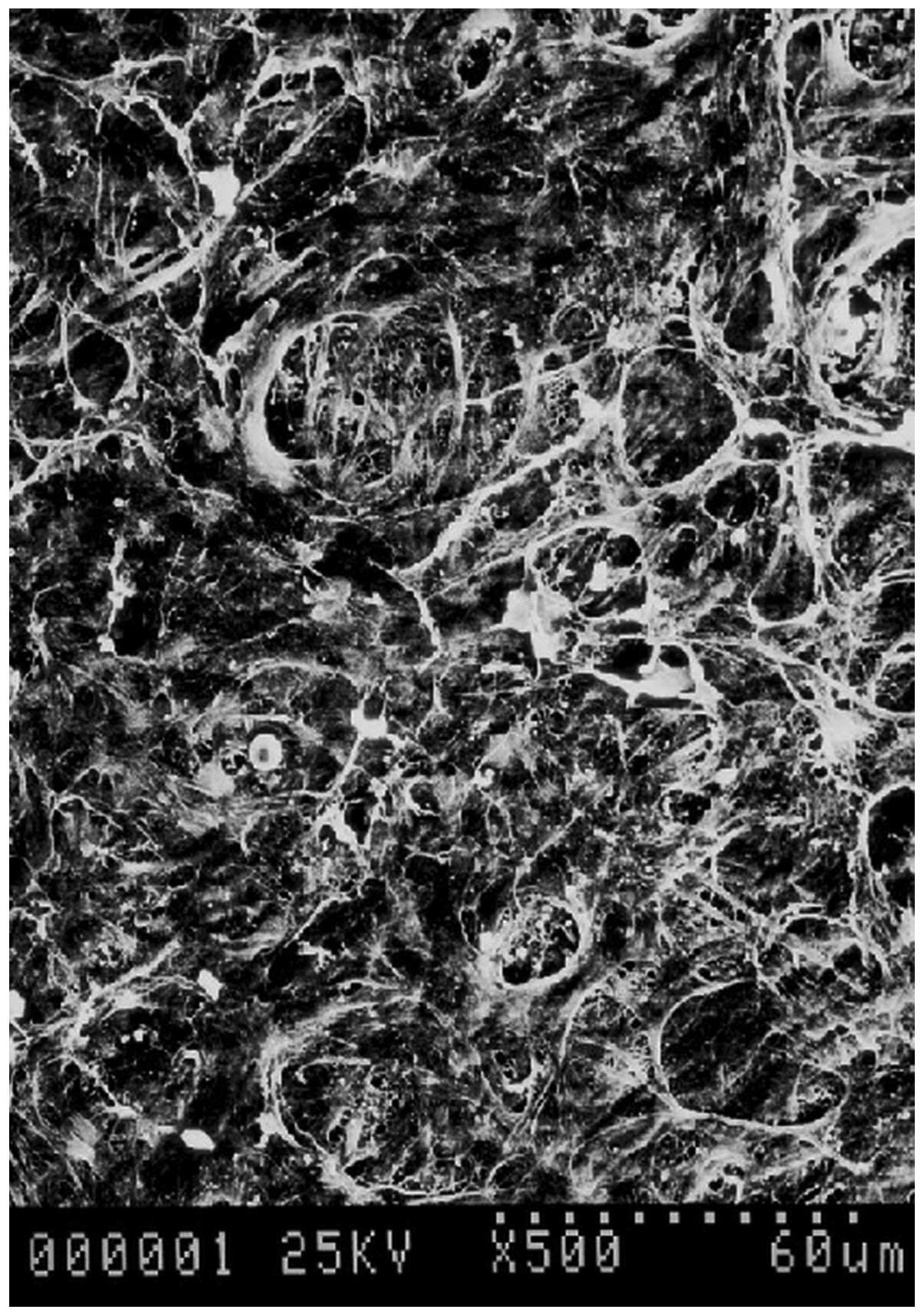
SEM observation showed a large number of fibrous and
membranous extracellular matrix components on the internal surface
of the autogenous living tissue biological tubes. The cell bodies
and fiber-like apophyses were dimly visible below the matrix. The
blood cell components were also occasionally visible (Fig. 5).
The immunohistochemical study results showed that
all the tube walls contained a number of α-SMA-positive cells,
whereas desmin staining of the mature smooth muscle cytoskeleton
was negative (Fig. 6).
Mechanical testing of the living
tissue biological tubes
The tensile strength and elongation rate at the
breaking point of the living tissue biological tubes were measured
to characterize the antitensile capability of the living tissue
material in the biological tubes and to represent the material
deformability. The tensile strength of the fresh autogenous living
tissue biological tubes at the breaking point was observed to be
4.7±2.3 MPa, whereas the elongation rate at the breaking point was
34.2±8.3%. Under the experimental conditions, the tensile strength
at the breaking point of the natural femoral arteries was 9.3±3.2
MPa, whereas the elongation rate at the breaking point was
91.5±27.1%.
The bursting strength was measured to determine the
maximum internal pressure that the constructed material was able to
withstand prior to breaking. This process ascertained if the
autogenous living tissue biological tubes had sufficient strength
to withstand physical tension. A total of 8 living tissue
biological tubes (four groups) from different animals were tested
and 4 natural femoral arteries from these corresponding animals
were used as the positive controls. The bursting strength of the
living tissue biological tubes was 1100±187 mmHg. This bursting
strength value was far larger than the internal pressure borne by
the natural blood vessels under normal conditions. The pressure
tolerance of the natural canine femoral arteries under the
experimental conditions was 2280±317 mmHg.
Vascular tissue tolerance to suture tensile force
may be defined by the suture tolerance strength. A 6-0 silk suture
was used for the tests in the present study. Specimens from the
four animals were tested and each specimen was tested three times.
The suture tolerance strength of the fresh living biological tubes
was observed to be 2.5±0.3 N. The suture tolerance strength of the
natural blood vessels was 3.2±0.4 N. The suture tolerance strength
values were similar, indicating that the living tissue biological
tubes in this group exhibited an adequate suture tolerance strength
and would be able to tolerate an end-to-end anastomosis in
vivo surgery.
Discussion
The present study attempted to develop a type of
autogenous living tissue biomaterial that would be useful for in
vitro vascular tissue engineering. The proposed material
contained only the autogenous cells and extracellular matrix
components of the patient and may be used for the in vitro
construction of tissue-engineered vessels. The tissue wrapping
phenomenon induced by an implanted inert allotransplant was
applied. First, silicone tubes were implanted into the body of the
experimental animals to obtain the pod-like biological tissue
formed by wrapping. In vitro trimming was then conducted to
form a tubular structure called an autogenous living tissue
biological tube. The histological components, structure, tensile
strength and elongation rate at the breaking point, bursting
strength, suture serviceability and other mechanical properties of
the proposed material were studied for the preliminary function of
wrapping the tubular tissue around a small-caliber artery.
The present study used a hydrophilic silicone tube
as a mould and implanted it into the canine body. The pod-like
structure wrapped around the implant was formed after one month.
The required living tissue biological tube was obtained by in
vitro trimming. No adhesion existed between the inner surface
of the tube and the implant. Implantation of the silicone tubes
into subcutaneous tissue was attempted during the preliminary
experiment. Similar wrapping tubes were obtained as a result.
However, the wrapping tubes were easily damaged during the
acquisition process as the wrapping tissue adhered to the
subcutaneous fascia. By contrast, the wrapping tubes implanted into
the abdominal cavity of the experimental animals were mostly free
and their boundaries with the surrounding tissues were clear.
Therefore, the present study adopted the method of implanting
silicone tubes into the abdominal cavity of the experimental
animals. The biological tube walls that were obtained were composed
of an extracellular matrix with plenty of collagen fibers and
myofibroblasts. The collagen-fiber network was observed as an
irregular concentric circle-like structure similar to normal
vascular lamellar-like structures (12). The tube wall thickness was 70–250
μm, which is in agreement with that of a biological
tube.
The varying common tissues and vascular tissue
engineering materials must have appropriate mechanical properties.
For example, sufficient strength to withstand a considerable
internal pressure without rupturing is required. The proposed
material should not bend or tangle due to postural changes and the
silicone tubes must be able to serve as sutures and be suitable for
use in clinical applications (13). Collagen accounts for 25–33% of the
total protein in the human body. Collagen is widely distributed in
the extracellular matrix and constitutes tissue organ support.
Collagen fibers are closely correlated with the biomechanical
properties of tissue organs. In addition, collagen is the basic
structural substance of vessels, skeleton, cartilage, muscle
tendons, ligaments and other organs. Collagen fibers also cause
organs to have a high tensile strength. Histological studies have
clearly shown that the static mechanical property of the
vasculature is maintained by connective tissues, including collagen
fibers. Collagen fibers are distributed in various layers of the
tube walls and are located among smooth muscle cells, the
subendothelial layer and the adventitia (14,15).
The living tissue biological tube constructed in the present study
was mainly composed of extracellular matrix components, including
collagen tissues (type III collagen was predominant), and is
similar to the natural blood vessels (with similar concentric
structures). Mechanical study confirms that the prepared living
tissue biological tube had an improved antitensile capacity,
deformability and adequate bursting strength. The living tissue
biological tubes were able to withstand bursting strength values of
>1000 mmHg and had surgical suture properties similar to those
of the natural arteries. These biological tubes also possessed good
mechanical properties.
Several studies have shown that the collagen
components of natural arteries are collagen fibers composed of
collagen types I and III (16).
The spiral directions of the collagen α-chain are contrary to one
another. Collagens connect end to end and polymerize in a parallel
manner. Collagen fibers exhibit a birefringence phenomenon under a
micropolariscope. In addition, the collagen fibers formed by the
various types of collagen are inconsistent in thickness. Collagen
fibers exhibit a range of colors under a micropolariscope,
therefore, estimation of the collagen type is feasible. Picrosirius
red is a highly acidic anionic dye that easily reacts with basic
groups in the collagen fiber to enhance birefringence and increase
resolution. Picrosirius red is a long, unfolded molecule that is
able to closely absorb collagen molecules to generate a stable
reaction. In addition, this dye does not fade subsequent to
staining and it also demonstrates specificity. Thus, picrosirius
red is presently the best dye for collagen staining. Collagen types
I, II, and III each exhibit various colors under a micropolariscope
subsequent to staining. Comparisons between the three collagen
types are clear and thus, they may be easily differentiated.
Several studies have indicated that, in comparison with current
common histological specific and immunohistochemistrical staining,
picrosirius red staining has several advantages, including its ease
of operation, time-saving qualities, low cost and high specificity
and sensitivity (17–19). The living tissue biological tube
constructed in the present study was mainly composed of type I and
III collagen fibers, which provided adequate strength to the
tube.
The extracellular matrix is a type of complete
protein molecule secreted outside of the cells following cell
synthesis. Matrix components may be generally classified into three
categories: glycosaminoglycans/proteoglycans, constructive proteins
and adhesive proteins. Studies in recent years have suggested that
the in vivo extracellular matrix is biologically significant
as it is not only a support structure and attachment site for a
variety of cells, but it also plays a significant role in the
regulation of cellular behaviour and gene expression, the
excitation of transmembrane signals and the transformation of cell
phenotype and function (20,21).
Collagen is a main component of the vascular extracellular matrix.
Collagen is located in the subendothelial layer and around the
vascular middle-layer of smooth muscle cells (22,23).
The living tissue biological tube constructed in the present study
was composed of autogenous living cells and natural extracellular
matrix components predominantly based on collagen and carrying a
number of biological signals. In addition, it is an ideal
base-frame material for constructing tissue engineering organs.
The inert allotransplant-induced wrapping phenomenon
was applied to implant silicone tubes into the abdominal cavities
of the experimental animals for four weeks to construct a type of
living tissue biological tube. Histological examination indicated
that the living tissue biological tube was composed of autogenous
myofibroblasts and extracellular matrix components predominantly
based on collagen types I and III. Mechanical testing showed that
the biological tube had a good mechanical strength, allowing it to
withstand opposing bursting strength values of >1000 mmHg. The
biological tube also exhibited a good suture strength. Therefore,
the biological tube obtained in the present study may be used as a
base-frame material for constructing tissue engineering
vessels.
References
|
1.
|
Peirce EC II: Autologous tissue tubes for
aortic grafts in dogs. Surgery. 33:648–657. 1953.PubMed/NCBI
|
|
2.
|
Sparks CH, Melgard MA and Raaf J: Carotid
artery replacement with reinforced autogenous vein grafts.
Angiology. 14:542–551. 1963. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Parsonnet V, Alpert J and Brief DK:
Autogenous polypropylene-supported collagen tubes for long-term
arterial replacement. Surgery. 70:935–939. 1971.PubMed/NCBI
|
|
4.
|
Sparks CH: Silicone mandril method of
femoropopliteal artery bypass. Clinical experience and surgical
technics. Am J Surg. 124:244–249. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Sparks CH: Silicone mandril method for
growing reinforced autogenous femoro-popliteal artery grafts in
situ. Ann Surg. 177:293–300. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
McKee JA, Banik SS, Boyer MJ, et al: Human
arteries engineered in vitro. EMBO Rep. 4:633–638. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Isenberg BC, Williams C and Tranquillo RT:
Small-diameter artificial arteries engineered in vitro. Circ Res.
98:25–35. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Campbell GR and Campbell JH: Development
of tissue engineered vascular grafts. Curr Pharm Biotechnol.
8:43–50. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Bailey MT, Pillarisetti S, Xiao H and
Vyavahare NR: Role of elastin in pathologic calcification of
xenograft heart valves. J Biomed Mater Res A. 66:93–102. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Kakisis JD, Liapis CD, Breuer C and Sumpio
BE: Artificial blood vessel: the Holy Grail of peripheral vascular
surgery. J Vasc Surg. 41:349–354. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
L’Heureux N, Dusserre N, Konig G, et al:
Human tissue-engineered blood vessel for adult arterial
revascularization. Nat Med. 12:361–365. 2006.PubMed/NCBI
|
|
12.
|
Konig G, McAllister TN, Dusserre N, et al:
Mechanical properties of completely autologous human tissue
engineered blood vessels compared to human saphenous vein and
mammary artery. Biomaterials. 30:1542–1550. 2009. View Article : Google Scholar
|
|
13.
|
Higgins SP, Solan AK and Niklason LE:
Effects of polyglycolic acid on porcine smooth muscle cell growth
and differentiation. J Biomed Mater Res A. 67:295–302. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Shekhonin BV, Domogatsky SP, Muzykantov
VR, Idelson GL and Rukosuev VS: Distribution of type I, III, IV and
V collagen in normal and atherosclerotic human arterial wall:
immunomorphological characteristics. Coll Relat Res. 5:355–368.
1985. View Article : Google Scholar
|
|
15.
|
Voss B and Rauterberg J: Localization of
collagen types I, III, IV and V, fibronectin and laminin in human
arteries by the indirect immunofluorescence method. Pathol Res
Pract. 181:568–575. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Morton LF and Barnes MJ: Collagen
polymorphism in the normal and diseased blood vessel wall.
Investigation of collagens types I, III and V. Atherosclerosis.
42:41–51. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Canham PB, Finlay HM, Kiernan JA and
Ferguson GG: Layered structure of saccular aneurysms assessed by
collagen birefringence. Neurol Res. 21:618–626. 1999.PubMed/NCBI
|
|
18.
|
Hou Y, Mao Z, Wei X, et al: The roles of
TGF-beta1 gene transfer on collagen formation during Achilles
tendon healing. Biochem Biophys Res Commun. 383:235–239. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Kaemmer D, Bozkurt A, Otto J, et al:
Evaluation of tissue components in the peripheral nervous system
using Sirius red staining and immunohistochemistry: a comparative
study (human, pig, rat). J Neurosci Methods. 190:112–116. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Chen C, Loe F, Blocki A, Peng Y and
Raghunath M: Applying macromolecular crowding to enhance
extracellular matrix deposition and its remodeling in vitro for
tissue engineering and cell-based therapies. Adv Drug Deliv Rev.
63:277–290. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Kim BS, Park IK, Hoshiba T, Jiang HL, Choi
YJ, Akaike T and Cho CS: Design of artificial extracellular
matrices for tissue engineering. Prog Polym Sci. 36:238–268. 2011.
View Article : Google Scholar
|
|
22.
|
Elliott JT, Woodward JT, Langenbach KJ,
Tona A, Jones PL and Plant AL: Vascular smooth muscle cell response
on thin films of collagen. Matrix Biol. 24:489–502. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Critser PJ, Kreger ST, Voytik-Harbin SL
and Yoder MC: Collagen matrix physical properties modulate
endothelial colony forming cell-derived vessels in vivo. Microvasc
Res. 80:23–30. 2010. View Article : Google Scholar : PubMed/NCBI
|