Introduction
Inflammatory bowel disease (IBD) consists of two
main forms: Crohn’s disease (CD) and ulcerative colitis (UC). These
conditions are characterized by chronically relapsing disorders of
the gastrointestinal tract (1–3). The
etiology of IBD is unclear and there are no effective long-term
treatments. Current treatment strategies depend on disease
severity, and the majority of them are focused on attenuating
disease symptoms rather than treating the disease. Furthermore,
numerous currently used treatment approaches for IBD may lead to
systemic immunosuppression which limits their long-term use.
Therefore, there is a need for the development of new therapeutic
agents. Complementary and alternative medicines (CAM), particularly
herbal therapies, including traditional Chinese medicine (TCM),
have received interest as they have relatively few side-effects and
have been used as alternative remedies for a variety of diseases,
including IBD (4–7).
The inflammatory response is highly regulated by
multiple cellular signal transduction pathways, including the
nuclear factor κB (NF-κB) pathway, which is capable of being
activated by various pathogens, including lipopolysaccharide (LPS).
As a main component of the outer membrane of gram-negative
bacteria, LPS has been hypothesized to form an important risk
factor of IBD (8–10). LPS activates the host Toll-like
receptor 4 (TLR4) via direct interaction, which subsequently
transduces immune-related signals to the nucleus via transcription
factors, including NF-κB (11,12),
leading to the positive regulation of the expression of various
pro-inflammatory cytokines, including TNF-α, IL-6 and IL-8
(13–19). Therefore, suppression of the NF-κB
pathway may provide an effective strategy for the treatment of
inflammatory diseases, including IBD.
Qing Hua Chang Yin (QHCY) is a well-known
traditional Chinese formulation that consists of a combination of
eleven herbs, including Herba et Gemma Agrimoniae, Coptis
chinensis Franch, Radix Sanguisorbae, Radix Paeoniae
Rubra, Elettaria cardamomum, Magnolia
officinalis, Artemisia capillaris Thunb, Herba
Eupaatorii Fortunei, Semen Coicis, Semen Dolichoris
Album and Poria cocos. Collectively, these components
confer QHCY with the properties of eliminating heat and dampness,
as well as strengthening the spleen to nourish vitality (known as
tonifying the Spleen Qi in Chinese). Since the accumulation of
toxic dampness and heat is one of the major causative factors in
the pathogenesis of UC in the TCM system, heat clearing and
eliminating dampness provides the basic principle behind the
treatment of inflammatory diseases with QHCY. QHCY has long been
used in China to clinically treat UC (20–25).
However, the precise mechanism of its anti-inflammatory activity
remains largely unclear. Using LPS-stimulated Caco-2 cells as an
in vitro inflammatory model of the human intestinal
epithelium, we evaluated the anti-inflammatory effects of QHCY and
investigated its underlying molecular mechanisms.
Materials and methods
Materials and reagents
Dulbecco’s modified Eagle’s medium (DMEM), fetal
bovine serum (FBS), penicillin-streptomycin and trypsin-EDTA were
purchased from Invitrogen Life Technologies (Carlsbad, CA, USA).
LPS from Escherichia coli serotype 055:B5 was purchased from
Sigma-Aldrich (St. Louis, MO, USA). Antibodies for Western blot
analysis were obtained from Cell Signaling Technology, Inc.
(Beverly, MA, USA). All other reagents, unless otherwise stated,
were obtained from Sigma Chemicals (St. Louis, MO, USA).
Preparation of QHCY
In total, 220 g dehydrated Herba et Gemma
Agrimoniae, 33 g Coptis chinensis Franch, 100 g Radix
Sanguisorbae, 110 g Radix Paeoniae Rubra, 56 g
Elettaria cardamomum, 110 g Magnolia officinalis, 110
g Artemisia capillaris Thunb, 110 g Herba Eupaatorii
Fortunei, 220 g Semen Coicis, 110 g Semen Dolichoris
Album and 220 g Poria cocos were extracted with boiling
water 3 times. The extracts were then combined and concentrated by
boiling to a final volume of 1,000 ml. The final concentration of
QHCY crude drug was ∼1.4 mg/ml.
Cell culture
Human colon cancer Caco-2 cells were purchased from
the American Type Culture Collection (Rockville, MA, USA). Cells
(passages 20–40) were grown in DMEM containing 10% (v/v) FBS, 1,000
mg/l of glucose, 50 U/ml penicillin and 50 μg/ml
streptomycin in a 37°C humidified incubator with 5% CO2.
Cells were subcultured at 85–90% confluence. Caco-2 cells usually
reached confluence 3 days after seeding and differentiated into
enterocyte-like cells 18–20 days post-confluence. Fully
differentiated cells were used for the experiments. On the day of
the experiment, the medium was removed and cells were washed twice
with DMEM supplemented with 0.5% FBS.
Enzyme-linked immunosorbent assay
(ELISA)
Differentiated Caco-2 cells (20 days
post-confluence) in 24-well plates were pre-incubated with various
concentrations of QHCY for 1 h prior to being stimulated with 1
μg/ml of LPS for 24 h. At the end of the experiment, the
supernatants were collected following centrifugation of the cell
culture media at 3,000 × g for 10 min. The secretion levels of
cytokines were measured using a human TNF-α ELISA kit (Invitrogen
Life Technologies, Camarillo, CA, USA) and a human IL-8 ELISA kit
(BD Pharmingen, San Diego, CA, USA), according to the
manufacturer’s instructions. All samples were assayed in
triplicate. Absorbance was read at 450 nm.
Evaluation of cell viability by
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay
Differentiated Caco-2 cells (20 days
post-confluence) in 96-well plates were treated with various
concentrations of QHCY for 24 h. The cytotoxic effect of QHCY on
the Caco-2 cells was examined using the MTT colorimetric assay. MTT
(100 μl, 0.5 mg/ml in PBS) was briefly added to each well
and the samples were incubated for an additional 4 h at 37°C. The
purple-blue MTT formazan precipitate was dissolved in 100 μl
DMSO. The absorbance was measured at 570 nm using a
spectrophotometer reader (Model ELX800; BioTek Instruments, Inc.,
Winooski, VT, USA).
Western blot analysis
Differentiated Caco-2 cells (20 days
post-confluence) in 6-well plates were pre-incubated with various
concentrations of QHCY for 1 h prior to being stimulated with 1
μg/ml of LPS for 30 mins. At the end of experiment, the
cells were washed with ice-cold phosphate-buffered saline (PBS).
The cells were then lysed with cell lysis buffer (50 mM Tris-HCl,
pH 7.4, 150 mM NaCl, 0.5% NP-40, 5 mM EDTA, 50 mM NaF and 1 mM
PMSF) containing protease and phosphatase inhibitor (PI) cocktails.
The cell lysate was centrifuged at 10,000 × g at 4°C for 10 min and
the supernatant was collected to examine the protein expression
levels of IĸB-α and p-IĸB-α. To determine NF-ĸB nuclear
translocation, a nuclear extract was prepared: prior to lysis, the
cells were incubated in 0.5 ml hypotonic buffer A (10 mM HEPES, pH
7.9, 0.75 mM spermidine, 0.15 mM spermine, 0.1 mM EDTA and 0.1 mM
EGTA) and allowed to swell at 4°C. The cells were then sedimented
at 300 × g for 10 min. The supernatant was removed and replaced
with 1 ml fresh hypotonic buffer A plus PI cocktail. Cells were
homogenized by 10–15 strokes in a Dounce homogenizer and then
incubated in 5 ml sucrose restoration buffer which was composed of
0.5 ml hypotonic buffer B (500 mM HEPES, pH 7.9, 7.5 mM spermidine,
1.5 mM spermine, 2 mM EDTA, 2 mM EGTA and 10 mM DTT) and 4.5 ml of
7.5% sucrose). Nuclei were sedimented by centrifugation at 14,000 ×
g for 1 min. The supernatant was removed and the pellet was
resuspended in lysis buffer. Protein concentrations in total
lysates or nuclear extracts were quantified using BCA. Equivalent
amounts of protein were resolved in 12% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels and
electroblotted. The PVDF membranes were blocked with 5% skimmed
milk and probed with primary antibodies against IĸB-α, p-IĸB-α,
p50, RelA and β-actin (1:1,000) overnight at 4°C and then with
appropriate HRP-conjugated secondary antibody followed by enhanced
chemiluminescence detection.
Statistical analysis
Data were analyzed using the SPSS package for
Windows (Version 11.5; SPSS, Inc., Chicago, IL, USA). Statistical
analysis of the data was performed with the Student’s t-test and
one-way ANOVA. P<0.05 was considered to indicate a statistically
significant result.
Results
QHCY inhibits LPS-induced inflammatory
response in intestinal epithelial cells
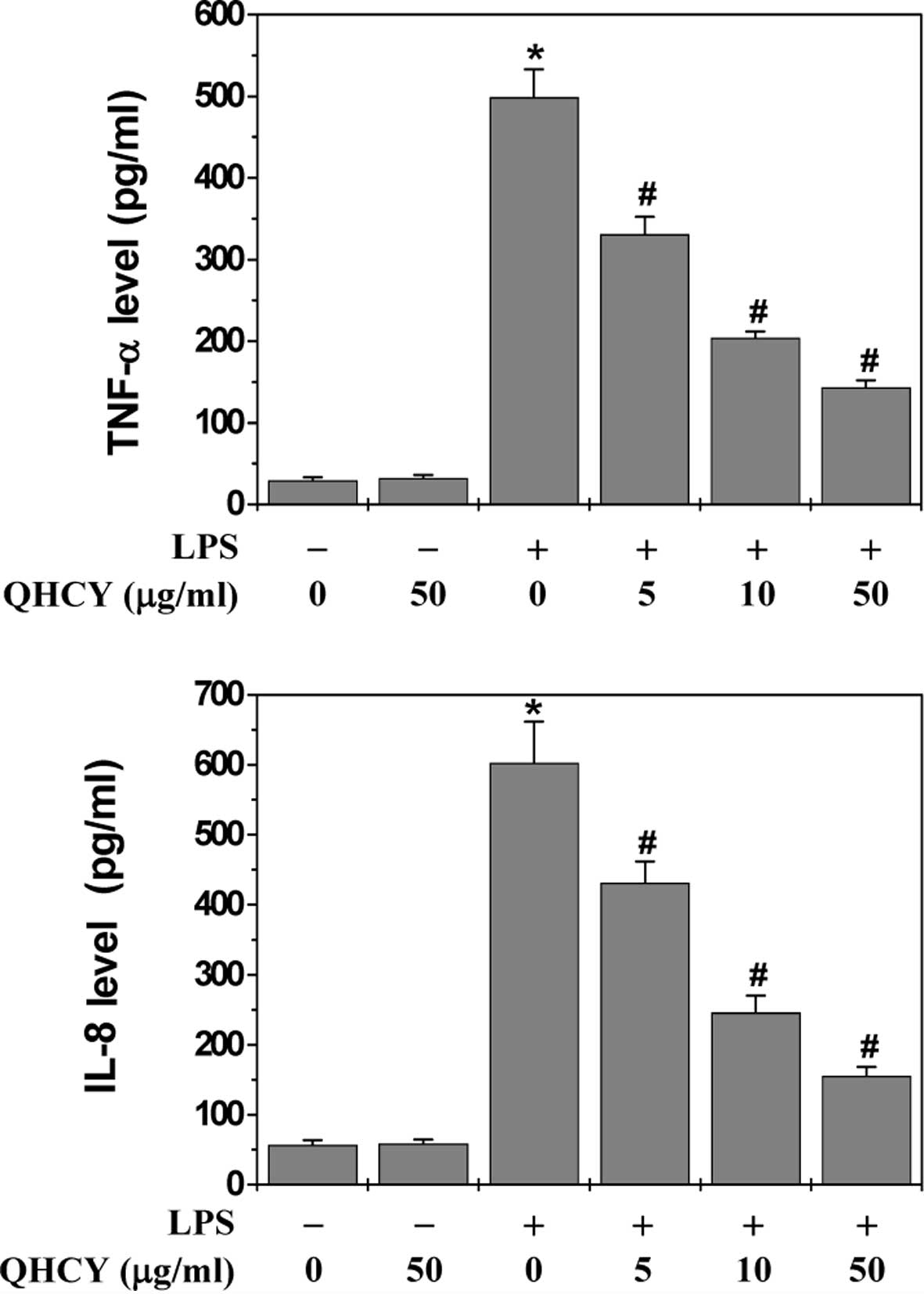
The effect of QHCY on LPS-induced inflammation in
differentiated Caco-2 cells was evaluated by measuring the
secretion levels of pro-inflammatory cytokines (TNF-α and IL-8)
since the release of cytokines is considered to represent an
indicator of inflammatory response. Following pretreatment with
various concentrations of QHCY for 1 h, differentiated Caco-2 cells
were stimulated with 1 μg/ml LPS for 24 h and the levels of
TNF-α and IL-8 in the culture supernatant were assessed by ELISA.
As shown in Fig. 1, LPS
stimulation significantly induced the release of TNF-α and IL-8 in
Caco-2 cells. However, QHCY treatment significantly and
concentration-dependently reduced the LPS-induced secretion of
TNF-α and IL-8, indicating that QHCY may inhibit inflammation in
intestinal epithelial cells.
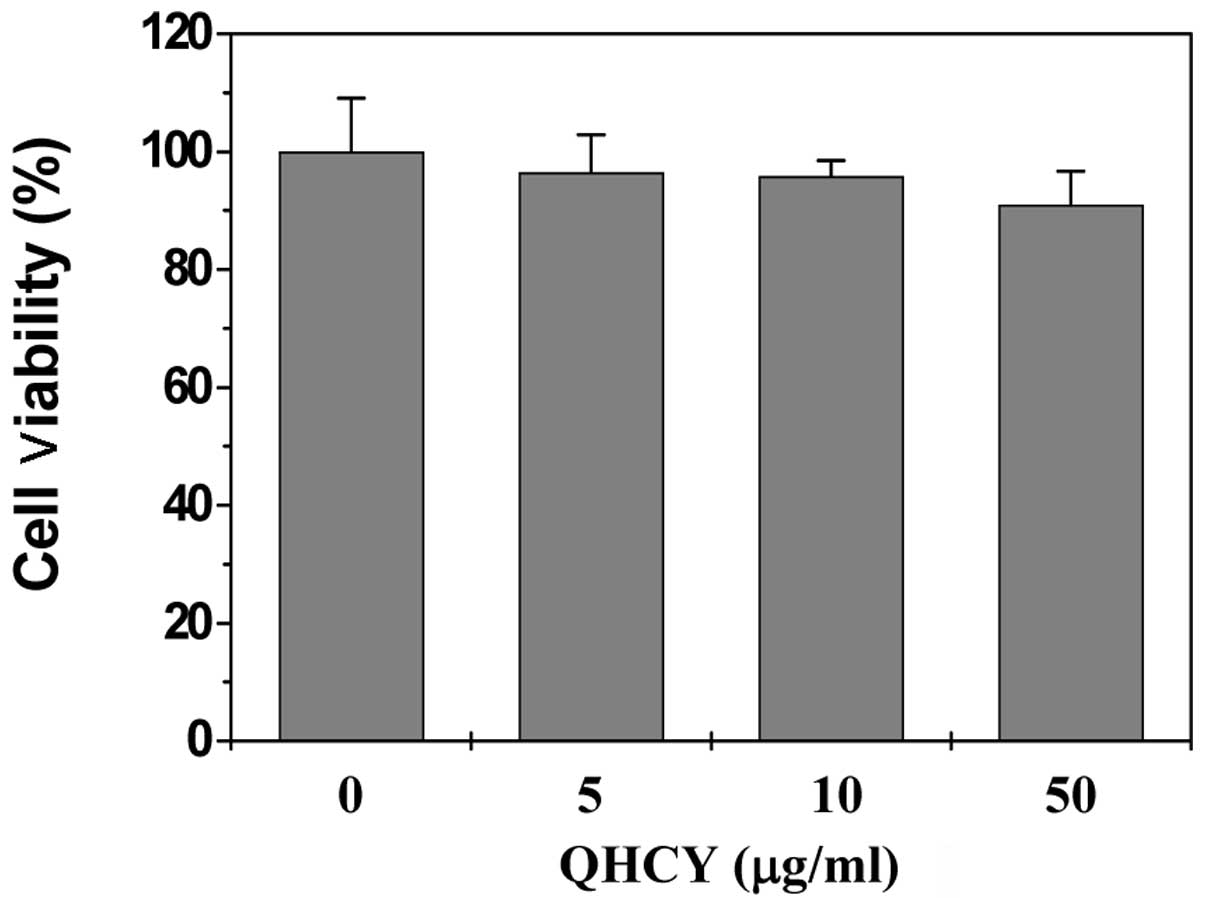
Cytotoxicity of QHCY in Caco-2 cells
To exclude the possibility that the
anti-inflammatory activity of QHCY was due to cytoxicity, we
determined its effect on Caco-2 cell viability using the MTT assay.
As shown in Fig. 2, the cell
viability was not affected by treatment with QHCY and/or LPS,
suggesting that the inhibitory effect of QHCY on LPS-induced
inflammation in intestinal epithelial cells did not result from a
cytotoxic action.
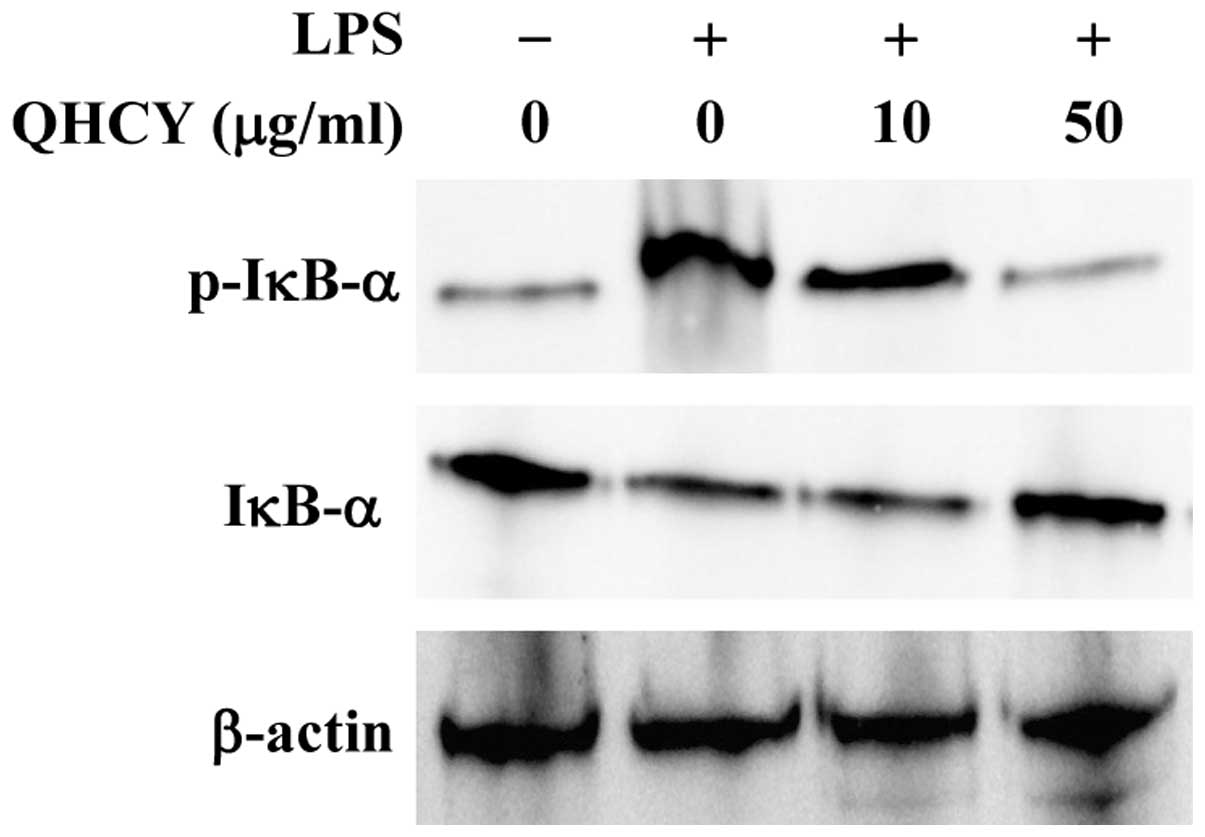
QHCY suppresses LPS-induced NF-ĸB
activation in intestinal epithelial cells
NF-κB is a critical transcription factor for
inflammation response. Activation of the NF-κB pathway consists of
several key processes, including the phosphorylation and
degradation of IκB and the subsequent nuclear translocation of
NF-κB. To investigate the mechanism of QHCY’s anti-inflammatory
activity, we examined its effect on LPS-induced activation of the
NF-κB pathway in intestinal epithelial cells. Differentiated Caco-2
cells were pretreated with QHCY for 1 h followed by stimulation
with LPS for another 30 min, and IκB phosphorylation was examined
by Western blotting. As shown in Fig.
3, upon LPS stimulation, the phosphorylation level of IκB
markedly increased and this increase was significantly attenuated
by QHCY in a concentration-dependent manner.
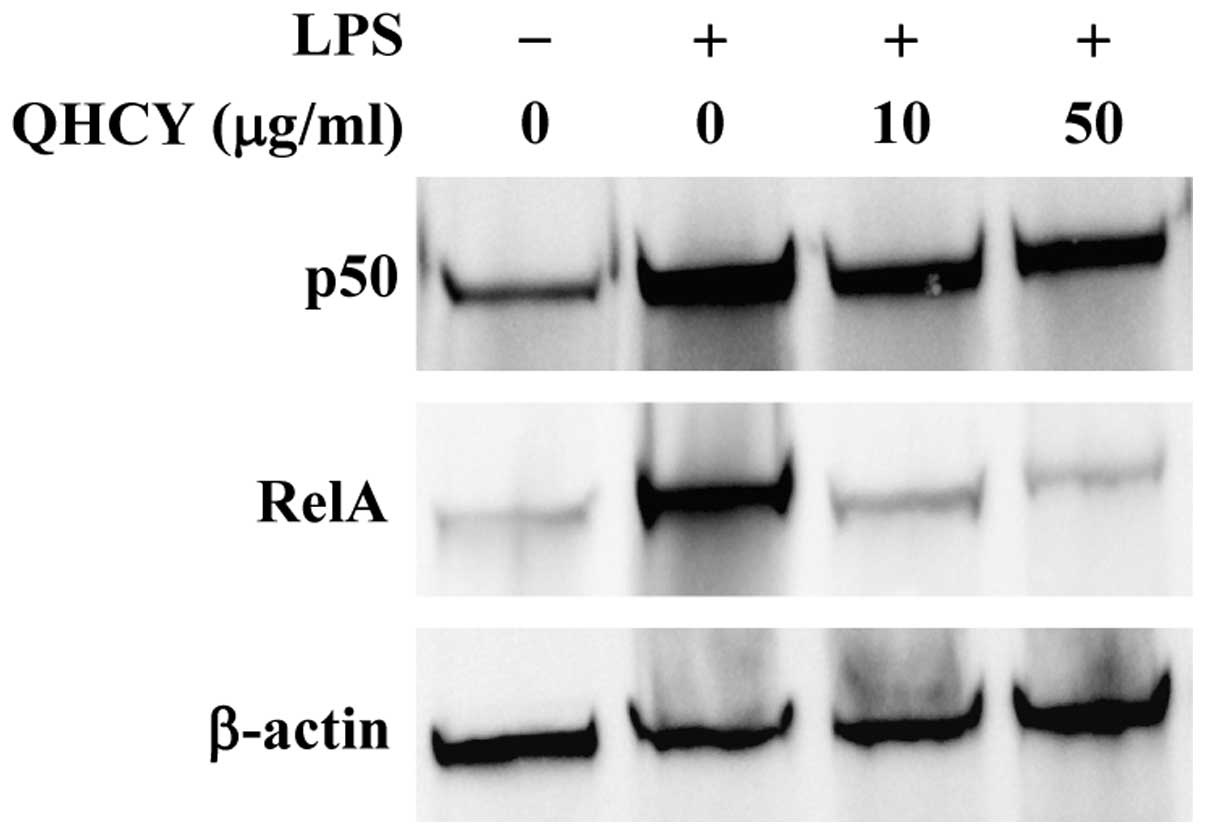
To verify these results, we examined the change in
nuclear content of two subunits of NF-κB, p50 and RelA, to evaluate
the effect of QHCY on LPS-induced NF-κB nuclear translocation,
which is an important step for NF-κB activation. In agreement with
the observed inhibitory effect on IĸB-α degradation, QHCY
concentration-dependently prevented the LPS-induced nuclear
translocation of p50 and RelA (Fig.
4).
Discussion
As a major form of IBD, UC is the result of a
chronic intestinal inflammatory response (1–3).
Since the precise etiology of UC remains unknown, there are no
effective long-term treatments. In addition, many currently used UC
treatments lead to the development of systemic immunosuppression.
Therefore, there is an urgent need for the development of new
therapeutic agents. Natural products, including TCM, have received
interest as they have relatively few side-effects and have been
used as alternative remedies for a variety of diseases, including
IBD (4–7). QHCY is a TCM formulation that has
been demonstrated to be effective in China for the clinical
treatment of UC (20–25). However, the molecular mechanism of
its anti-inflammatory activity remains to be elucidated. Therefore,
prior to the development of QHCY as an anti-UC agent, the mode of
its anti-inflammatory action requires elucidation.
Pro-inflammatory cytokines produced in the
intestine, including IL-8 and TNF-α, are important in the
pathogenesis of IBD; the release of pro-inflammatory cytokines is
therefore considered to represent an indicator of the inflammatory
response. Using LPS-stimulated Caco-2 cells as an in vitro
inflammatory model of the human intestinal epithelium, we observed
that QHCY significantly and concentration-dependently reduced the
LPS-induced secretion of TNF-α and IL-8, demonstrating that QHCY
inhibited the inflammatory response in intestinal epithelial cells.
The inflammatory response is tightly regulated by TLRs, a family of
pattern-recognition receptors (PRRs), which enable immune systems
to recognize pathogen-associated molecular patterns (PAMPs).
Different TLRs recognize different PAMPs, including LPS that
functions as a specific ligand for TLR4 (8–10,26–29).
Following activation by ligand binding, TLR4 transduces the
immune-related signals to the nucleus via transcription factors,
including nuclear factor κB. As one of the most significant nuclear
transcription factors, NF-κB is involved in the control of several
important physiological processes, particularly the immune and
inflammatory responses. In unstimulated cells, NF-κB is sequestered
in the cytosol via interaction with inhibitory IκB proteins.
However, when cells receive pathological stimuli, IκB proteins are
phosphorylated by IκB kinase (IKK). Phosphorylation of IκB proteins
results in their ubiquitination and degradation, which subsequently
releases sequestered NF-κB, leading to its translocation to the
nucleus where it induces the expression of various pro-inflammatory
cytokines (15–19). Using Western blotting, we observed
that QHCY treatment inhibited the phosphorylation of IκB and the
nuclear translocation of NF-κB in Caco-2 cells in a
concentration-dependent manner, suggesting that QHCY suppresses the
activation of the NF-κB signaling pathway.
In conclusion, in the present study we demonstrated
that QHCY ameliorates the inflammatory response by inhibiting the
activation of the NF-κB pathway. Our results further suggest that
QHCY may be an effective traditional Chinese formulation for the
treatment of UC and other inflammatory conditions.
Abbreviations:
|
QHCY
|
Qing Hua Chang Yin
|
|
UC
|
ulcerative colitis
|
|
IBD
|
inflammatory bowel disease
|
|
NF-κB
|
nuclear factor κB
|
|
TLR4
|
Toll-like receptor 4
|
|
LPS
|
lipopolysaccharide
|
|
TCM
|
traditional Chinese medicine
|
Acknowledgements
This study was supported by the
National Natural Science Foundation of China (no. 81173432).
References
|
1.
|
Rezaie A, Parker RD and Abdollahi M:
Oxidative stress and pathogenesis of inflammatory bowel disease: an
epiphenomenon or the cause? Dig Dis Sci. 52:2015–2021. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Podolsky DK: Inflammatory bowel disease. N
Engl J Med. 347:417–429. 2002. View Article : Google Scholar
|
|
3.
|
Odashima M, Otaka M, Jin M, et al:
Successful treatment of refractory duodenal Crohn’s disease with
infliximab. Dig Dis Sci. 52:31–32. 2007.PubMed/NCBI
|
|
4.
|
Treasure J: Herbal medicine and cancer: an
introductory overview. Semin Oncol Nurs. 21:177–183. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Stickel F and Schuppan D: Herbal medicine
in the treatment of liver diseases. Dig Liver Dis. 39:293–304.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Langmead L and Rampton DS: Review article:
complementary and alternative therapies for inflammatory bowel
disease. Aliment Pharmacol Ther. 23:341–349. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Bensoussan M, Jovenin N, Garcia B, et al:
Complementary and alternative medicine use by patients with
inflammatory bowel disease: results from a postal survey.
Gastroenterol Clin Biol. 30:14–23. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Caradonna L, Amati L, Magrone T,
Pellegrino M, Jirillo E and Caccavo D: Enteric bacteria,
lipopolysaccharides and related cytokines in inflammatory bowel
disease: biological and clinical significance. J Endotoxin Res.
6:205–214. 2000.
|
|
9.
|
Böcker U, Yezerskyy O, Feick P, et al:
Responsiveness of intestinal epithelial cell lines to
lipopolysaccharide is correlated with Toll-like receptor 4 but not
Toll-like receptor 2 or CD14 expression. Int J Colorectal Dis.
18:25–32. 2003.
|
|
10.
|
Bruewer M, Utech M, Ivanov AI, Hopkins AM,
Parkos CA and Nusrat A: Interferon-gamma induces internalization of
epithelial tight junction proteins via a macropinocytosis-like
process. FASEB J. 19:923–933. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Schreiber S, Nikolaus S and Hampe J:
Activation of nuclear factor kappa B inflammatory bowel disease.
Gut. 42:477–484. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Andresen L, Jørgensen VL, Perner A, Hansen
A, Eugen-Olsen J and Rask-Madsen J: Activation of nuclear factor
kappaB in colonic mucosa from patients with collagenous and
ulcerative colitis. Gut. 54:503–509. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Lakatos PL, Fischer S, Lakatos L, et al:
Current concept on the pathogenesis of inflammatory bowel
disease-crosstalk between genetic and microbial factors: pathogenic
bacteria and altered bacterial sensing or changes in mucosal
integrity take ‘toll’? World J Gastroenterol. 12:1829–1841.
2006.
|
|
14.
|
Lu YC, Yeh WC and Ohashi PS: LPS/TLR4
signal transduction pathway. Cytokine. 42:145–151. 2008. View Article : Google Scholar
|
|
15.
|
Snape WJ Jr and Kao HW: Role of
inflammatory mediators in colonic smooth muscle function in
ulcerative colitis. Dig Dis Sci. 33(Suppl 3): 65S–70S. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Neuman MG: Immune dysfunction in
inflammatory bowel disease. Transl Res. 149:173–186. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Eckmann L, Jung HC, Schürer-Maly C, Panja
A, Morzycka-Wroblewska E and Kagnoff MF: Differential cytokine
expression by human intestinal epithelial cell lines: regulated
expression of interleukin 8. Gastroenterol. 105:1689–1697.
1993.PubMed/NCBI
|
|
18.
|
Wang D, DuBois RN and Richmond A: The role
of chemokines in intestinal inflammation and cancer. Curr Opin
Pharmacol. 9:688–696. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Brynskov J, Foegh P, Pedersen G, et al:
Tumour necrosis factor alpha converting enzyme (TACE) activity in
the colonic mucosa of patients with inflammatory bowel disease.
Gut. 51:37–43. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Wang XY and Tian DL: Etiological and
pathological characteristics of ulcerative colitis and TCM
differentiation and treatment. Beijing Zhong Yi Yao Da Xue Xue Bao.
30:554–559. 2007.(In Chinese).
|
|
21.
|
Gong YP, Liu W, Ma GT, et al: Randomized
control study of ‘Qingchang Suppository’ on ulcerative colitis.
Shanghai Zhong Yi Yao Da Xue Xue Bao. 21:33–36. 2007.(In
Chinese).
|
|
22.
|
Fu NL and Huang JY: Progress of clinical
research of traditional Chinese medicine for the treatment of
ulcerative colitis. Journal of Traditional Chinese Medicine.
40:501–503. 1999.(In Chinese).
|
|
23.
|
Li QG: An idea about treatment of
ulcerative colitis by TCM methods. Beijing Zhong Yi. 23:149–150.
2004.(In Chinese).
|
|
24.
|
Wang CH, Gao WY, Li YF, et al: Study of
Fufangkushen colon-release capsule on ulcerative colitis of
endo-retention of damp heat type. Xian Dai Zhong Xi Yi Jie He Za
Zhi. 18:13–15. 2009.(In Chinese).
|
|
25.
|
Chen JT, Ke X, Fu XY, et al: The clinical
study of heat-clearing and damp-drying on the treatment of
damp-heat ulcerative colitis. Zhongguo Zhong Xi Yi Jie He Xiao Hua
Za Zhi. 17:256–257. 2009.(In Chinese).
|
|
26.
|
Kawai T and Akira S: TLR signaling. Cell
Death Differ. 13:816–825. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Johnson GB, Brunn GJ, Kodaira Y and Platt
JL: Receptor-mediated monitoring of tissue well-being via detection
of soluble heparan sulfate by Toll-like receptor 4. J Immunol.
168:5233–5239. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Lehnardt S, Schott E and Trimbuch T: A
vicious cycle involving release of heat shock protein 60 from
injured cells and activation of Toll-like receptor 4 mediates
neurodegeneration in the CNS. J Neurosci. 28:2320–2331. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Smiley ST, King JA and Hancock WW:
Fibrinogen stimulates macrophage chemokine secretion through
Toll-like receptor 4. J Immunol. 167:2887–2894. 2001. View Article : Google Scholar : PubMed/NCBI
|