Introduction
With the continuous improvement in the standard of
living worldwide, the population of individuals with hyperlipidemia
has expanded. Hyperlipidemia damages multiple organ systems,
particularly the cardiovascular system, and eventually leads to
atherosclerosis (AS), a complex vascular inflammatory disease. The
common pathological process of AS is the formation of an
atheromatous plaque, accompanied by the disorder of lipid
metabolism and damage to endothelial function (1). Hyperglycemia, hypertriglyceridemia,
hypercholesterolemia and hyperinsulinemia have been correlated with
a higher risk of heart and cerebrovascular diseases (2). There are >15 million fatalities
worldwide due to cardio- and cerebrovascular diseases every year,
making these diseases the leading cause of mortality (3). There is therefore a fundamental
requirement to decrease the levels of glucose and lipids.
In recent years, peroxisome proliferator-activated
receptors (PPARs) have been observed to be expressed in regions of
atherosclerotic injury (4).
Following activation, PPARs are able to protect endothelial cells
and improve the function of the endothelium through a variety of
pathways. PPARγ agonists have been demonstrated to inhibit the
expression of CD68, monocyte chemoattractant protein-1 (MCP-l),
vascular cell adhesion molecule-1 (VCAM-l) and tumor necrosis
factor-α (TNF-α) in rats with low-density lipoprotein (LDL)
receptor-deficiency, in addition to hindering the
monocyte-macrophage accumulation in the vascular wall (5). Furthermore, PPARγ downregulates the
expression of the MCP-l receptor, C-C chemokine receptor type-2
(CCR2) (6) and thereby reduces the
inflammatory reaction in local vessels. PPARγ ligands may be
mediated by nitric oxide (NO), which effectively inhibits the
expression of matrix metalloproteinase (MMP)-9 in macrophages
(7). As a member of the nuclear
receptor superfamily, PPARγ participates in the formation of fats,
and lipid and glucose metabolism. In addition, it has a major
impact on vascular biology and inflammation, particularly in the
development of atherosclerosis. PPARγ is able to protect blood
vessels by preserving vascular endothelial function, regulating
inflammatory cytokine and adhesion molecule expression, inhibiting
macrophage activation, promoting the reversal of cholesterol
transport, inhibiting vascular smooth muscle cell proliferation,
and migrating and stabilizing atherosclerotic plaques (8).
Caspases are a group of cysteine proteases with
aspartic acid-specific restriction sites, which cause apoptosis
through protein lysis (9). The
caspase family of proteases may directly initiate the
disintegration of apoptotic cells, and is thus important in the
molecular mechanisms of apoptosis (10). The caspases serve as convergent
points in a number of apoptotic pathways, and the majority of
caspase family proteases act as significant promoters or effectors
of apoptosis (11). At present, 14
types of caspases are recognized, and these are divided into three
categories: i) apoptosis-initiating factors, including caspase-8;
ii) apoptosis effectors, including caspase-3, which may be
activated by an upstream promoter; iii) those mainly involved in
cytokine-mediated inflammatory response and play a supporting role
in the death receptor-mediated apoptosis pathway. Once activated,
the caspases act on specific substrates and induce biochemical and
morphological changes in the cells, resulting in apoptosis.
Caspase-3 is the primary effector molecule and has critical
functions (12). Animal
experiments have demonstrated that the apoptosis of endothelial
cells may be induced by homocysteine (Hcy), and that a large number
of apoptotic endothelial cells are present in plaques. Thus,
Hcy-induced endothelial cell apoptosis may be closely correlated
with caspases. In this process, caspase-8 acts as the
apoptosis-initiating factor, while caspase-3 acts as the apoptotic
effector. However, the molecular mechanism(s) that initiates and
triggers the caspase-induced apoptosis of endothelial cells has not
yet been elucidated, and futher investigations are therefore
required.
The aim of the current study was to examine the
effect of folic acid (FA) and vitamin B12 (VB12) on the
mRNA expression of PPARγ, and caspase-3 and -8 in the abdominal
aortas of rats with hyperlipidemia. The hyperlipidemic rat model
was established with the artificial feeding of a high-fat diet. The
changes in the level of PPARγ mRNA expression, and the effects of
caspase-3 and -8 on endothelial cell apoptosis were examined via a
reverse transcription-polymerase chain reaction (RT-PCR) assay. The
protective effects of FA and VB12 on the vascular
endothelial cells was studied through drug intervention, in order
to provide new methods and a theoretical basis for the clinical
treatment of cardio- and cerebrovascular diseases.
Materials and methods
Experimental animals
Sixty healthy 4-week-old male Sprague Dawley (SD)
rats, weighing 110±10 g, were provided by the Experimental Animal
Center of Henan Province (Zhengzhou, China). The rats were randomly
divided into five groups (each n=12): the normal control (NC),
high-fat diet (HL), FA, VB12 and FA+VB12
groups. Following one week of adaptive feeding, the rats in the FA,
VB12 and FA+VB12 groups were fed with a high-fat diet,
and injected intraperitoneally with FA (0.5 mg/day), VB12 (0.05
mg/day) and FA+VB12 (0.5 mg/day and 0.05 mg/day),
respectively. The rats in the NC group were injected with 0.9% NaCl
solution (0.5 ml/day) and fed a normal diet, whereas those in the
HL group were fed a high-fat diet only. During the experiment, one
rat treated with FA, two rats treated with VB12 and two
rats treated with FA+VB12 died. The remaining rats survived and
were considered to be in a good condition. The study was approved
by the ethics committee of Xinxiang Medical University (Xinxiang,
China).
Serum preparation and blood lipid
determination
At the end of week 12, the rats were weighed and
injected intraperitoneally with 10% chloral hydrate (0.3 ml/100g).
Following anesthesia, blood samples were taken from the hearts of
the rats, and were centrifuged at 5000 × g for 5 min at 4°C. The
supernatant was stored at −20°C, prior to blood lipid
determination. The levels of total cholesterol (TC), triglycerides
(TG) and LDL and high-density lipoprotein (HDL) cholesterol were
quantified using an Architect CI8200 Automatic Biochemistry
Analyzer (Abbott Laboratories, Abbott Park, IL, USA).
RT-PCR assay of PPARγ and caspase-3 and
-8 mRNA levels in the abdominal aorta
Total RNA was extracted from the blood vessel using
TRIzol® reagent (Invitrogen Life Technologies, Carlsbad,
CA, USA). The extracted RNA was transformed to cDNA by RT-PCR,
using three pairs of primers: PPAR-γ, forward:
5′-CACAAGAGCTGACCCAATGGT TGCTG-3′ and reverse:
5′-CGCAGATCAGCAGACTCT GGGTTC-3′ (product size: 345 bp); caspase-8,
forward: 5′-AATGTTGGAGGAAAGCAATC-3′ and reverse: 5′-CAT
AGTCGTTGATTATCTTCAGC-3′ (product size: 380 bp); and caspase-3,
forward: 5′-TGTCATCTCGCTCTGGTACG-3′ and reverse:
5′-AAATGACCCCTTCATCACCA-3′ (product size: 279 bp). The PCR mixture
contained 20 μl reaction solution, including 2.0 μl
cDNA, 10 μl 1X Taq reaction buffer and 0.2 μM primer
(0.4 μl forward and reverse primers, respectively; Bioer
Technology Co., Ltd., Hangzhou, China). The PCR amplification was
performed at 94°C for 46 sec, 58°C for 46 sec and 72°C for 45 sec
(35 cycles). The PCR products were separated using 1.5% agarose gel
electrophoresis.
Statistical analysis
The mRNA levels were quantitatively analyzed using
the high-resolution Motic Images Advanced Software (Motic, Xiamen,
China). The data are presented as the arithmetic mean value ±
standard deviation. The Student’s t-test and analysis of variance
(ANOVA) were conducted using SPSS 13.0 software (SPSS, Inc.,
Chicago, IL, USA) for the comparison of two groups and multiple
groups, respectively. P<0.05 was considered to indicate a
statistically significant difference.
Results
Blood lipid levels in adult rats
The TC, TG and LDL and HDL cholesterol levels were
0.92±0.85, 3.93±0.23, 0.73±0.12 and 0.49±0.01 mmol/l, respectively,
in the rats of the HL group. Of these results, the TC, TG and LDL
cholesterol levels were significantly higher in the HL than in the
NC group (P<0.05), whereas the HDL level was significantly lower
in the HL than in the NC group (P<0.05). There were no
statistically significant differences observed between the blood
lipid levels of the rats in the HL group and the rats in the groups
that received drug intervention, i.e. FA, VB12 and
FA+VB12 (Table I). This
indicated that the application of FA and/or VB12 had no
significant effect on the blood lipid levels in adult rats.
 | Table I.Blood lipid levels in the serum of
adult rats following 12 weeks of treatment with FA and/or
VB12, combined with a high-fat diet. |
Table I.
Blood lipid levels in the serum of
adult rats following 12 weeks of treatment with FA and/or
VB12, combined with a high-fat diet.
| Group | n | TC (mmol/l) | TG (mmol/l) | LDL cholesterol
(mmol/l) | HDL cholesterol
(mmol/l) |
|---|
| NC | 12 | 0.40±0.05 | 1.65±0.98 | 0.50±0.05 | 0.87±0.10 |
| HL | 12 | 0.92±0.85a | 3.93±0.23a | 0.73±0.12a | 0.49±0.01a |
| FA | 11 | 0.92±0.10 | 3.83±0.31 | 0.76±0.15 | 0.48±0.04 |
|
FA+VB12 | 10 | 0.91±0.06 | 3.89±0.23 | 0.69±0.10 | 0.52±0.08 |
| VB12 | 10 | 0.90±0.08 | 3.91±0.21 | 0.74±0.07 | 0.48±0.04 |
| F statistic | - | 102.898 | 292.381 | 12.833 | 66.800 |
| P-value | | <0.05 | <0.05 | <0.05 | <0.05 |
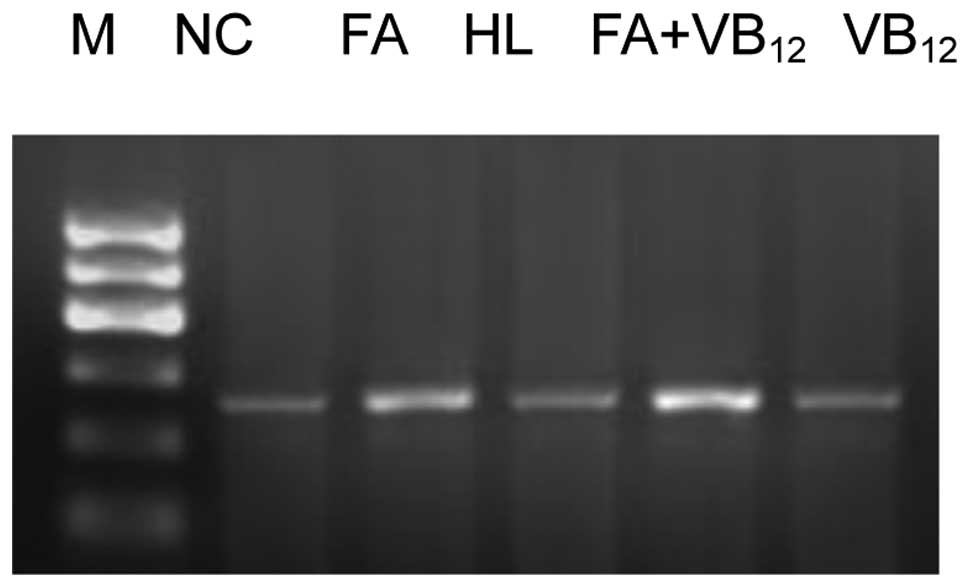
PPARγ and caspase-3 and -8 mRNA
expression in the vascular walls of adult rats PPARγ mRNA
expression
The levels of PPARγ mRNA expression in the rats of
the FA and FA+VB12 groups were significantly higher than
those of the HL and VB12 groups (P<0.05). In
addition, a statistically significant difference was observed
between the PPARγ mRNA expression levels in the rats of the FA and
FA+VB12 groups (P<0.05). The level of PPARγ mRNA
expression in the rats was significantly different between the NC
and HL groups (P<0.05), but not between the VB12 and
HL groups (P>0.05; Fig. 1).
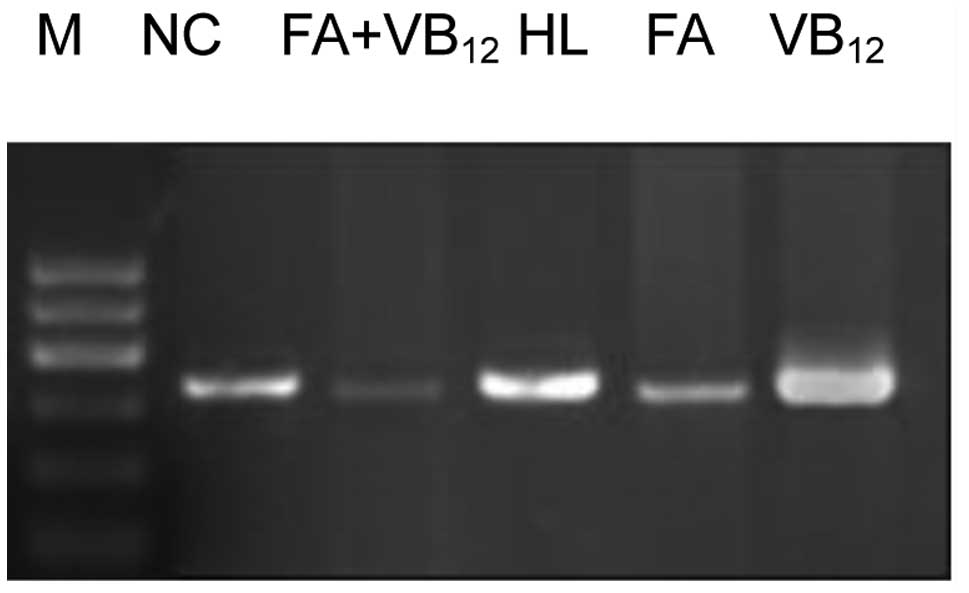
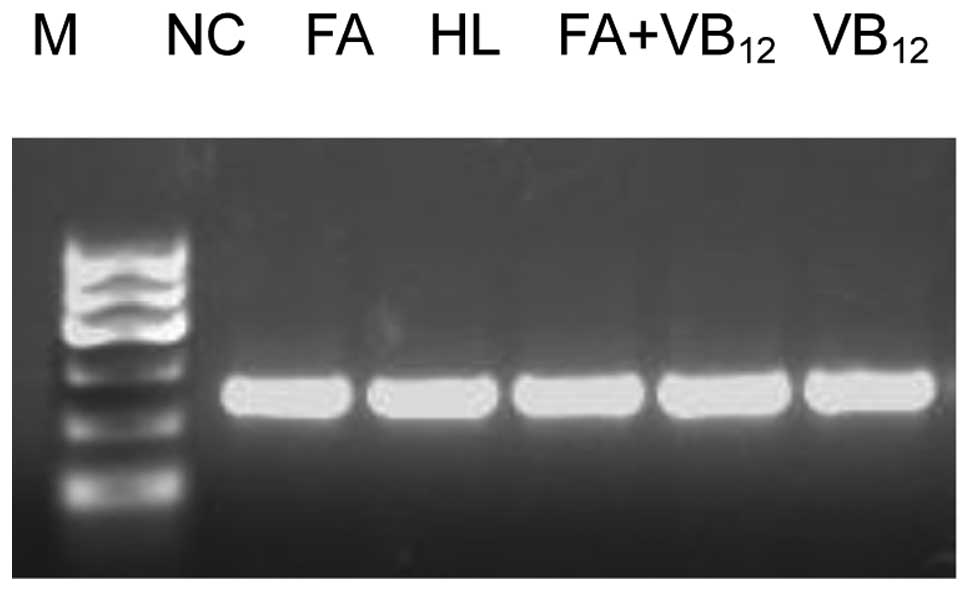
Caspase-3 and -8 mRNA expression
The caspase-3 and -8 mRNA expression levels in the
rats of the FA and FA+VB12 groups were significantly
lower than those in the rats of the HL and VB12 groups
(P<0.05). Furthermore, a significant difference was observed in
the levels of expression of these caspases between the FA and
FA+VB12 group rats (P<0.05). No significant
differences were observed in the levels of caspase-3 and -8 mRNA
expression between the rats of the VB12 and HL groups
(P>0.05), although the levels were significantly higher in the
HL group than in the NC group (P<0.05; Figs. 2–4).
Discussion
There are a number of risk factors correlated with
coronary heart disease (CHD), including immutable factors,
including age, gender and a family history of premature CHD, and
major modifiable factors, including hypertension, hyperglycemia,
hyperlipidemia, smoking and obesity. In the present study, a animal
model of CHD was established using SD rats fed a high-fat diet, and
FA and VB12 interventions were administered to the rats,
in order to explore the causative factors of CHD and the relevant
disease mechanisms, in addition to the potential clinical
application of FA and VB12(13). The results demonstrated that the
rats with hyperlipidemia (group HL) had significantly higher serum
TC, TG and LDL cholesterol levels, and lower serum HDL cholesterol
levels compared with the control rats (group NC, Table I). These results indicated that the
rats with hyperlipidemia were at risk of obesity, leading to
endothelial cell damage and eventually the induction and
acceleration of CHD occurrence and development.
By contrast, the FA, VB12 and FA+VB12
treatments did not exert any significant effects on the TC, TG and
LDL and HDL cholesterol levels in the rats with hyperlipidemia
(Table I), which demonstrated that
FA and VB12 are not suitable for the clinical treatment
of obesity or blood lipid metabolism.
With regard to the effect of FA and VB12
on the levels of caspase-3 and -8 mRNA expression in the abdominal
aorta of adult rats, it was revealed that the caspase-3 and -8 mRNA
levels increased in the HL and VB12-treated rats,
whereas the levels were reduced in the rats treated with FA and
FA+VB12, in comparison with the control group. These observations
suggested that FA is able to effectively inhibit Hcy-induced
endothelial cell apoptosis, thereby protecting the integrity of
endothelial function and preventing endothelial injury. Studies
have indicated that there are at least three apoptotic signaling
pathways that are related to the activation of caspases: i) the
mitochondrial/cytochrome c pathway; ii) the death receptor
pathway and iii) the endoplasmic reticulum pathway (14,15).
The signal for apoptosis may be conveyed through one or more
channels, thereby activating caspases and eventually inducing
apoptosis. It was revealed by Schuerwegh that NO is important in
the TNF-α-induced apoptosis of bovine chondrocytes, and it has been
confirmed that there is a close interrelation between TNF-α and
caspase-3, as well as between caspase-3 and NO, in the process of
apoptosis (16). High Hcy-induced
endothelial cell apoptosis is commonly accompanied by changes in
vascular endothelial growth factor (VEGF), endothelin (ET) and NO,
which affect the expression of apoptosis-related caspase enzymes,
and further induce the apoptosis of endothelial cells. FA is able
to reduce the Hcy concentration in the blood and change the VEGF,
ET and NO levels. As a result, this affects the expression of
caspase enzymes, and ultimately has an impact on the apoptosis of
endothelial cells (17).
In the present study, the levels of PPARγ mRNA were
observed be significantly lower in the HL and
VB12-treated rats than in the control group, which was
indicative of endothelial cell damage and dysfunction (Table II). By contrast, the PPARγ mRNA
levels increased significantly in the rats treated with FA and
FA+VB12 compared with those in the HL group, which
suggested that FA effectively reduced the Hcy level and prevented
endothelial cell dysfunction, thus protecting the integrity of
endothelial function and preventing endothelial injury. However,
further investigations are required, in order to study the detailed
molecular mechanisms of these processes, and to determine whether
FA and VB12 are PPARγ agonists. PPARγ agonists are
capable of activating PPARγ, thus protecting the endothelium, and
affecting the complex changes in the cytokines and inflammatory
factors that occur following endothelial damage, in the process of
AS (5,6).
 | Table II.Relative mRNA expression levels (gray
value ratio) in the abdominal aortas of adult rats following 12
weeks of treatment with FA and/or VB12, combined with a
high-fat diet. |
Table II.
Relative mRNA expression levels (gray
value ratio) in the abdominal aortas of adult rats following 12
weeks of treatment with FA and/or VB12, combined with a
high-fat diet.
| Group | n |
Caspase-8/β-actin |
Caspase-3/β-actin | PPARγ/β-actin |
|---|
| NC | 12 | 0.45±0.07 | 0.43±0.07 | 0.66±0.04 |
| HL | 12 | 0.70±0.12a | 0.70±0.13a | 0.44±0.04a |
| FA | 11 | 0.40±0.07b | 0.40±0.07b | 0.71±0.02b |
|
FA+VB12 | 10 | 0.25±0.02bc | 0.25±0.02bc | 0.80±0.01bc |
| VB12 | 10 | 0.71±0.02d | 0.72±0.12d | 0.46±0.04d |
| F statistic | | 51.962 | 52.031 | 254.531 |
| P-value | | <0.05 | <0.05 | <0.05 |
In conclusion, the removal of CHD-promoting factors
and the protection of endothelial cell function are required for
the treatment of CHD. This study demonstrated that FA intervention
and treatment was effective in reducing the Hcy concentration in
the serum of rats fed with a high-fat diet, and with elevated VEGF
levels and AS. In addition to an intensive lipid-lowering
treatment, the supplementary application of an appropriate quantity
of FA and VB12 may effectively protect the function of
the vascular endothelium, thus contributing to the prevention and
treatment of CHD.
Acknowledgements
This study was supported by the
Funding Project for Young First-Class Teachers of Colleges and
Universities in Henan Province, China (grant no. 2009GGJS-085).
References
|
1.
|
de Carvalho JF, Viana VS, Neto EF, et al:
Anti-lipoprotein lipase antibodies in patients with
hypertriglyceridemia without associated autoimmune disease. Isr Med
Assoc J. 13:350–353. 2011.PubMed/NCBI
|
|
2.
|
Díaz-Castro L, Cabello-Rangel H,
Cuevas-Pineda GJ, et al: Prevalence of the metabolic syndrome in a
psychiatric hospital in Mexico. Actas Esp Psiquiatr. 39:115–122.
2011.PubMed/NCBI
|
|
3.
|
Drechsler C, Grootendorst DC, Pilz S, et
al: Wasting and sudden cardiac death in hemodialysis patients: a
post hoc analysis of 4D (Die Deutsche Diabetes Dialyse Studie). Am
J Kidney Dis. 58:599–607. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Soskić SS, Dobutović BD, Sudar EM, et al:
Peroxisome proliferator-activated receptors and atherosclerosis.
Angiology. 62:523–534. 2011.PubMed/NCBI
|
|
5.
|
Sugawara A, Uruno A, Kudo M, et al:
Effects of PPARγ on hypertension, atherosclerosis, and chronic
kidney disease. Endocr J. 57:847–852. 2010.
|
|
6.
|
Pfützner A, Schöndorf T, Hanefeld M and
Forst T: High-sensitivity C-reactive protein predicts
cardiovascular risk in diabetic and nondiabetic patients: effects
of insulin-sensitizing treatment with pioglitazone. J Diabetes Sci
Technol. 4:706–716. 2010.
|
|
7.
|
Eberhardt W, Akool el-S, Rebhan J, et al:
Inhibition of cytokine-induced matrix metalloproteinase 9
expression by peroxisome proliferator-activated receptor alpha
agonists is indirect and due to a NO-mediated reduction of mRNA
stability. J Biol Chem. 277:33518–33528. 2002. View Article : Google Scholar
|
|
8.
|
Yuan X, Zhang Z, Gong K, et al: Inhibition
of reactive oxygen species/extracellular signal-regulated kinases
pathway by pioglitazone attenuates advanced glycation end
products-induced proliferation of vascular smooth muscle cells in
rats. Biol Pharm Bull. 34:618–623. 2011. View Article : Google Scholar
|
|
9.
|
Mazumder S, Plesca D and Almasan A:
Caspase-3 activation is a critical determinant of genotoxic
stress-induced apoptosis. Methods Mol Biol. 414:13–21.
2008.PubMed/NCBI
|
|
10.
|
Cao X, Pobezinskaya YL, Morgan MJ and Liu
ZG: The role of TRADD in TRAIL-induced apoptosis and signaling.
FASEB J. 25:1353–1358. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Stepień A, Izdebska M and Grzanka A: The
types of cell death. Postepy Hig Med Dosw (Online). 61:420–428.
2007.(In Polish).
|
|
12.
|
Rajesh M, Mukhopadhyay P, Bátkai S, et al:
Cannabidiol attenuates cardiac dysfunction, oxidative stress,
fibrosis, and inflammatory and cell death signaling pathways in
diabetic cardiomyopathy. J Am Coll Cardiol. 56:2115–2125. 2010.
View Article : Google Scholar
|
|
13.
|
Drenos F, Talmud PJ, Casas JP, et al:
Integrated associations of genotypes with multiple blood biomarkers
linked to coronary heart disease risk. Hum Mol Genet. 18:2305–2316.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Shafikhani SH, Morales C and Engel J: The
Pseudomonas aeruginosa type III secreted toxin ExoT is
necessary and sufficient to induce apoptosis in epithelial cells.
Cell Microbiol. 10:994–1007. 2008.
|
|
15.
|
Narasimhan P, Liu J, Song YS, et al: VEGF
Stimulates the ERK 1/2 signaling pathway and apoptosis in cerebral
endothelial cells after ischemic conditions. Stroke. 40:1467–1473.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Schuerwegh AJ, Dombrecht EJ, Stevens WJ,
et al: Influence of pro-inflammatory (IL-1 alpha, IL-6, TNF-alpha,
IFN-gamma) and anti-inflammatory (IL-4) cytokines on chondrocyte
function. Osteoarthritis Cartilage. 11:681–687. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Guerzoni AR, Biselli PM, Godoy MF, et al:
Homocysteine and MTHFR and VEGF gene polymorphisms: impact on
coronary artery disease. Arq Bras Cardiol. 92:263–268. 2009.
View Article : Google Scholar : PubMed/NCBI
|